Sutureless Aortic Valve Replacement with Perceval Bioprosthesis Superior to Transcatheter Aortic Valve Implantation: A Promising Option for the Gray-Zone of Aortic Valve Replacement Procedures—A State-of-the-Art Systematic Review, Meta-Analysis, and Future Directions
Abstract
1. Introduction
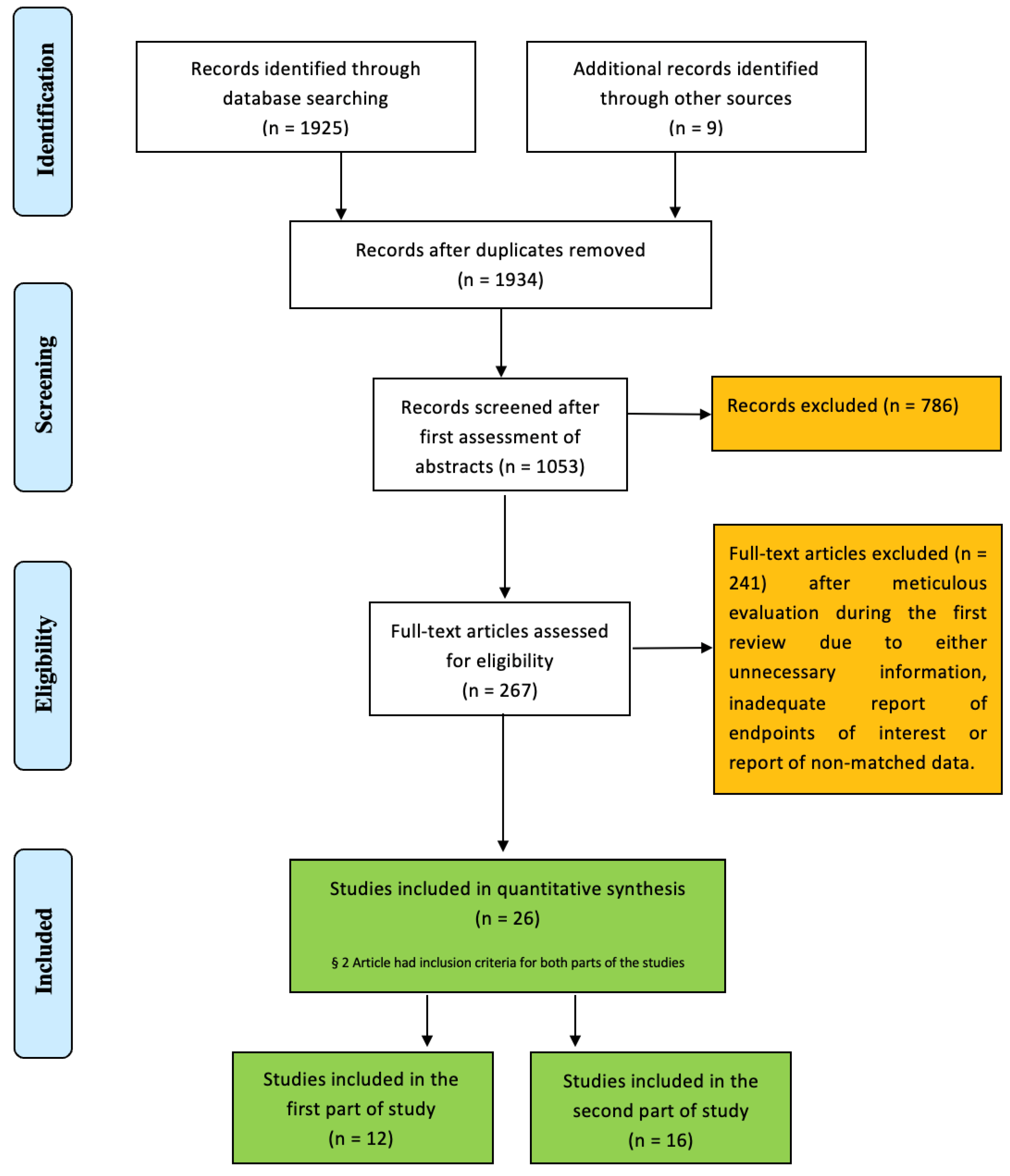
2. Materials and Methods
- -
- Eligibility criteria
- Population: all patients suffered from aortic valve pathologies;
- Intervention: SUAVR with Perceval bioprosthesis;
- Comparator: TAVI;
- Outcome: early and mid-term outcomes and complications after both procedures;
- Study design: Original articles were included in the initial assessment. Experimental studies, case reports, conference summaries, letters, editorials, reviews, and general overviews were excluded.
- -
- Literature search
- -
- Data extraction
- -
- Outcome measures
- -
- Ethics
- -
- Statistical analysis
3. Results
- First Part of the Study
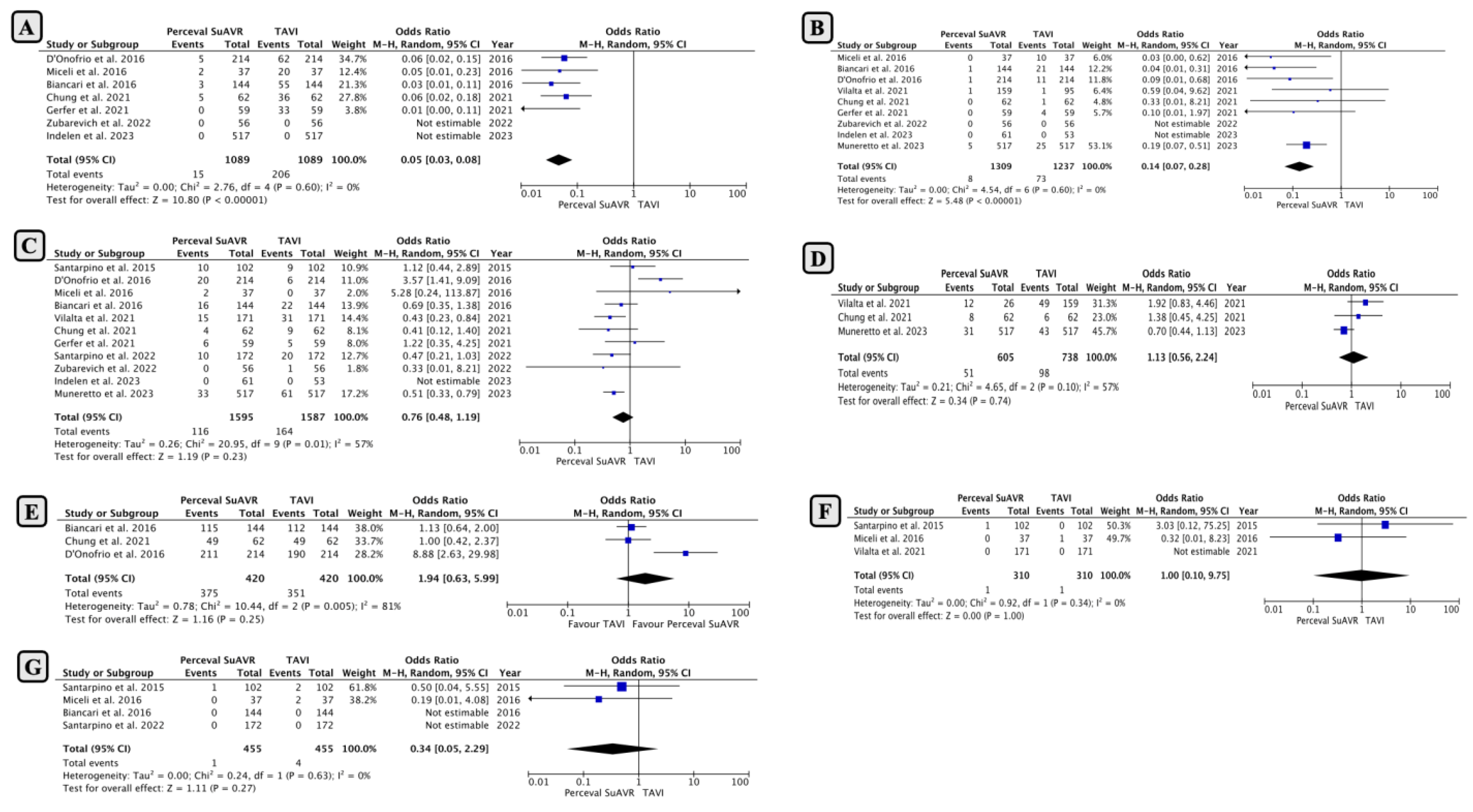
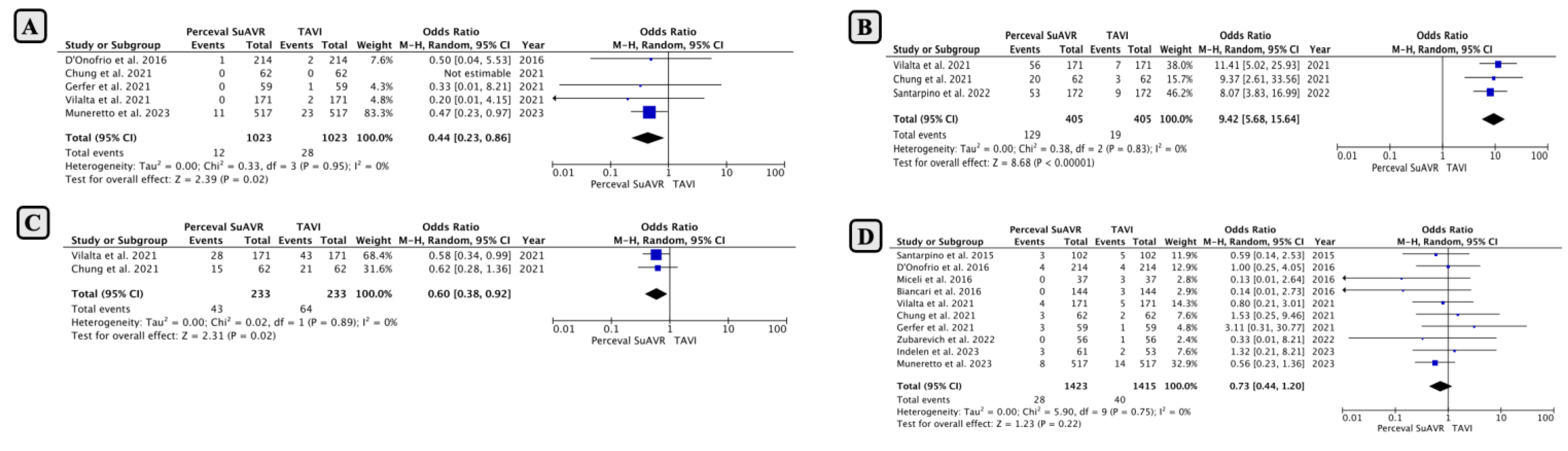
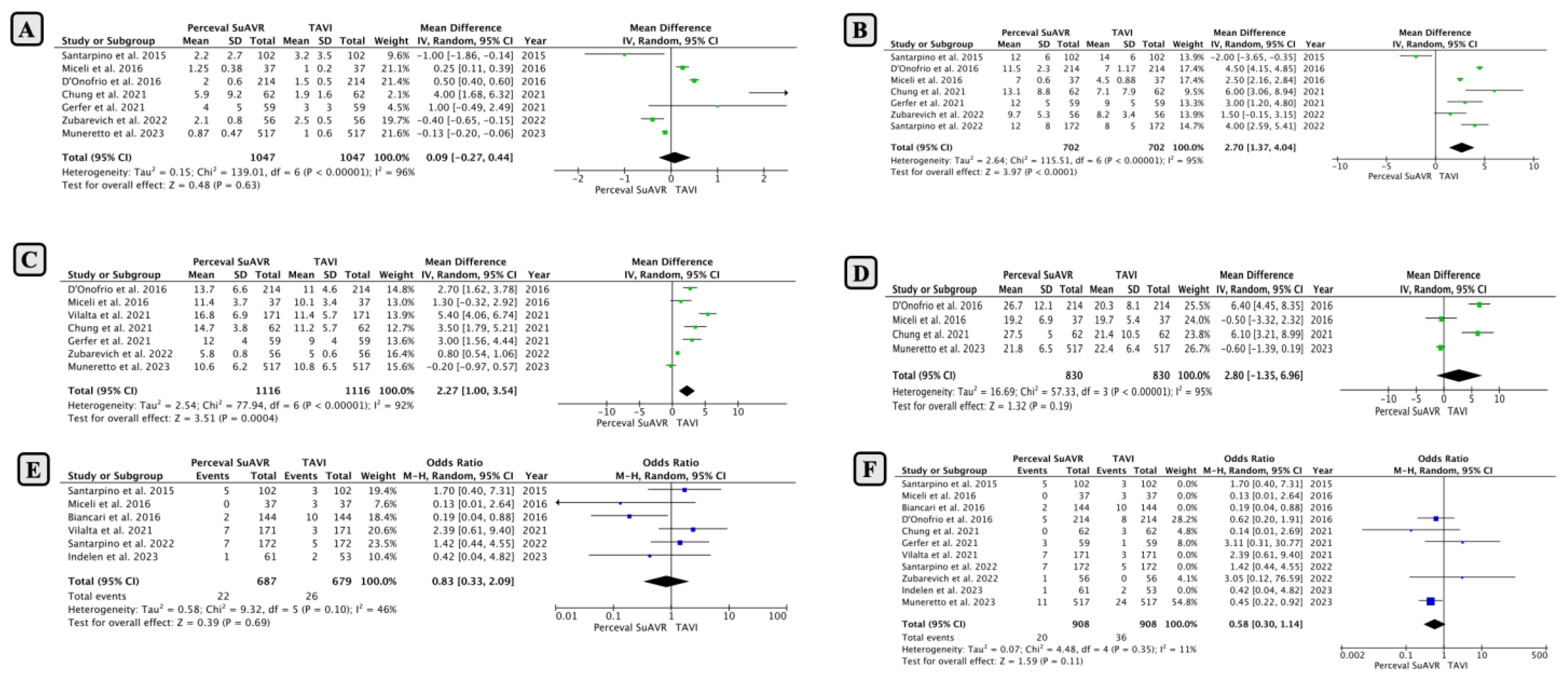
- Comparison of Early Outcomes between SUAVR and TAVI
- Subgroup Analysis Based on Minimally Invasive SUAVR
- Subgroup Analysis Based on Intermediate-High-Risk Clinical Profiles
- Comparison of Mid-Term Outcomes Between SUAVR and TAVI
- Second Part of the Study
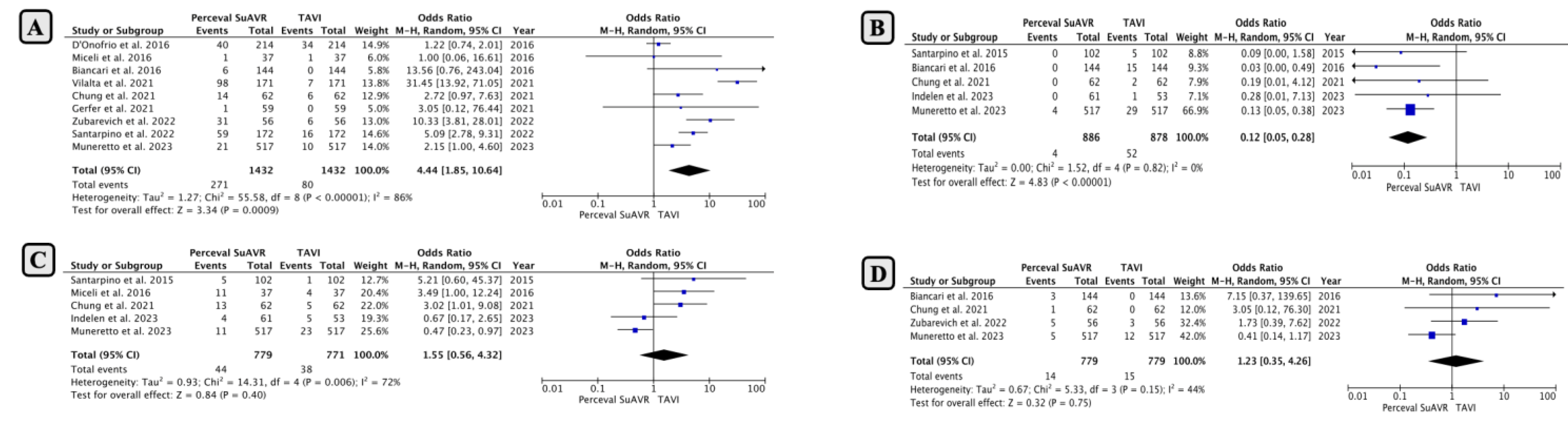
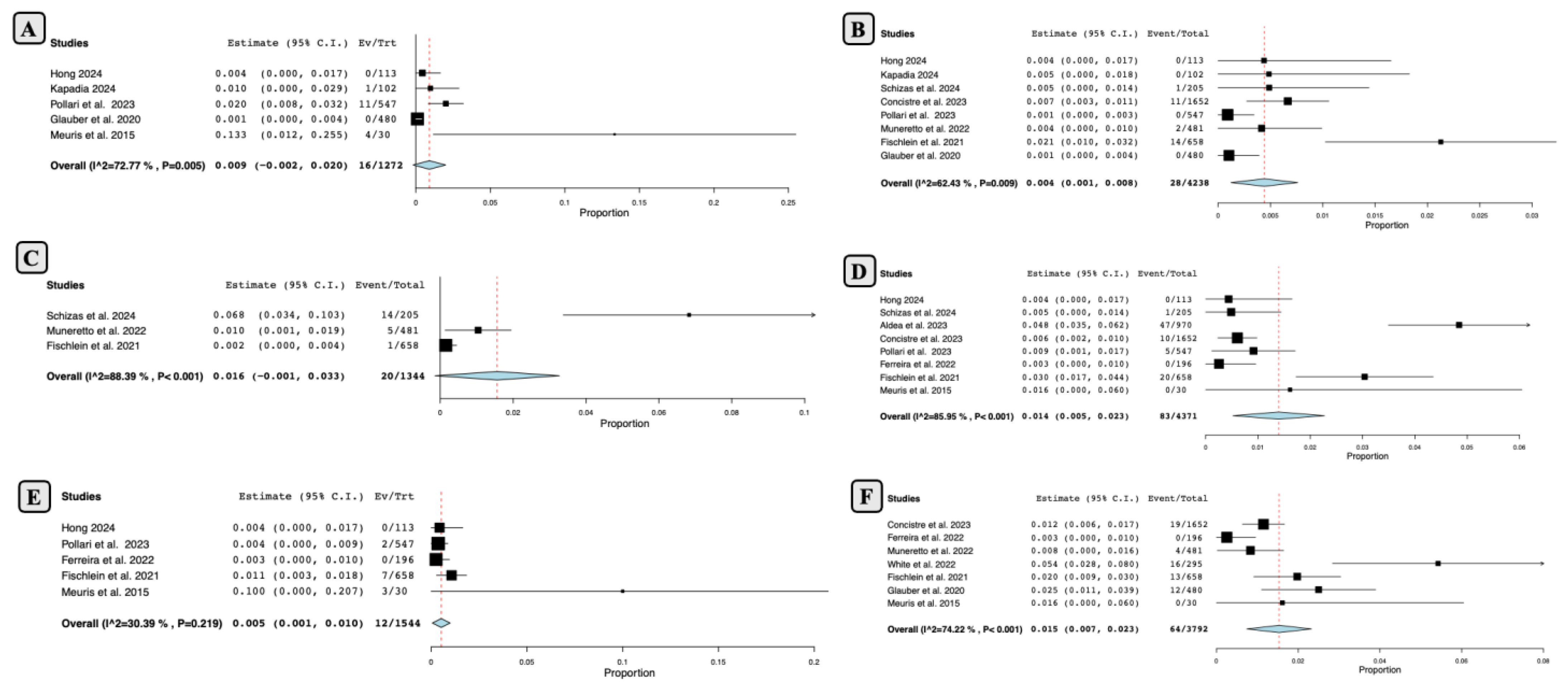
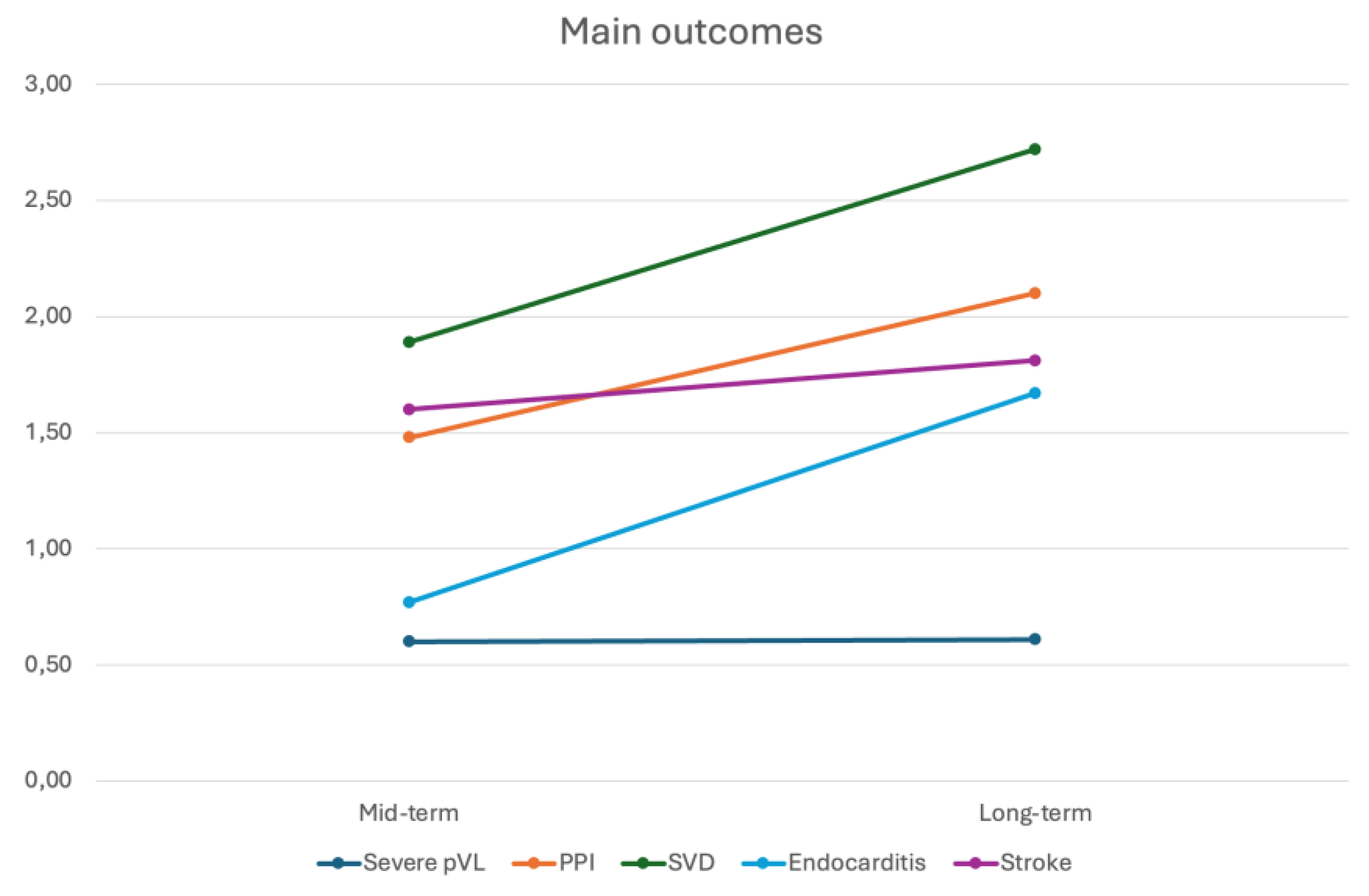
- Mid-term Outcomes Following SUAVR
- Long-term Outcomes Following SUAVR
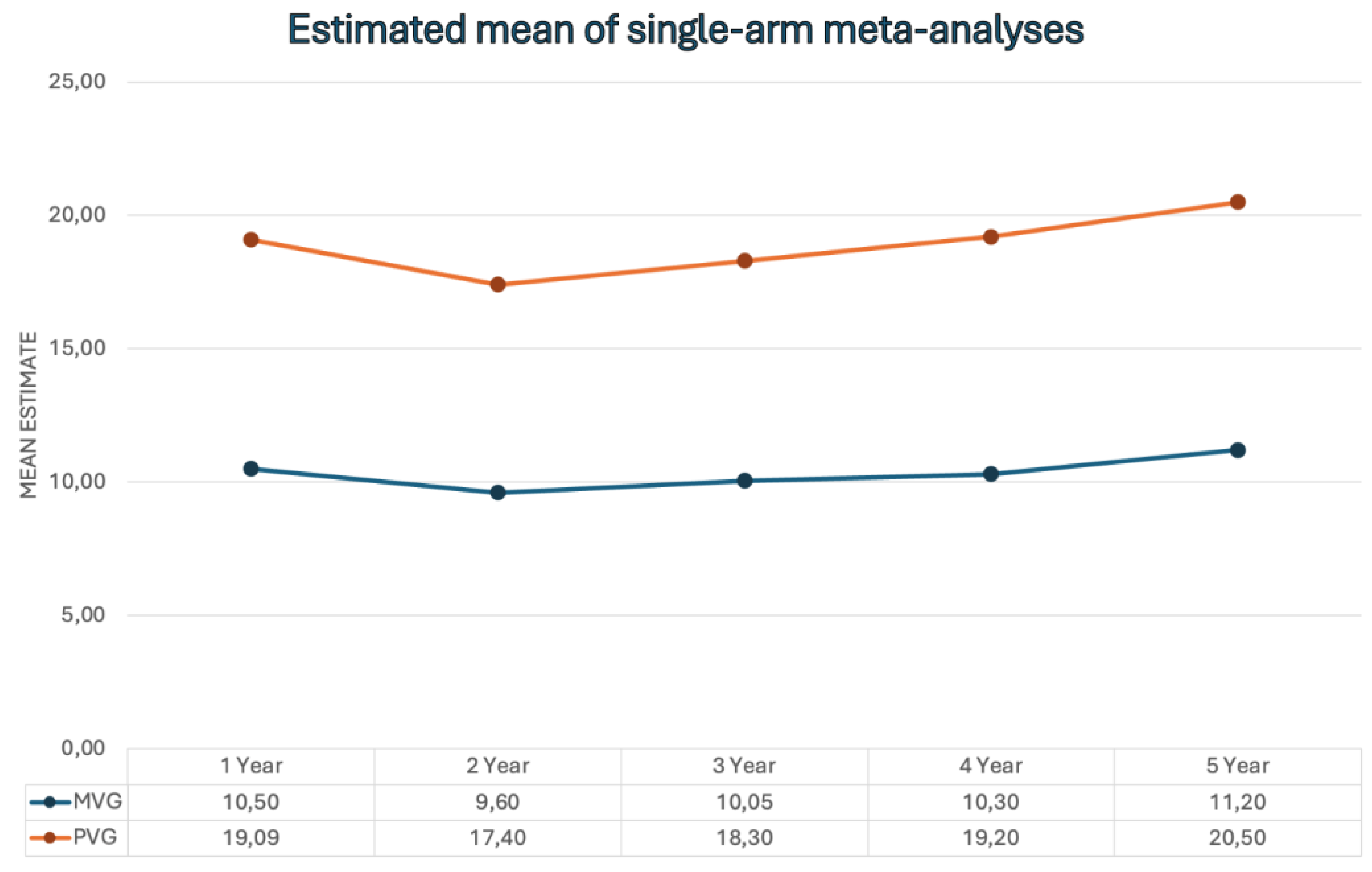
- Mid-Term and Long-Term Echocardiographic Findings After SUAVR
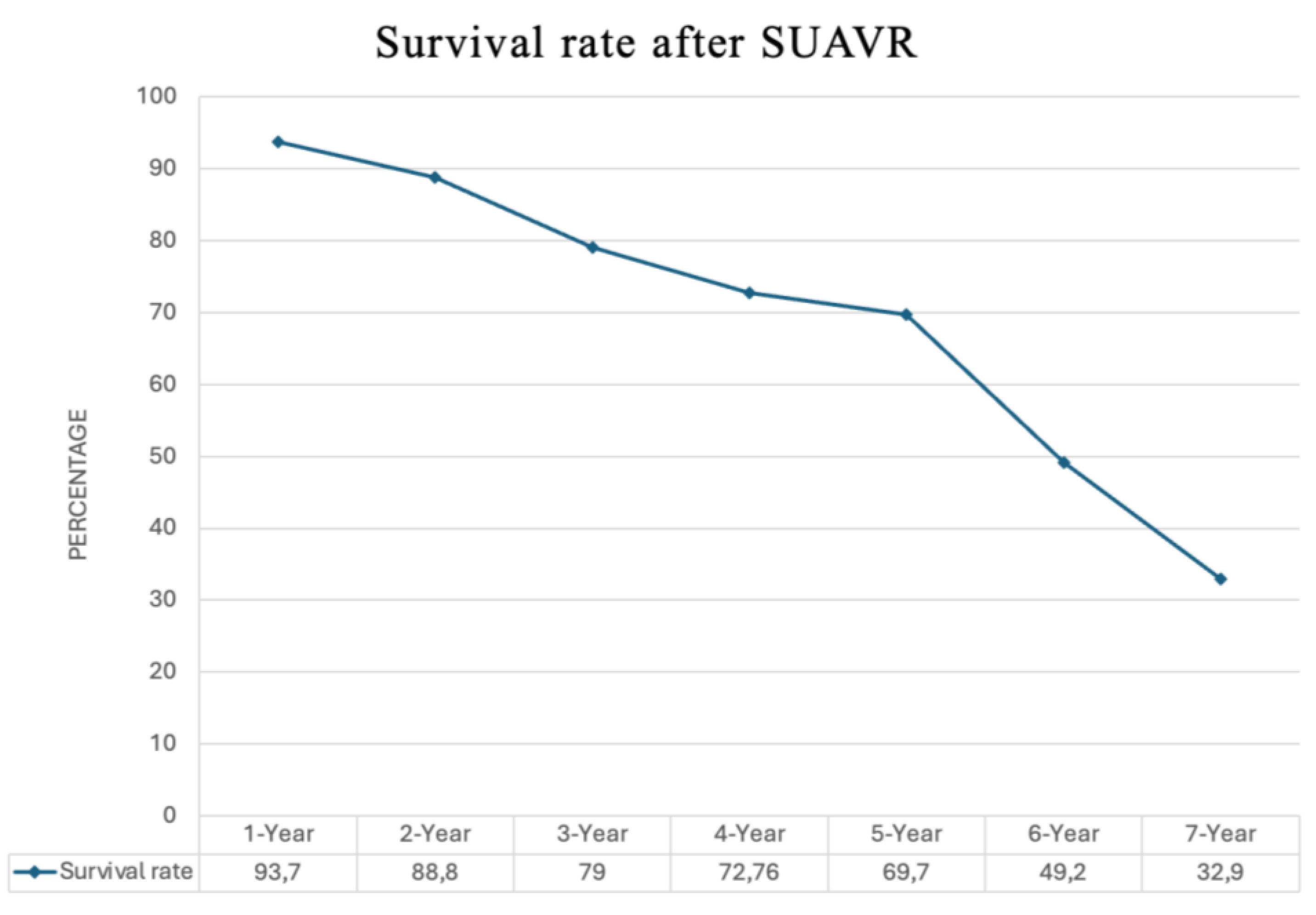
- Life Expectancy After SUAVR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Généreux, P.; Sharma, R.P.; Cubeddu, R.J.; Aaron, L.; Abdelfattah, O.M.; Koulogiannis, K.P.; Marcoff, L.; Naguib, M.; Kapadia, S.R.; Makkar, R.R.; et al. The Mortality Burden of Untreated Aortic Stenosis. J. Am. Coll. Cardiol. 2023, 82, 2101–2109. [Google Scholar] [CrossRef]
- Rahman, A.; Rowe, M.K. Aortic stenosis: Update in monitoring and management. Aust. J. Gen. Pract. 2024, 53, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Krasniqi, L.; Brandes, A.; Erik Mortensen, P.; Gerke, O.; Riber, L. Severe aortic stenosis treated with transcatheter aortic valve implantation or surgical aortic valve replacement with perimount in Western Denmark 2016–2022: A nationwide retrospective study. Interdiscip. CardioVasc. Thorac. Surg. 2024, 39, ivae122. [Google Scholar] [CrossRef] [PubMed]
- Auer, J.; Krotka, P.; Reichardt, B.; Traxler, D.; Wendt, R.; Mildner, M.; Ankersmit, H.J.; Graf, A. Selection for transcatheter versus surgical aortic valve replacement and mid-term survival: Results of the AUTHEARTVISIT study. Eur. J. Cardiothorac. Surg. 2024, 66, ezae214. [Google Scholar] [CrossRef]
