Role of Mechanical Circulatory Support in Complex High-Risk and Indicated Percutaneous Coronary Intervention: Current Indications, Device Options, and Potential Complications
Abstract
1. Introduction
2. Available MCS Devices and Their Rationale
2.1. Type of MCS Devices
2.2. Proposed Decision Algorithm
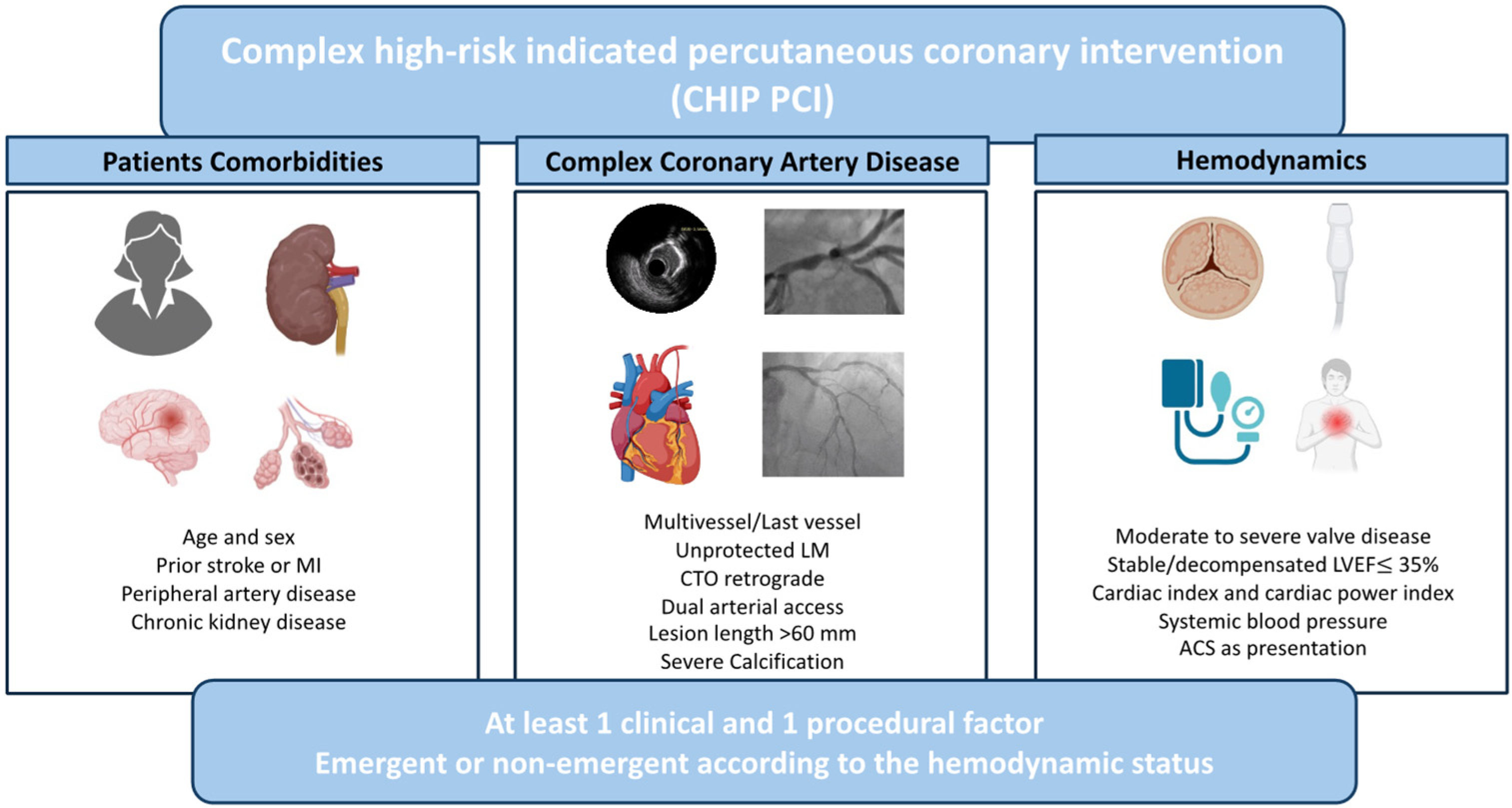
3. Clinical Scenarios for the Use of MCS during CHIPs
3.1. Severe LV Dysfunction
3.2. Unprotected Left Main and Severe Multivessel Disease
3.3. Chronic Total Occlusions
3.4. Severely Calcified Disease Requiring Calcium Modification Techniques
3.5. Severe Concomitant Heart Valve Disease
4. Risk and Complications of MCS Devices
4.1. Vascular Complications
4.2. Contrast Associated Acute Kidney Injury
4.3. Hemolysis
4.4. Anticoagulation Management: Balancing between Thromboembolic and Bleeding Events
5. Unmet Needs and Future Directions
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Neumann, F.-J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.-P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on Myocardial Revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Byrne, R.A.; Fremes, S.; Capodanno, D.; Czerny, M.; Doenst, T.; Emberson, J.R.; Falk, V.; Gaudino, M.; McMurray, J.J.V.; Mehran, R.; et al. 2022 Joint ESC/EACTS Review of the 2018 Guideline Recommendations on the Revascularization of Left Main Coronary Artery Disease in Patients at Low Surgical Risk and Anatomy Suitable for PCI or CABG. Eur. Heart J. 2023, 44, 4310–4320. [Google Scholar] [CrossRef] [PubMed]
- Truesdell, A.G.; Davies, R.; Eltelbany, M.; Megaly, M.; Rosner, C.; Cilia, L.A. Mechanical Circulatory Support for Complex High-Risk Percutaneous Coronary Intervention. US Cardiol. Rev. 2023, 17, e03. [Google Scholar] [CrossRef]
- Chieffo, A.; Burzotta, F.; Pappalardo, F.; Briguori, C.; Garbo, R.; Masiero, G.; Nicolini, E.; Ribichini, F.; Trani, C.; Álvarez, B.C.; et al. Clinical Expert Consensus Document on the Use of Percutaneous Left Ventricular Assist Support Devices during Complex High-Risk Indicated PCI. Int. J. Cardiol. 2019, 293, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Protty, M.; Sharp, A.S.P.; Gallagher, S.; Farooq, V.; Spratt, J.C.; Ludman, P.; Anderson, R.; McEntegart, M.M.; Hanratty, C.; Walsh, S.; et al. Defining Percutaneous Coronary Intervention Complexity and Risk: An Analysis of the United Kingdom BCIS Database 2006–2016. JACC Cardiovasc. Interv. 2022, 15, 39–49. [Google Scholar] [CrossRef]
- van Nunen, L.X.; Noc, M.; Kapur, N.K.; Patel, M.R.; Perera, D.; Pijls, N.H.J. Usefulness of Intra-Aortic Balloon Pump Counterpulsation. Am. J. Cardiol. 2016, 117, 469–476. [Google Scholar] [CrossRef]
- Patterson, T.; Perera, D.; Redwood, S.R. Intra-Aortic Balloon Pump for High-Risk Percutaneous Coronary Intervention. Circ. Cardiovasc. Interv. 2014, 7, 712–720. [Google Scholar] [CrossRef]
- Perera, D.; Stables, R.; Clayton, T.; De Silva, K.; Lumley, M.; Clack, L.; Thomas, M.; Redwood, S. Long-Term Mortality Data From the Balloon Pump–Assisted Coronary Intervention Study (BCIS-1). Circulation 2013, 127, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Chieffo, A.; Dudek, D.; Hassager, C.; Combes, A.; Gramegna, M.; Halvorsen, S.; Huber, K.; Kunadian, V.; Maly, J.; Møller, J.E.; et al. Joint EAPCI/ACVC Expert Consensus Document on Percutaneous Ventricular Assist Devices. EuroIntervention 2021, 17, e274–e286. [Google Scholar] [CrossRef]
- Lüsebrink, E.; Kellnar, A.; Krieg, K.; Binzenhöfer, L.; Scherer, C.; Zimmer, S.; Schrage, B.; Fichtner, S.; Petzold, T.; Braun, D.; et al. Percutaneous Transvalvular Microaxial Flow Pump Support in Cardiology. Circulation 2022, 145, 1254–1284. [Google Scholar] [CrossRef]
- Burzotta, F.; Trani, C.; Doshi, S.N.; Townend, J.; van Geuns, R.J.; Hunziker, P.; Schieffer, B.; Karatolios, K.; Møller, J.E.; Ribichini, F.L.; et al. Impella Ventricular Support in Clinical Practice: Collaborative Viewpoint from a European Expert User Group. Int. J. Cardiol. 2015, 201, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Alqarqaz, M.; Basir, M.; Alaswad, K.; O’Neill, W. Effects of Impella on Coronary Perfusion in Patients With Critical Coronary Artery Stenosis. Circ. Cardiovasc. Interv. 2018, 11, e005870. [Google Scholar] [CrossRef] [PubMed]