- López Martínez, H.; Vilalta, V.; Farjat-Pasos, J.; Ferrer-Sistach, E.; Mohammadi, S.; Escabia, C.; Kalavrouziotis, D.; Resta, H.; Borrellas, A.; Dumont, E.; et al. Heart failure hospitalization following surgical or transcatheter aortic valve implantation in low-risk aortic stenosis. ESC Heart Fail. 2024. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, A.; Messina, A.; Lorusso, R.; Alfieri, O.R.; Fusari, M.; Rubino, P.; Rinaldi, M.; Di Bartolomeo, R.; Glauber, M.; Troise, G.; et al. Sutureless aortic valve replacement as an alternative treatment for patients belonging to the “gray zone” between transcatheter aortic valve implantation and conventional surgery: A propensity-matched, multicenter analysis. J. Thorac. Cardiovasc. Surg. 2012, 144, 1010–1016. [Google Scholar] [CrossRef]
- Muneretto, C.; Bisleri, G.; Moggi, A.; Di Bacco, L.; Tespili, M.; Repossini, A.; Rambaldini, M. Treating the patients in the ‘grey-zone’ with aortic valve disease: A comparison among conventional surgery, sutureless valves and transcatheter aortic valve replacement. Interact. Cardiovasc. Thorac. Surg. 2015, 20, 90–95. [Google Scholar] [CrossRef]
- Fialka, N.M.; El-Andari, R.; Wang, S.; Dokollari, A.; Kent, W.D.T.; Fatehi Hassanabad, A. The Perceval Sutureless Bioprosthetic Aortic Valve: Evolution of Surgical Valve Technology. Innovations 2024, 19, 125–135. [Google Scholar] [CrossRef]
- Vasanthan, V.; Kent, W.; Gregory, A.; Maitland, A.; Cutrara, C.; Bouchard, D.; Asch, F.; Adams, C. Perceval Valve Implantation: Technical Details and Echocardiographic Assessment. Ann. Thorac. Surg. 2019, 107, e223–e225. [Google Scholar] [CrossRef] [PubMed]
- Bowdish, M.E.; Habib, R.H.; Kaneko, T.; Thourani, V.H.; Badhwar, V. Cardiac Surgery After Transcatheter Aortic Valve Replacement: Trends and Outcomes. Ann. Thorac. Surg. 2024, 118, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.J.I.J.S. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2010, 8, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Vilalta, V.; Alperi, A.; Cediel, G.; Mohammadi, S.; Fernández-Nofrerias, E.; Kalvrouziotis, D.; Delarochellière, R.; Paradis, J.M.; González-Lopera, M.; Fadeuilhe, E.; et al. Midterm Outcomes Following Sutureless and Transcatheter Aortic Valve Replacement in Low-Risk Patients with Aortic Stenosis. Circ. Cardiovasc. Interv. 2021, 14, e011120. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.H.; Lee, S.H.; Ko, Y.G.; Lee, S.; Shim, C.Y.; Ahn, C.M.; Hong, G.R.; Shim, J.K.; Kwak, Y.L.; Hong, M.K. Transcatheter Aortic Valve Replacement versus Sutureless Aortic Valve Replacement: A Single Center Retrospective Cohort Study. Yonsei Med. J. 2021, 62, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Gerfer, S.; Mauri, V.; Kuhn, E.; Adam, M.; Eghbalzadeh, K.; Djordjevic, I.; Ivanov, B.; Gaisendrees, C.; Frerker, C.; Schmidt, T.; et al. Comparison of Self-Expanding RDV Perceval S versus TAVI ACURATE neo/TF. Thorac. Cardiovasc. Surg. 2021, 69, 420–427. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, A.; Salizzoni, S.; Rubino, A.S.; Besola, L.; Filippini, C.; Alfieri, O.; Colombo, A.; Agrifoglio, M.; Fischlein, T.; Rapetto, F.; et al. The rise of new technologies for aortic valve stenosis: A comparison of sutureless and transcatheter aortic valve implantation. J. Thorac. Cardiovasc. Surg. 2016, 152, 99–109.e2. [Google Scholar] [CrossRef]