- Meani, P.; Lorusso, R.; Pappalardo, F. ECPella: Concept, Physiology and Clinical Applications. J. Cardiothorac. Vasc. Anesth. 2022, 36, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, H.; Saku, K.; Nishikawa, T.; Yokota, S.; Sato, K.; Morita, H.; Yoshida, Y.; Fukumitsu, M.; Uemura, K.; Kawada, T.; et al. The Impact of ECPELLA on Haemodynamics and Global Oxygen Delivery: A Comprehensive Simulation of Biventricular Failure. Intensive Care Med. Exp. 2024, 12, 13. [Google Scholar] [CrossRef]
- O’Neill, W.W.; Kleiman, N.S.; Moses, J.; Henriques, J.P.S.; Dixon, S.; Massaro, J.; Palacios, I.; Maini, B.; Mulukutla, S.; Džavík, V.; et al. A Prospective, Randomized Clinical Trial of Hemodynamic Support With Impella 2.5 Versus Intra-Aortic Balloon Pump in Patients Undergoing High-Risk Percutaneous Coronary Intervention. Circulation 2012, 126, 1717–1727. [Google Scholar] [CrossRef]
- Kovacic, J.C.; Kini, A.; Banerjee, S.; Dangas, G.; Massaro, J.; Mehran, R.; Popma, J.; O’neill, W.W.; Sharma, S.K. Patients with 3-Vessel Coronary Artery Disease and Impaired Ventricular Function Undergoing PCI with Impella 2.5 Hemodynamic Support Have Improved 90-Day Outcomes Compared to Intra-Aortic Balloon Pump: A Sub-Study of The PROTECT II Trial. J. Interv. Cardiol. 2015, 28, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Ameloot, K.; Bastos, M.B.; Daemen, J.; Schreuder, J.; Boersma, E.; Zijlstra, F.; Van Mieghem, N.M. New-Generation Mechanical Circulatory Support during High-Risk PCI: A Cross-Sectional Analysis. EuroIntervention 2019, 15, 427–433. [Google Scholar] [CrossRef]
- O’Neill, W.W.; Anderson, M.; Burkhoff, D.; Grines, C.L.; Kapur, N.K.; Lansky, A.J.; Mannino, S.; McCabe, J.M.; Alaswad, K.; Daggubati, R.; et al. Improved Outcomes in Patients with Severely Depressed LVEF Undergoing Percutaneous Coronary Intervention with Contemporary Practices. Am. Heart J. 2022, 248, 139–149. [Google Scholar] [CrossRef]
- Han, J.J.; Swain, J.D. The Perfect ECMO Candidate. J. Am. Coll. Cardiol. 2018, 71, 1178–1182. [Google Scholar] [CrossRef]
- Allen, S.; Holena, D.; McCunn, M.; Kohl, B.; Sarani, B. A Review of the Fundamental Principles and Evidence Base in the Use of Extracorporeal Membrane Oxygenation (ECMO) in Critically Ill Adult Patients. J Intensive Care Med. 2011, 26, 13–26. [Google Scholar] [CrossRef]
- Tomasello, S.D.; Boukhris, M.; Ganyukov, V.; Galassi, A.R.; Shukevich, D.; Haes, B.; Kochergin, N.; Tarasov, R.; Popov, V.; Barbarash, L. Outcome of Extracorporeal Membrane Oxygenation Support for Complex High-Risk Elective Percutaneous Coronary Interventions: A Single-Center Experience. Heart Lung 2015, 44, 309–313. [Google Scholar] [CrossRef]
- van den Brink, F.S.; Meijers, T.A.; Hofma, S.H.; van Boven, A.J.; Nap, A.; Vonk, A.; Symersky, P.; Sjauw, K.D.; Knaapen, P. Prophylactic Veno-Arterial Extracorporeal Membrane Oxygenation in Patients Undergoing High-Risk Percutaneous Coronary Intervention. Neth. Heart J. 2020, 28, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Al-Husami, W.; Yturralde, F.; Mohanty, G.; Pastore, C.; Lotun, K.; Venesy, D.; Waxman, S.; Pyne, C.; Gossman, D.; Nesto, R.; et al. Single-Center Experience with the Tandem Heart Percutaneous Ventricular Assist Device to Support Patients Undergoing High-Risk Percutaneous Coronary Intervention. J. Invasive Cardiol. 2008, 20, 319–322. [Google Scholar] [PubMed]
- Van Mieghem, N.M.; Daemen, J.; Lenzen, M.J.; Zandstra, R.; Malkin, O.; van Geuns, R.-J.M. The PulseCath iVAC 2L Left Ventricular Assist Device: Conversion to a Percutaneous Transfemoral Approach. EuroIntervention 2015, 11, 835–839. [Google Scholar] [CrossRef]
- Bavishi, C.; Lemor, A.; Trivedi, V.; Chatterjee, S.; Moreno, P.; Lasala, J.; Aronow, H.D.; Dawn Abbott, J. Etiologies and Predictors of 30-Day Readmissions in Patients Undergoing Percutaneous Mechanical Circulatory Support–Assisted Percutaneous Coronary Intervention in the United States: Insights from the Nationwide Readmissions Database. Clin. Cardiol. 2018, 41, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Geppert, A.; Mashayekhi, K.; Huber, K. The Use of Mechanical Circulatory Support in Elective High-Risk Percutaneous Coronary Interventions: A Literature-Based Review. Eur. Heart J. Open 2024, 4, oeae007. [Google Scholar] [CrossRef]
- Turkiewicz, K.; Rola, P.; Kulczycki, J.J.; Włodarczak, S.; Jastrzębski, A.; Pęcherzewski, M.; Furtan, Ł.; Barycki, M.; Doroszko, A.; Włodarczak, A.; et al. High-Risk PCI Facilitated by Levosimendan Infusion and Impella CP Support in ACS Cohort-Pilot Study. Pol. Heart J. 2024. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, T.M.; Ohman, E.M.; O’Neill, W.W.; Rab, T.; Cigarroa, J.E. A Practical Approach to Mechanical Circulatory Support in Patients Undergoing Percutaneous Coronary Intervention: An Interventional Perspective. JACC Cardiovasc. Interv. 2016, 9, 871–883. [Google Scholar] [CrossRef]
- Maini, B.; Naidu, S.S.; Mulukutla, S.; Kleiman, N.; Schreiber, T.; Wohns, D.; Dixon, S.; Rihal, C.; Dave, R.; O’Neill, W. Real-World Use of the Impella 2.5 Circulatory Support System in Complex High-Risk Percutaneous Coronary Intervention: The USpella Registry. Catheter. Cardiovasc. Interv. 2012, 80, 717–725. [Google Scholar] [CrossRef]
- Wollmuth, J.; Patel, M.P.; Dahle, T.; Bharadwaj, A.; Waggoner, T.E.; Chambers, J.W.; Ruiz-Rodriguez, E.; Mahmud, E.; Thompson, C.; Morris, D.L. Ejection Fraction Improvement Following Contemporary High-Risk Percutaneous Coronary Intervention: RESTORE EF Study Results. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100350. [Google Scholar] [CrossRef]
- Romagnoli, E.; Burzotta, F.; Cerracchio, E.; Russo, G.; Aurigemma, C.; Pedicino, D.; Locorotondo, G.; Graziani, F.; Leone, A.M.; D’Amario, D.; et al. Impact of Impella Protected-Percutaneous Coronary Intervention on Left Ventricle Function Recovery of Patients with Extensive Coronary Disease and Poor Left Ventricular Function. Int. J. Cardiol. 2023, 387, 131098. [Google Scholar] [CrossRef]
- Hanson, L.; Vogrin, S.; Noaman, S.; Dinh, D.; Zheng, W.; Lefkovits, J.; Brennan, A.; Reid, C.; Stub, D.; Duffy, S.J.; et al. Long-Term Outcomes of Unprotected Left Main Percutaneous Coronary Intervention in Centers Without Onsite Cardiac Surgery. Am. J. Cardiol. 2022, 168, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Fajadet, J.; Capodanno, D.; Stone, G.W. Management of Left Main Disease: An Update. Eur. Heart J. 2019, 40, 1454–1466. [Google Scholar] [CrossRef]
- Stone, G.W.; Kappetein, A.P.; Sabik, J.F.; Pocock, S.J.; Morice, M.C.; Puskas, J.; Kandzari, D.E.; Karmpaliotis, D.; Brown, W.M., III; Lembo, N.J.; et al. Five-Year Outcomes after PCI or CABG for Left Main Coronary Disease. N. Engl. J. Med. 2019, 381, 1820–1830. [Google Scholar] [CrossRef] [PubMed]
- Holm, N.R.; Mäkikallio, T.; Lindsay, M.M.; Spence, M.S.; Erglis, A.; Menown, I.B.A.; Trovik, T.; Kellerth, T.; Kalinauskas, G.; Mogensen, L.J.H.; et al. Percutaneous Coronary Angioplasty versus Coronary Artery Bypass Grafting in the Treatment of Unprotected Left Main Stenosis: Updated 5-Year Outcomes from the Randomised, Non-Inferiority NOBLE Trial. Lancet 2020, 395, 191–199. [Google Scholar] [CrossRef]
- Thuijs, D.J.F.M.; Kappetein, A.P.; Serruys, P.W.; Mohr, F.-W.; Morice, M.-C.; Mack, M.J.; Holmes, D.R.; Curzen, N.; Davierwala, P.; Noack, T.; et al. Percutaneous Coronary Intervention versus Coronary Artery Bypass Grafting in Patients with Three-Vessel or Left Main Coronary Artery Disease: 10-Year Follow-up of the Multicentre Randomised Controlled SYNTAX Trial. Lancet 2019, 394, 1325–1334. [Google Scholar] [CrossRef]
- Park, D.-W.; Ahn, J.-M.; Park, H.; Yun, S.-C.; Kang, D.-Y.; Lee, P.H.; Kim, Y.-H.; Lim, D.-S.; Rha, S.-W.; Park, G.-M.; et al. Ten-Year Outcomes After Drug-Eluting Stents Versus Coronary Artery Bypass Grafting for Left Main Coronary Disease. Circulation 2020, 141, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Briguori, C.; Sarais, C.; Pagnotta, P.; Airoldi, F.; Liistro, F.; Sgura, F.; Spanos, V.; Carlino, M.; Montorfano, M.; Di Mario, C.; et al. Elective versus Provisional Intra-Aortic Balloon Pumping in High-Risk Percutaneous Transluminal Coronary Angioplasty. Am. Heart J. 2003, 145, 700–707. [Google Scholar] [CrossRef]