- Miceli, A.; Gilmanov, D.; Murzi, M.; Marchi, F.; Ferrarini, M.; Cerillo, A.G.; Quaini, E.; Solinas, M.; Berti, S.; Glauber, M. Minimally invasive aortic valve replacement with a sutureless valve through a right anterior mini-thoracotomy versus transcatheter aortic valve implantation in high-risk patients. Eur. J. Cardiothorac. Surg. 2016, 49, 960–965. [Google Scholar] [CrossRef]
- Muneretto, C.; Solinas, M.; Folliguet, T.; Di Bartolomeo, R.; Repossini, A.; Laborde, F.; Rambaldini, M.; Santarpino, G.; Di Bacco, L.; Fischlein, T. Sutureless versus transcatheter aortic valves in elderly patients with aortic stenosis at intermediate risk: A multi-institutional study. J. Thorac. Cardiovasc. Surg. 2022, 163, 925–935.e5. [Google Scholar] [CrossRef]
- Muneretto, C.; Di Bacco, L.; Pollari, F.; Baudo, M.; Solinas, M.; D’Alonzo, M.; Di Eusanio, M.; Rosati, F.; Folliguet, T.; Fischlein, T. Sutureless versus transcatheter valves in patients with aortic stenosis at intermediate risk: A multi-institutional European study. Surgery 2023, 174, 1153–1160. [Google Scholar] [CrossRef]
- Zubarevich, A.; Szczechowicz, M.; Amanov, L.; Arjomandi Rad, A.; Osswald, A.; Torabi, S.; Ruhparwar, A.; Weymann, A. Non-Inferiority of Sutureless Aortic Valve Replacement in the TAVR Era: David versus Goliath. Life 2022, 12, 979. [Google Scholar] [CrossRef] [PubMed]
- Santarpino, G.; Lorusso, R.; Moscarelli, M.; Mikus, E.; Wisniewski, K.; Dell’Aquila, A.M.; Margari, V.; Carrozzo, A.; Barbato, L.; Fiorani, V.; et al. Sutureless versus transcatheter aortic valve replacement: A multicenter analysis of “real-world” data. J. Cardiol. 2022, 79, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Santarpino, G.; Pfeiffer, S.; Jessl, J.; Dell’Aquila, A.; Vogt, F.; von Wardenburg, C.; Schwab, J.; Sirch, J.; Pauschinger, M.; Fischlein, T. Clinical Outcome and Cost Analysis of Sutureless Versus Transcatheter Aortic Valve Implantation with Propensity Score Matching Analysis. Am. J. Cardiol. 2015, 116, 1737–1743. [Google Scholar] [CrossRef] [PubMed]
- Indelen, C.; Bas, T.; Kar, A.; Ergenç, E.; Karademir, B.C.; Sismanoglu, M.; Kirali, K. Cost-Effectiveness and Clinical Outcome of Transcatheter Versus Sutureless Aortic Valve Replacement. Heart Surg. Forum 2023, 26, e284–e291. [Google Scholar] [CrossRef] [PubMed]
- Biancari, F.; Barbanti, M.; Santarpino, G.; Deste, W.; Tamburino, C.; Gulino, S.; Immè, S.; Di Simone, E.; Todaro, D.; Pollari, F.; et al. Immediate outcome after sutureless versus transcatheter aortic valve replacement. Heart Vessels 2016, 31, 427–433. [Google Scholar] [CrossRef][Green Version]