- Schreiber, T.; Htun, W.; Blank, N.; Telila, T.; Mercado, N.; Briasoulis, A.; Kaki, A.; Kondur, A.; Munir, A.; Grines, C. Real-World Supported Unprotected Left Main Percutaneous Coronary Intervention with Impella Device; Data from the USpella Registry: Percutaneous Assist Devices for Unprotected LM. Catheter. Cardiovasc. Interv. 2017, 90, 576–581. [Google Scholar] [CrossRef]
- Jabbar, A.A.; Jbara, Y.; Ebrahimi, A.J.; Mufti, O.; Ali, O.; Markert, R.; Joffe, D.; Fishbein, G. Left Ventricular Support for Unprotected Left Main Coronary Artery Interventions (The Dayton Heart and Vascular Impella Registry). Heart Views 2022, 23, 150–156. [Google Scholar] [CrossRef]
- Riley, R.F.; McCabe, J.M.; Kalra, S.; Lazkani, M.; Pershad, A.; Doshi, D.; Kirtane, A.J.; Nicholson, W.; Kearney, K.; Demartini, T.; et al. Impella-Assisted Chronic Total Occlusion Percutaneous Coronary Interventions: A Multicenter Retrospective Analysis. Catheter. Cardiovasc. Interv. 2018, 92, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Neupane, S.; Basir, M.; Alqarqaz, M.; O’Neill, W.; Alaswad, K. TCT-224 High-Risk Chronic Total Occlusion Percutaneous Coronary Interventions Assisted With Tandem Heart. J. Am. Coll. Cardiol. 2019, 74, B223. [Google Scholar] [CrossRef]
- Karacsonyi, J.; Deffenbacher, K.; Benzuly, K.H.; Flaherty, J.D.; Alaswad, K.; Basir, M.; Megaly, M.S.; Jaffer, F.; Doshi, D.; Poommipanit, P.; et al. Use of Mechanical Circulatory Support in Chronic Total Occlusion Percutaneous Coronary Intervention. Am. J. Cardiol. 2023, 189, 76–85. [Google Scholar] [CrossRef] [PubMed]
- De Maria, G.L.; Scarsini, R.; Banning, A.P. Management of Calcific Coronary Artery Lesions: Is It Time to Change Our Interventional Therapeutic Approach? JACC Cardiovasc. Interv. 2019, 12, 1465–1478. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, C.S.; Wilson, S.J.; Bogle, R.; Hanratty, C.G.; Williams, R.; Walsh, S.J.; McEntegart, M.B.; Spratt, J.C. Intravascular Lithotripsy for Lesion Preparation in Patients with Calcific Distal Left Main Disease. EuroIntervention 2020, 16, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Shlofmitz, E.; Jeremias, A.; Shlofmitz, R.A.; Ali, Z.A. Lesion Preparation with Orbital Atherectomy. Interv. Cardiol. Rev. 2019, 14, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Shlofmitz, R.A.; Galougahi, K.K.; Jeremias, A.; Shlofmitz, E.; Thomas, S.V.; Ali, Z.A. Calcium Modification in Percutaneous Coronary Interventions. Interv. Cardiol. Clin. 2022, 11, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Yarusi, B.; Jagadeesan, V.; Hussain, S.; Jivan, A.; Tesch, A.; Flaherty, J.; Schimmel, D.; Benzuly, K. Combined Coronary Orbital Atherectomy and Intravascular Lithotripsy for the Treatment of Severely Calcified Coronary Stenoses: The First Case Series. J. Invasive Cardiol. 2022, 34, E210–E217. [Google Scholar] [CrossRef]
- Sardella, G.; Stefanini, G.; Leone, P.P.; Boccuzzi, G.; Fovero, N.T.; Van Mieghem, N.; Giacchi, G.; Escaned, J.; Fineschi, M.; Testa, L.; et al. Coronary Lithotripsy as Elective or Bail-Out Strategy After Rotational Atherectomy in the Rota-Shock Registry. Am. J. Cardiol. 2023, 198, 1–8. [Google Scholar] [CrossRef]
- Alkhalil, A.; Hajjar, R.; Ibrahim, H.; Ruiz, C.E. Mechanical Circulatory Support in Transcatheter Aortic Valve Implantation in the United States (from the National Inpatient Sample). Am. J. Cardiol. 2019, 124, 1615–1620. [Google Scholar] [CrossRef]