- Aldea, G.S.; Burke, C.R.; Fischlein, T.; Heimansohn, D.A.; Haverich, A.; Suri, R.M.; Ad, N. Does valve size impact hemodynamic, left ventricular mass regression, and prosthetic valve deterioration with a sutureless aortic valve? J. Thorac. Cardiovasc. Surg. 2023, 168, 502–509. [Google Scholar] [CrossRef]
- Concistré, G.; Baghai, M.; Santarpino, G.; Royse, A.; Scherner, M.; Troise, G.; Glauber, M.; Solinas, M. Clinical and hemodynamic outcomes of the Perceval sutureless aortic valve from a real-world registry. Interdiscip. Cardiovasc. Thorac. Surg. 2023, 36, ivad103. [Google Scholar] [CrossRef]
- Dokollari, A.; Margaryan, R.; Torregrossa, G.; Sicouri, S.; Cameli, M.; Mandoli, G.E.; Prifti, E.; Veshti, A.; Bonacchi, M.; Gelsomino, S. Risk predictors that impact long-term prognosis in patients undergoing aortic valve replacement with the Perceval sutureless bioprosthesis. Cardiovasc. Revasc. Med. 2023, 55, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.; Rua, N.; Sena, A.; Velho, T.R.; Gonçalves, J.; Junqueira, N.; Almeida, A.G.; Nobre, Â.; Pinto, F. Sutureless bioprosthesis for aortic valve replacement: Surgical and clinical outcomes. J. Card. Surg. 2022, 37, 4774–4782. [Google Scholar] [CrossRef]
- Fischlein, T.; Meuris, B.; Folliguet, T.; Hakim-Meibodi, K.; Misfeld, M.; Carrel, T.; Zembala, M.; Cerutti, E.; Asch, F.M.; Haverich, A. Midterm outcomes with a sutureless aortic bioprosthesis in a prospective multicenter cohort study. J. Thorac. Cardiovasc. Surg. 2022, 164, 1772–1780.e11. [Google Scholar] [CrossRef] [PubMed]
- Glauber, M.; Di Bacco, L.; Cuenca, J.; Di Bartolomeo, R.; Baghai, M.; Zakova, D.; Fischlein, T.; Troise, G.; Viganò, G.; Solinas, M. Minimally Invasive Aortic Valve Replacement with Sutureless Valves: Results from an International Prospective Registry. Innovations 2020, 15, 120–130. [Google Scholar] [CrossRef]
- Hong, S.; Son, J.W.; Yoon, Y. Clinical Midterm Results of Surgical Aortic Valve Replacement with Sutureless Valves. J. Chest Surg. 2024, 57, 255. [Google Scholar] [CrossRef]
- Kapadia, S.J.; Salmasi, M.Y.; Zientara, A.; Roussin, I.; Quarto, C.; Asimakopoulos, G. Perceval sutureless bioprosthesis versus Perimount sutured bioprosthesis for aortic valve replacement in patients with aortic stenosis: A retrospective, propensity-matched study. J. Cardiothorac. Surg. 2024, 19, 95. [Google Scholar] [CrossRef]
- Lamberigts, M.; Szecel, D.; Rega, F.; Verbrugghe, P.; Dubois, C.; Meuris, B. Sutureless aortic valves in isolated and combined procedures: Thirteen years of experience in 784 patients. J. Thorac. Cardiovasc. Surg. 2024, 167, 1724–1732.e1. [Google Scholar] [CrossRef] [PubMed]
- Meuris, B.; Flameng, W.J.; Laborde, F.; Folliguet, T.A.; Haverich, A.; Shrestha, M. Five-year results of the pilot trial of a sutureless valve. J. Thorac. Cardiovasc. Surg. 2015, 150, 84–88. [Google Scholar] [CrossRef]