- Martinez, C.A.; Singh, V.; Londoño, J.C.; Cohen, M.G.; Alfonso, C.E.; O’Neill, W.W.; Heldman, A.W. Percutaneous Retrograde Left Ventricular Assist Support for Interventions in Patients with Aortic Stenosis and Left Ventricular Dysfunction. Catheter. Cardiovasc. Interv. 2012, 80, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Almajed, M.R.; Mahmood, S.; Obri, M.; Nona, P.; Gonzalez, P.E.; Chiang, M.; Wang, D.D.; Frisoli, T.; Lee, J.; Basir, M.; et al. Application of Impella Mechanical Circulatory Support Devices in Transcatheter Aortic Valve Replacement and Balloon Aortic Valvuloplasty: A Single-Center Experience. Cardiovasc. Revascularization Med. 2023, 53, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Van Edom, C.J.; Gramegna, M.; Baldetti, L.; Beneduce, A.; Castelein, T.; Dauwe, D.; Frederiks, P.; Giustino, G.; Jacquemin, M.; Janssens, S.P.; et al. Management of Bleeding and Hemolysis During Percutaneous Microaxial Flow Pump Support: A Practical Approach. JACC Cardiovasc. Interv. 2023, 16, 1707–1720. [Google Scholar] [CrossRef] [PubMed]
- Salter, B.S.; Gross, C.R.; Weiner, M.M.; Dukkipati, S.R.; Serrao, G.W.; Moss, N.; Anyanwu, A.C.; Burkhoff, D.; Lala, A. Temporary Mechanical Circulatory Support Devices: Practical Considerations for All Stakeholders. Nat. Rev. Cardiol. 2023, 20, 263–277. [Google Scholar] [CrossRef]
- Lemor, A.; Basir, M.B.; Truesdell, A.G.; Tamis-Holland, J.E.; Alqarqaz, M.; Grines, C.L.; Villablanca, P.A.; Alaswad, K.; Pinto, D.S.; O’Neill, W. Trends in the Outcomes of High-Risk Percutaneous Ventricular Assist Device-Assisted Percutaneous Coronary Intervention, 2008-2018. Am. J. Cardiol. 2021, 156, 65–71. [Google Scholar] [CrossRef]
- Lemor, A.; Dabbagh, M.F.; Cohen, D.; Villablanca, P.; Tehrani, B.; Alaswad, K.; Alqarqaz, M.; Lasorda, D.; Kaki, A.; Genereux, P.; et al. Rates and Impact of Vascular Complications in Mechanical Circulatory Support. Catheter. Cardiovasc. Interv. 2022, 99, 1702–1711. [Google Scholar] [CrossRef]
- Blanke, P.; Weir-McCall, J.R.; Achenbach, S.; Delgado, V.; Hausleiter, J.; Jilaihawi, H.; Marwan, M.; Nørgaard, B.L.; Piazza, N.; Schoenhagen, P.; et al. Computed Tomography Imaging in the Context of Transcatheter Aortic Valve Implantation (TAVI)/Transcatheter Aortic Valve Replacement (TAVR): An Expert Consensus Document of the Society of Cardiovascular Computed Tomography. JACC Cardiovasc. Imaging 2019, 12, 1–24. [Google Scholar] [CrossRef]
- Meijers, T.A.; Nap, A.; Aminian, A.; Schmitz, T.; Dens, J.; Teeuwen, K.; van Kuijk, J.-P.; van Wely, M.; Bataille, Y.; Kraaijeveld, A.O.; et al. Ultrasound-Guided versus Fluoroscopy-Guided Large-Bore Femoral Access in PCI of Complex Coronary Lesions: The International, Multicentre, Randomised ULTRACOLOR Trial. EuroIntervention 2024, 20, e876–e886. [Google Scholar] [CrossRef] [PubMed]
- Seto, A.H.; Abu-Fadel, M.S.; Sparling, J.M.; Zacharias, S.J.; Daly, T.S.; Harrison, A.T.; Suh, W.M.; Vera, J.A.; Aston, C.E.; Winters, R.J.; et al. Real-Time Ultrasound Guidance Facilitates Femoral Arterial Access and Reduces Vascular Complications: FAUST (Femoral Arterial Access With Ultrasound Trial). JACC Cardiovasc. Interv. 2010, 3, 751–758. [Google Scholar] [CrossRef]
- Wollmuth, J.; Korngold, E.; Croce, K.; Pinto, D.S. The Single-Access for Hi-Risk PCI (SHiP) Technique. Catheter. Cardiovasc. Interv. 2020, 96, 114–116. [Google Scholar] [CrossRef]
- Bazarbashi, N.; Ahuja, K.; Gad, M.M.; Sammour, Y.M.; Kaur, M.; Karrthik, A.; Saad, A.M.; Khubber, S.; Dhaliwal, K.; Mick, S.L.; et al. The Utilization of Single versus Double Perclose Devices for Transfemoral Aortic Valve Replacement Access Site Closure: Insights from Cleveland Clinic Aortic Valve Center. Catheter. Cardiovasc. Interv. 2020, 96, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Eltelbany, M.; Fabbri, M.; Batchelor, W.B.; Cilia, L.; Ducoffe, A.; Endicott, K.; Epps, K.; McBurnie, A.; Neville, R.; Rosner, C.; et al. Best Practices for Vascular Arterial Access and Closure: A Contemporary Guide for the Cardiac Catheterization Laboratory. Front. Cardiovasc. Med. 2024, 11, 1349480. [Google Scholar] [CrossRef]
- Rastan, A.J.; Tillmann, E.; Subramanian, S.; Lehmkuhl, L.; Funkat, A.K.; Leontyev, S.; Doenst, T.; Walther, T.; Gutberlet, M.; Mohr, F.W. Visceral Arterial Compromise During Intra-Aortic Balloon Counterpulsation Therapy. Circulation 2010, 122, S92–S99. [Google Scholar] [CrossRef] [PubMed]
- Azzalini, L.; Spagnoli, V.; Ly, H.Q. Contrast-Induced Nephropathy: From Pathophysiology to Preventive Strategies. Can. J. Cardiol. 2016, 32, 247–255. [Google Scholar] [CrossRef]
- Giustino, G.; Chieffo, A.; Palmerini, T.; Valgimigli, M.; Feres, F.; Abizaid, A.; Costa, R.A.; Hong, M.-K.; Kim, B.-K.; Jang, Y.; et al. Efficacy and Safety of Dual Antiplatelet Therapy After Complex PCI. J. Am. Coll. Cardiol. 2016, 68, 1851–1864. [Google Scholar] [CrossRef] [PubMed]
- Azzalini, L.; Poletti, E.; Lombardo, F.; Laricchia, A.; Beneduce, A.; Moscardelli, S.; Bellini, B.; Maccagni, D.; Cappelletti, A.; Ancona, M.B.; et al. Risk of Contrast-Induced Nephropathy in Patients Undergoing Complex Percutaneous Coronary Intervention. Int. J. Cardiol. 2019, 290, 59–63. [Google Scholar] [CrossRef]
- Mehran, R.; Owen, R.; Chiarito, M.; Baber, U.; Sartori, S.; Cao, D.; Nicolas, J.; Pivato, C.A.; Nardin, M.; Krishnan, P.; et al. A Contemporary Simple Risk Score for Prediction of Contrast-Associated Acute Kidney Injury after Percutaneous Coronary Intervention: Derivation and Validation from an Observational Registry. Lancet 2021, 398, 1974–1983. [Google Scholar] [CrossRef] [PubMed]
- Laskey, W.K.; Jenkins, C.; Selzer, F.; Marroquin, O.C.; Wilensky, R.L.; Glaser, R.; Cohen, H.A.; Holmes, D.R. Volume-to-Creatinine Clearance Ratio: A Pharmacokinetically Based Risk Factor for Prediction of Early Creatinine Increase After Percutaneous Coronary Intervention. J. Am. Coll. Cardiol. 2007, 50, 584–590. [Google Scholar] [CrossRef]
- Trivedi, H.S.; Moore, H.; Nasr, S.; Aggarwal, K.; Agrawal, A.; Goel, P.; Hewett, J. A Randomized Prospective Trial to Assess the Role of Saline Hydration on the Development of Contrast Nephrotoxicity. Nephron Clin. Pract. 2004, 93, c29–c34. [Google Scholar] [CrossRef]
- Roberts, N.; Chandrasekaran, U.; Das, S.; Qi, Z.; Corbett, S. Hemolysis Associated with Impella Heart Pump Positioning: In Vitro Hemolysis Testing and Computational Fluid Dynamics Modeling. Int. J. Artif. Organs. 2020, 43, 710–718. [Google Scholar] [CrossRef]
- Nakamura, M.; Imamura, T.; Hida, Y.; Kinugawa, K. Pulmonary Artery Pulsatility Index and Hemolysis during Impella-Incorporated Mechanical Circulatory Support. J. Clin. Med. 2022, 11, 1206. [Google Scholar] [CrossRef] [PubMed]
- Dvanajscak, Z.; Walker, P.D.; Cossey, L.N.; Messias, N.C.; Boils, C.L.; Kuperman, M.B.; Larsen, C.P. Hemolysis-Associated Hemoglobin Cast Nephropathy Results from a Range of Clinicopathologic Disorders. Kidney Int. 2019, 96, 1400–1407. [Google Scholar] [CrossRef]
- Vermeulen Windsant, I.C.; Snoeijs, M.G.; Hanssen, S.J.; Altintas, S.; Heijmans, J.H.; Koeppel, T.A.; Schurink, G.W.H.; Buurman, W.A.; Jacobs, M.J. Hemolysis Is Associated with Acute Kidney Injury during Major Aortic Surgery. Kidney Int. 2010, 77, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Dufour, N.; Radjou, A.; Thuong, M. Hemolysis and Plasma Free Hemoglobin during Extracorporeal Membrane Oxygenation Support: From Clinical Implications to Laboratory Details. ASAIO J. 2020, 66, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Montisci, A.; Bertoldi, L.; Price, S.; Hassager, C.; Møller, J.; Pappalardo, F. Intensive Care Unit Management of Percutaneous Mechanical Circulatory Supported Patients: The Role of Imaging. Eur. Heart J. Suppl. 2021, 23, A15–A22. [Google Scholar] [CrossRef] [PubMed]
- Zein, R.; Patel, C.; Mercado-Alamo, A.; Schreiber, T.; Kaki, A. A Review of the Impella Devices. Interv. Cardiol. 2022, 17, e05. [Google Scholar] [CrossRef]
- Vincent, J.-L.; Cecconi, M.; De Backer, D. The Fluid Challenge. Crit. Care 2020, 24, 703. [Google Scholar] [CrossRef]
- Vandenbriele, C.; Arachchillage, D.J.; Frederiks, P.; Giustino, G.; Gorog, D.A.; Gramegna, M.; Janssens, S.; Meyns, B.; Polzin, A.; Scandroglio, M.; et al. Anticoagulation for Percutaneous Ventricular Assist Device-Supported Cardiogenic Shock. J. Am. Coll. Cardiol. 2022, 79, 1949–1962. [Google Scholar] [CrossRef]