- Pollari, F.; Mamdooh, H.; Hitzl, W.; Grossmann, I.; Vogt, F.; Fischlein, T. Ten years’ experience with the sutureless aortic valve replacement: Incidence and predictors for survival and valve durability at follow-up. Eur. J. Cardiothorac. Surg. 2023, 63, ezac572. [Google Scholar] [CrossRef] [PubMed]
- Schizas, N.; Samiotis, I.; Nazou, G.; Iliopoulos, D.C.; Anagnostopoulos, I.; Kousta, M.; Papaioannou, N.; Argiriou, M.; Dedeilias, P. Perceval-S over time. Clinical outcomes after ten years of usage. J. Cardiothorac. Surg. 2024, 19, 192. [Google Scholar] [CrossRef]
- Szecel, D.; Eurlings, R.; Rega, F.; Verbrugghe, P.; Meuris, B. Perceval Sutureless Aortic Valve Implantation: Midterm Outcomes. Ann. Thorac. Surg. 2021, 111, 1331–1337. [Google Scholar] [CrossRef]
- White, A.; Bozso, S.J.; Lakey, O.; Hong, Y.Z.; Wang, S.H.; Nagendran, J.; Moon, M.C. Rapid deployment valves versus conventional tissue valves for aortic valve replacement. J. Thorac. Cardiovasc. Surg. 2022, 163, 2036–2042. [Google Scholar] [CrossRef]
- Sá, M.P.; Jabagi, H.; Dokollari, A.; Awad, A.K.; Van den Eynde, J.; Malin, J.H.; Sicouri, S.; Torregrossa, G.; Ruhparwar, A.; Weymann, A.; et al. Early and late outcomes of surgical aortic valve replacement with sutureless and rapid-deployment valves versus transcatheter aortic valve implantation: Meta-analysis with reconstructed time-to-event data of matched studies. Catheter. Cardiovasc. Interv. 2022, 99, 1886–1896. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xie, Y.; Zhang, H.; Yang, P.; Zhang, Y.; Lu, C.; Liu, Y.; Wang, H.; Xu, Z.; Hu, J. Sutureless vs. rapid-deployment valve: A systemic review and meta-analysis for a direct comparison of intraoperative performance and clinical outcomes. Front. Cardiovasc. Med. 2023, 10, 1123487. [Google Scholar] [CrossRef]
- D’Onofrio, A.; Salizzoni, S.; Filippini, C.; Tessari, C.; Bagozzi, L.; Messina, A.; Troise, G.; Dalla Tomba, M.; Rambaldini, M.; Dalén, M.; et al. Surgical aortic valve replacement with new-generation bioprostheses: Sutureless versus rapid-deployment. J. Thorac. Cardiovasc. Surg. 2020, 159, 432–442. [Google Scholar] [CrossRef]
- Al-Sarraf, N.; Thalib, L.; Hughes, A.; Houlihan, M.; Tolan, M.; Young, V.; McGovern, E. Cross-clamp time is an independent predictor of mortality and morbidity in low- and high-risk cardiac patients. Int. J. Surg. 2011, 9, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Iino, K.; Miyata, H.; Motomura, N.; Watanabe, G.; Tomita, S.; Takemura, H.; Takamoto, S. Prolonged Cross-Clamping During Aortic Valve Replacement Is an Independent Predictor of Postoperative Morbidity and Mortality: Analysis of the Japan Cardiovascular Surgery Database. Ann. Thorac. Surg. 2017, 103, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Okuno, T.; Tomii, D.; Heg, D.; Lanz, J.; Praz, F.; Stortecky, S.; Reineke, D.; Windecker, S.; Pilgrim, T. Five-year outcomes of mild paravalvular regurgitation after transcatheter aortic valve implantation. EuroIntervention 2022, 18, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Laakso, T.; Laine, M.; Moriyama, N.; Dahlbacka, S.; Airaksinen, J.; Virtanen, M.; Husso, A.; Tauriainen, T.; Niemelä, M.; Mäkikallio, T.; et al. Impact of paravalvular regurgitation on the mid-term outcome after transcatheter and surgical aortic valve replacement. Eur. J. Cardiothorac. Surg. 2020, 58, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Niinami, H.; Sawa, Y.; Shimokawa, T.; Domoto, S.; Nakamura, Y.; Sakaguchi, T.; Ito, T.; Toda, K.; Amano, A.; Gersak, B. 1-year outcomes of patients implanted with the Perceval sutureless valve: The Japanese post-marketing surveillance study. Heart Vessels 2023, 38, 949–956. [Google Scholar] [CrossRef]
- Dokollari, A.; Ramlawi, B.; Torregrossa, G.; Sá, M.P.; Sicouri, S.; Prifti, E.; Gelsomino, S.; Bonacchi, M. Benefits and Pitfalls of the Perceval Sutureless Bioprosthesis. Front. Cardiovasc. Med. 2021, 8, 789392. [Google Scholar] [CrossRef] [PubMed]
- Cerillo, A.G.; Amoretti, F.; Mariani, M.; Cigala, E.; Murzi, M.; Gasbarri, T.; Solinas, M.; Chiappino, D. Increased Gradients After Aortic Valve Replacement with the Perceval Valve: The Role of Oversizing. Ann. Thorac. Surg. 2018, 106, 121–128. [Google Scholar] [CrossRef]
- Woldendorp, K.; Doyle, M.P.; Bannon, P.G.; Misfeld, M.; Yan, T.D.; Santarpino, G.; Berretta, P.; Di Eusanio, M.; Meuris, B.; Cerillo, A.G.; et al. Aortic valve replacement using stented or sutureless/rapid deployment prosthesis via either full-sternotomy or a minimally invasive approach: A network meta-analysis. Ann. Cardiothorac. Surg. 2020, 9, 347–363. [Google Scholar] [CrossRef]
- Kondo, N.; Totsugawa, T.; Hiraoka, A.; Tamura, K.; Yoshitaka, H.; Sakaguchi, T. Left Atrial Appendage Resection During Minimally Invasive Aortic Valve Surgery via Right Minithoracotomy. Innovations 2017, 12, 378–379. [Google Scholar] [CrossRef] [PubMed]
- Yanagawa, B.; Cruz, J.; Boisvert, L.; Bonneau, D. A simple modification to lower incidence of heart block with sutureless valve implantation. J. Thorac. Cardiovasc. Surg. 2016, 152, 630–632. [Google Scholar] [CrossRef]
- Siontis, G.C.; Jüni, P.; Pilgrim, T.; Stortecky, S.; Büllesfeld, L.; Meier, B.; Wenaweser, P.; Windecker, S. Predictors of permanent pacemaker implantation in patients with severe aortic stenosis undergoing TAVR: A meta-analysis. J. Am. Coll. Cardiol. 2014, 64, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Nazif, T.M.; Dizon, J.M.; Hahn, R.T.; Xu, K.; Babaliaros, V.; Douglas, P.S.; El-Chami, M.F.; Herrmann, H.C.; Mack, M.; Makkar, R.R.; et al. Predictors and clinical outcomes of permanent pacemaker implantation after transcatheter aortic valve replacement: The PARTNER (Placement of AoRtic TraNscathetER Valves) trial and registry. JACC Cardiovasc. Interv. 2015, 8 Pt A, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, S.; Nguyen, C.T.N.; Kim, K.M.; Yang, B.; Ailawadi, G.; Patel, H.J.; Deeb, G.M. Aortic valve reintervention after transcatheter aortic valve replacement. J. Thorac. Cardiovasc. Surg. 2023, 165, 1321–1332.e4. [Google Scholar] [CrossRef] [PubMed]