- Chieffo, A.; Ancona, M.B.; Burzotta, F.; Pazzanese, V.; Briguori, C.; Trani, C.; Piva, T.; De Marco, F.; Di Biasi, M.; Pagnotta, P.; et al. Observational Multicentre Registry of Patients Treated with IMPella Mechanical Circulatory Support Device in ITaly: The IMP-IT Registry. EuroIntervention 2020, 15, e1343–e1350. [Google Scholar] [CrossRef] [PubMed]
- Gorog, D.A.; Price, S.; Sibbing, D.; Baumbach, A.; Capodanno, D.; Gigante, B.; Halvorsen, S.; Huber, K.; Lettino, M.; Leonardi, S.; et al. Antithrombotic Therapy in Patients with Acute Coronary Syndrome Complicated by Cardiogenic Shock or Out-of-Hospital Cardiac Arrest: A Joint Position Paper from the European Society of Cardiology (ESC) Working Group on Thrombosis, in Association with the Acute Cardiovascular Care Association (ACCA) and European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J.-Cardiovasc. Pharmacother. 2021, 7, 125–140. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the Management of Acute Coronary Syndromes: Developed by the Task Force on the Management of Acute Coronary Syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the Diagnosis and Management of Chronic Coronary Syndromes: The Task Force for the Diagnosis and Management of Chronic Coronary Syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Vandenbriele, C.; Balthazar, T.; Engelen, M.; Adriaenssens, T.; Verhamme, P.; Peerlinck, K.; Janssens, S.; Jacquemin, M.; Coagulation Group, University of Leuven. Acquired von Willebrand Syndrome in Left Impella Supported Cardiogenic Shock Patients. Eur. Heart J. 2020, 41, ehaa946.1538. [Google Scholar] [CrossRef]
- Lockie, C.J.A.; Gillon, S.A.; Barrett, N.A.; Taylor, D.; Mazumder, A.; Paramesh, K.; Rowland, K.; Daly, K.; Camporota, L.; Meadows, C.I.S.; et al. Severe Respiratory Failure, Extracorporeal Membrane Oxygenation, and Intracranial Hemorrhage. Crit. Care Med. 2017, 45, 1642–1649. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.P.; Spertus, J.A.; Curtis, J.P.; Desai, N.; Masoudi, F.A.; Bach, R.G.; McNeely, C.; Al-Badarin, F.; House, J.A.; Kulkarni, H.; et al. The Evolving Landscape of Impella Use in the United States Among Patients Undergoing Percutaneous Coronary Intervention With Mechanical Circulatory Support. Circulation 2020, 141, 273–284. [Google Scholar] [CrossRef]
- Dhruva, S.S.; Ross, J.S.; Mortazavi, B.J.; Hurley, N.C.; Krumholz, H.M.; Curtis, J.P.; Berkowitz, A.; Masoudi, F.A.; Messenger, J.C.; Parzynski, C.S.; et al. Association of Use of an Intravascular Microaxial Left Ventricular Assist Device vs Intra-Aortic Balloon Pump With In-Hospital Mortality and Major Bleeding Among Patients With Acute Myocardial Infarction Complicated by Cardiogenic Shock. JAMA 2020, 323, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Pietrasik, A.; Gąsecka, A.; Jasińska-Gniadzik, K.; Szwed, P.; Grygier, M.; Pawłowski, T.; Sacha, J.; Kochman, J. Roadmap towards an Institutional Impella Programme for High-Risk Coronary Interventions. ESC Heart Fail. 2023, 10, 2200–2213. [Google Scholar] [CrossRef]
- Riley Robert, F. Complex, Higher-Risk, and Indicated PCI (CHIP) Fellowship. J. Am. Coll. Cardiol. 2020, 75, 980–984. [Google Scholar] [CrossRef]
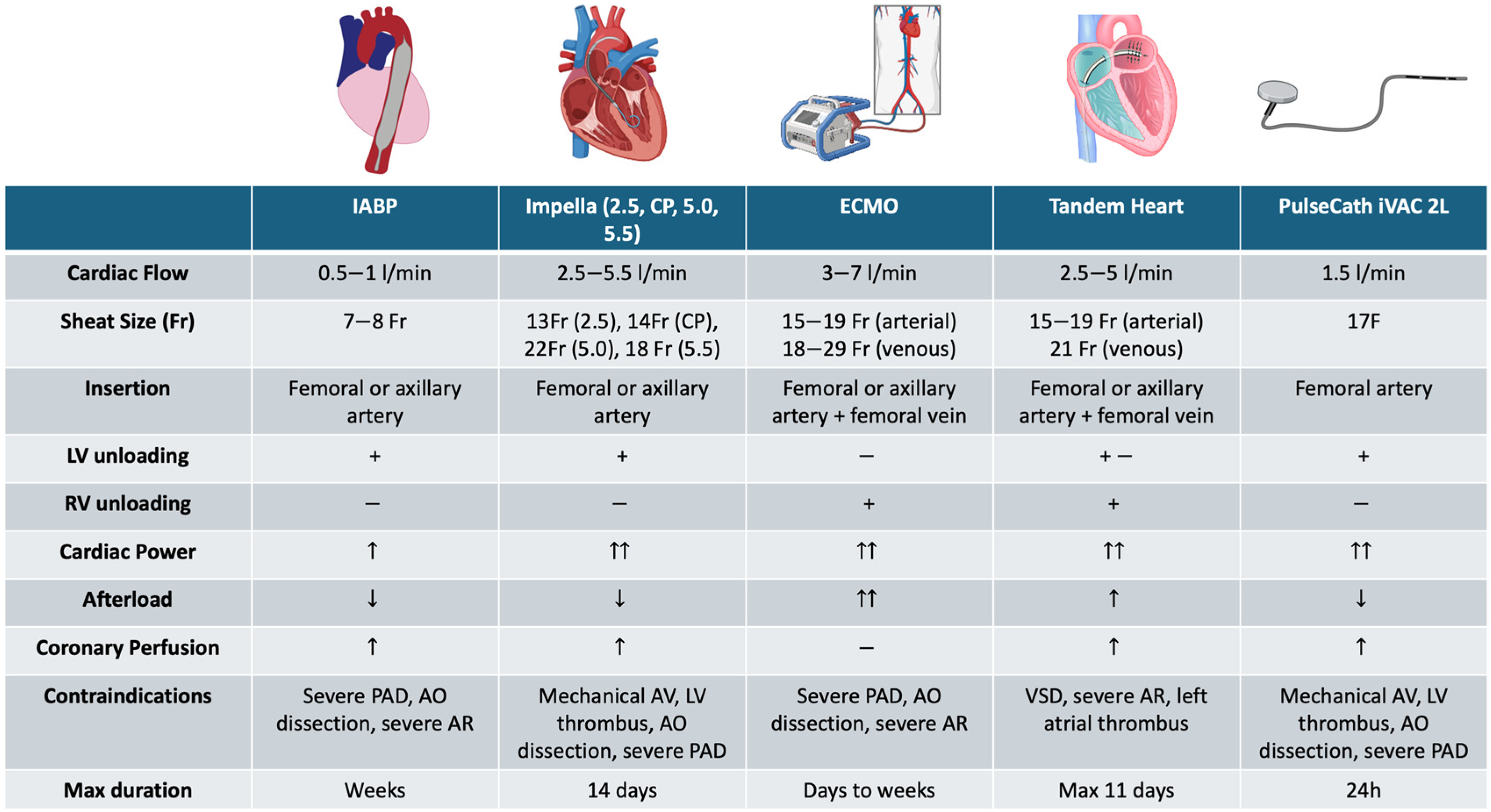
| Device | Advantages | Disadvantages | Indications |
|---|---|---|---|
| IABP | Easy insertion and low risk of complications. | Slight hemodynamic support inadequate in case of severe or inotropic-refractory cardiogenic shock. |
|
| Impella | Greater hemodynamic support and possibility of choosing the appropriate flow rate. | Complex insertion procedure high risk of complications. |
|
| ECMO | Maximum support for patients with severe cardiac and respiratory failure. | Complex insertion procedure high risk of complications. |
|
| TandemHeart | Robust hemodynamic support. | Complex insertion and procedure technical experience required to perform transeptal puncture and high risk of complications. |
|
| PulseCath iVAC | Increase the mean arterial pressure while not influencing mean pulmonary pressure and mixed venous O2 saturation. | Complex insertion procedure high risk of complications. |
|
| Scenario | Recommendation |
|---|---|