| First Author | Year | Study Period | Country | Surgical Center | Study Type | Median Follow-Up (Months) | Propensity-Machted |
|---|---|---|---|---|---|---|---|
| Vilalta et al. [12] | 2021 | 2011–2020 | Canada/Spain | Multicenter | Prospective non-randomized | 24 (12–36) | Yes |
| Chung et al. [13] | 2021 | 2011–2019 | Republic of Korea | Single-Center | Non-prospective non-randomized | 12.9 (4.1–26.5) | Yes |
| Gerfer et al. [14] | 2021 | 2012–2017 | Germany | Single-Center | Non-prospective non-randomized | ND | Yes |
| Dónofrio et al. [15] | 2016 | 2007–2012 | Italy | Multicenter | Non-prospective non-randomized | At least 1 Year | Yes |
| Miceli et al. [16] | 2016 | 2008–2013 | Italy | Single-Center | Non-prospective non-randomized | 13 (7–25) | Yes |
| Muneretto et al. [17] | 2020 | 2008–2015 | Italy | Multicenter | Non-prospective non-randomized | 60 months | Yes |
| Biancari et al. [23] | 2016 | 2007–2014 | Itay/Germany/Sweden/Belgium | Multicenter | Non-prospective non-randomized | Until Discharge | Yes |
| Zubarevich et al. [19] | 2022 | 2018–2021 | Germany | Single-Center | Non-prospective non-randomized | 18.1 ± 12.3 | Yes |
| Santarpino et al. [20] | 2022 | 2010–2018 | Italy | Multicenter | Non-prospective non-randomized | 42.87 ± 21.69 | Yes |
| Santarpino et al. [21] | 2015 | 2010–2015 | Germany | Single-Center | Randomized non-prospective | 24.5 ± 13.8 | Yes |
| Indelen et al. [22] | 2023 | 2015–2020 | Turkey | Single-Center | Non-prospective non-randomized | ND | Yes |
| Muneretto et al. [18] | 2023 | 2013–2020 | Italy/Germany/France | Multicenter | Non-prospective non-randomized | 51.6 (13.2–88.8) | Yes |
| First Name | Year | County | Center | Study Period | Type of Study | Sample Size | Median-Follow-UP (Year) |
|---|---|---|---|---|---|---|---|
| Aldea et al. [24] | 2023 | USA | Multicenter | 2010–2015 | Retrospective observational study | 970 | 4 years |
| Concistre et al. [25] | 2023 | Italy | Single-Center | 2011–2021 | Prospective cohort study | 1652 | 1 year (up to 8 years) |
| Dokollari et al. [26] | 2023 | The Netherlands | Single-Center | 2013–2020 | Retrospective observational study | 101 | 7 years |
| Ferreira et al. [27] | 2022 | Portugal | Single-Center | 2015–2020 | Retrospective observational study | 196 | Up to 5 years |
| Fischlein et al. [28] | 2021 | Germany | Multicenter | 2010–2013 | Prospective cohort study | 658 | 3.8 years |
| Glauber et al. [29] | 2020 | Italy | Multicenter | 2011–2018 | Prospective cohort study | 480 | 2.4 years |
| Hong et al. [30] | 2024 | Korea | Single-Center | 2015–2020 | Retrospective observational study | 113 | 51.19 ± 20.6 |
| Kapadia et al. [31] | 2024 | UK | Single-Center | 2014–2020 | Retrospective observational study | 102 | ND |
| Lamberigts et al. [32] | 2022 | Belgium | Single-Center | 2007–2019 | Retrospective observational study | 784 | 7.03 years |
| Meuris et al. [33] | 2015 | Belgium | Multicenter | 2007–2008 | Prospective cohort study | 30 | 4.2 years |