| Unprotected Left Main and Severe Coronary Artery Disease | Percutaneous MCS is strongly encouraged in case of unprotected distal LM associated with SYNTAX score ≥ 33 and severe LV dysfunction (LVEF ≤ 35%) when surgical approach is not possible. Consider a Percutaneous MCS in case of non-emergent CHIP for unprotected distal LM associated with SYNTAX score > 22 and severe LV dysfunction (LVEF < 35%). |
| Complete Revascularization | Percutaneous MCS pre PCI is indicated in patients undergoing non-emergent CHIP, in case of complex procedures in patients with severe LV dysfunction in the attempt to obtain complete revascularization. |
| Complex CTO | Percutaneous MCS is indicated as preventive strategy in symptomatic or ischemic patients with (1) severely reduced LVEF and complex anatomical setting, if not amenable for surgery, including high-risk CTO features; (2) less than severe LVEF and complex anatomical settings as second attempt after a failed CTO-PCI because of hemodynamic instability or CTO-PCI retrograde from last remaining vessel. |
| Last Remaining Vessel | Percutaneous MCS protected PCI is strongly indicated as life-saving strategy, in last remaining vessel revascularization non-amenable for CABG associated with LVEF dysfunction. |
| Diffuse and Calcified Lesions | Percutaneous MCS non-emergent protected PCI is indicated in patients at risk because of the coronary disease and severe LV dysfunction especially when rotational atherectomy is required. |
| High-Risk Slow-No Reflow | Percutaneous MCS use in this setting is indicated as bail-out strategy in case of slow-no reflow not promptly responsive to drugs and associated with hemodynamic decay of the CHIP patients. |
| Hemodynamic Instability | Apply Percutaneous MCS in hemodynamic instability to prevent or support hemodynamic compromise. |
| Severe LV Dysfunction and Heart Failure | Percutaneous MCS should be considered in a heart team approach and should be implanted prior to intervention in an effort to avoid “crashing onto support” and to enable complete revascularization when feasible in patients without a surgical revascularization option. |
| Severe Concomitant Heart Valve Disease | Percutaneous MCS use in patients with severe heart valve disease is actually not recommended. Percutaneous MCS bail-out use to stabilize patients who crushed after aortic valvuloplasty may be considered in experienced centers when further valve treatments are considered feasible. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Muro, F.M.; Bellino, M.; Esposito, L.; Attisano, T.; Meucci, F.; Mattesini, A.; Galasso, G.; Vecchione, C.; Di Mario, C. Role of Mechanical Circulatory Support in Complex High-Risk and Indicated Percutaneous Coronary Intervention: Current Indications, Device Options, and Potential Complications. J. Clin. Med. 2024, 13, 4931. https://doi.org/10.3390/jcm13164931
Di Muro FM, Bellino M, Esposito L, Attisano T, Meucci F, Mattesini A, Galasso G, Vecchione C, Di Mario C. Role of Mechanical Circulatory Support in Complex High-Risk and Indicated Percutaneous Coronary Intervention: Current Indications, Device Options, and Potential Complications. Journal of Clinical Medicine. 2024; 13(16):4931. https://doi.org/10.3390/jcm13164931
Chicago/Turabian StyleDi Muro, Francesca Maria, Michele Bellino, Luca Esposito, Tiziana Attisano, Francesco Meucci, Alessio Mattesini, Gennaro Galasso, Carmine Vecchione, and Carlo Di Mario. 2024. "Role of Mechanical Circulatory Support in Complex High-Risk and Indicated Percutaneous Coronary Intervention: Current Indications, Device Options, and Potential Complications" Journal of Clinical Medicine 13, no. 16: 4931. https://doi.org/10.3390/jcm13164931
APA StyleDi Muro, F. M., Bellino, M., Esposito, L., Attisano, T., Meucci, F., Mattesini, A., Galasso, G., Vecchione, C., & Di Mario, C. (2024). Role of Mechanical Circulatory Support in Complex High-Risk and Indicated Percutaneous Coronary Intervention: Current Indications, Device Options, and Potential Complications. Journal of Clinical Medicine, 13(16), 4931. https://doi.org/10.3390/jcm13164931