| Muneretto et al. [17] | 2022 | Italy | Multicenter | 2008–2015 | Retrospective observational study | 481 | 5 years |
| Pollari et al. [34] | 2023 | Germany | Single-Center | 2010–2020 | Retrospective observational study | 547 | 4.53 years |
| Santarpino et al. [20] | 2022 | Italy | Multicenter | 2010–2018 | Retrospective observational study | 172 | 6.1 years |
| Schizas et al. [35] | 2024 | Greece | Single-Center | 2013–2020 | Retrospective observational study | 205 | 6.27 ± 2.03 |
| Szecel et al. [36] | 2021 | Belgium | Single-Center | 2007–2017 | Retrospective observational study | 468 | 3.1 ± 2 up to 11.2 year |
| White et al. [37] | 2022 | Canada | Single-Center | 2013–2019 | Retrospective observational study | 295 | 2.4 years |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali-Hasan-Al-Saegh, S.; Takemoto, S.; Shafiei, S.; Yavuz, S.; Arjomandi Rad, A.; Amanov, L.; Merzah, A.S.; Salman, J.; Ius, F.; Kaufeld, T.; et al. Sutureless Aortic Valve Replacement with Perceval Bioprosthesis Superior to Transcatheter Aortic Valve Implantation: A Promising Option for the Gray-Zone of Aortic Valve Replacement Procedures—A State-of-the-Art Systematic Review, Meta-Analysis, and Future Directions. J. Clin. Med. 2024, 13, 4887. https://doi.org/10.3390/jcm13164887
Ali-Hasan-Al-Saegh S, Takemoto S, Shafiei S, Yavuz S, Arjomandi Rad A, Amanov L, Merzah AS, Salman J, Ius F, Kaufeld T, et al. Sutureless Aortic Valve Replacement with Perceval Bioprosthesis Superior to Transcatheter Aortic Valve Implantation: A Promising Option for the Gray-Zone of Aortic Valve Replacement Procedures—A State-of-the-Art Systematic Review, Meta-Analysis, and Future Directions. Journal of Clinical Medicine. 2024; 13(16):4887. https://doi.org/10.3390/jcm13164887
Chicago/Turabian StyleAli-Hasan-Al-Saegh, Sadeq, Sho Takemoto, Saeed Shafiei, Senol Yavuz, Arian Arjomandi Rad, Lukman Amanov, Ali Saad Merzah, Jawad Salman, Fabio Ius, Tim Kaufeld, and et al. 2024. "Sutureless Aortic Valve Replacement with Perceval Bioprosthesis Superior to Transcatheter Aortic Valve Implantation: A Promising Option for the Gray-Zone of Aortic Valve Replacement Procedures—A State-of-the-Art Systematic Review, Meta-Analysis, and Future Directions" Journal of Clinical Medicine 13, no. 16: 4887. https://doi.org/10.3390/jcm13164887
APA StyleAli-Hasan-Al-Saegh, S., Takemoto, S., Shafiei, S., Yavuz, S., Arjomandi Rad, A., Amanov, L., Merzah, A. S., Salman, J., Ius, F., Kaufeld, T., Schmack, B., Popov, A.-F., Sabashnikov, A., Ruhparwar, A., Zubarevich, A., & Weymann, A. (2024). Sutureless Aortic Valve Replacement with Perceval Bioprosthesis Superior to Transcatheter Aortic Valve Implantation: A Promising Option for the Gray-Zone of Aortic Valve Replacement Procedures—A State-of-the-Art Systematic Review, Meta-Analysis, and Future Directions. Journal of Clinical Medicine, 13(16), 4887. https://doi.org/10.3390/jcm13164887









