Pulmonary Manifestations of IBD: Case Report and Review of the Literature
Abstract
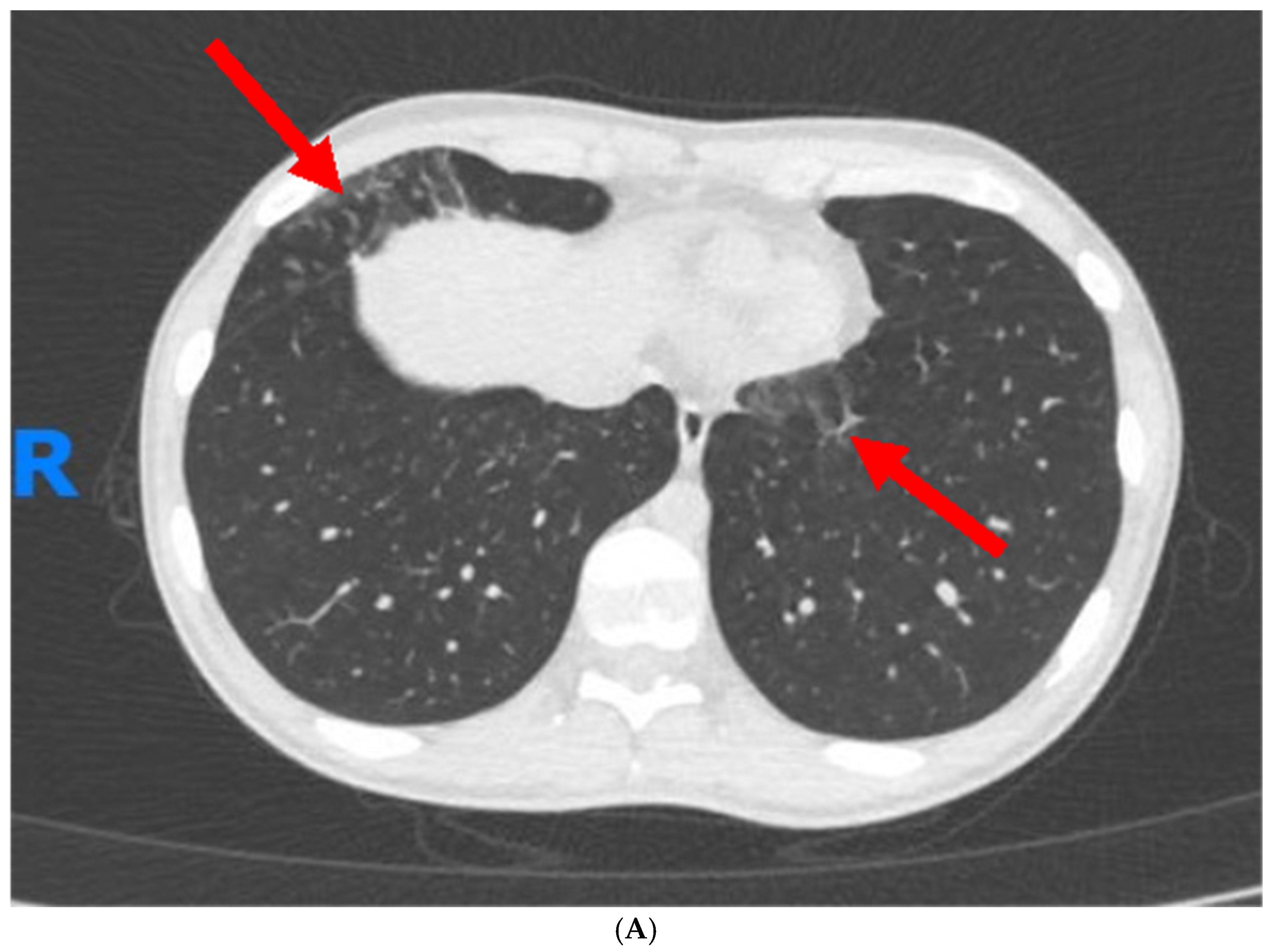
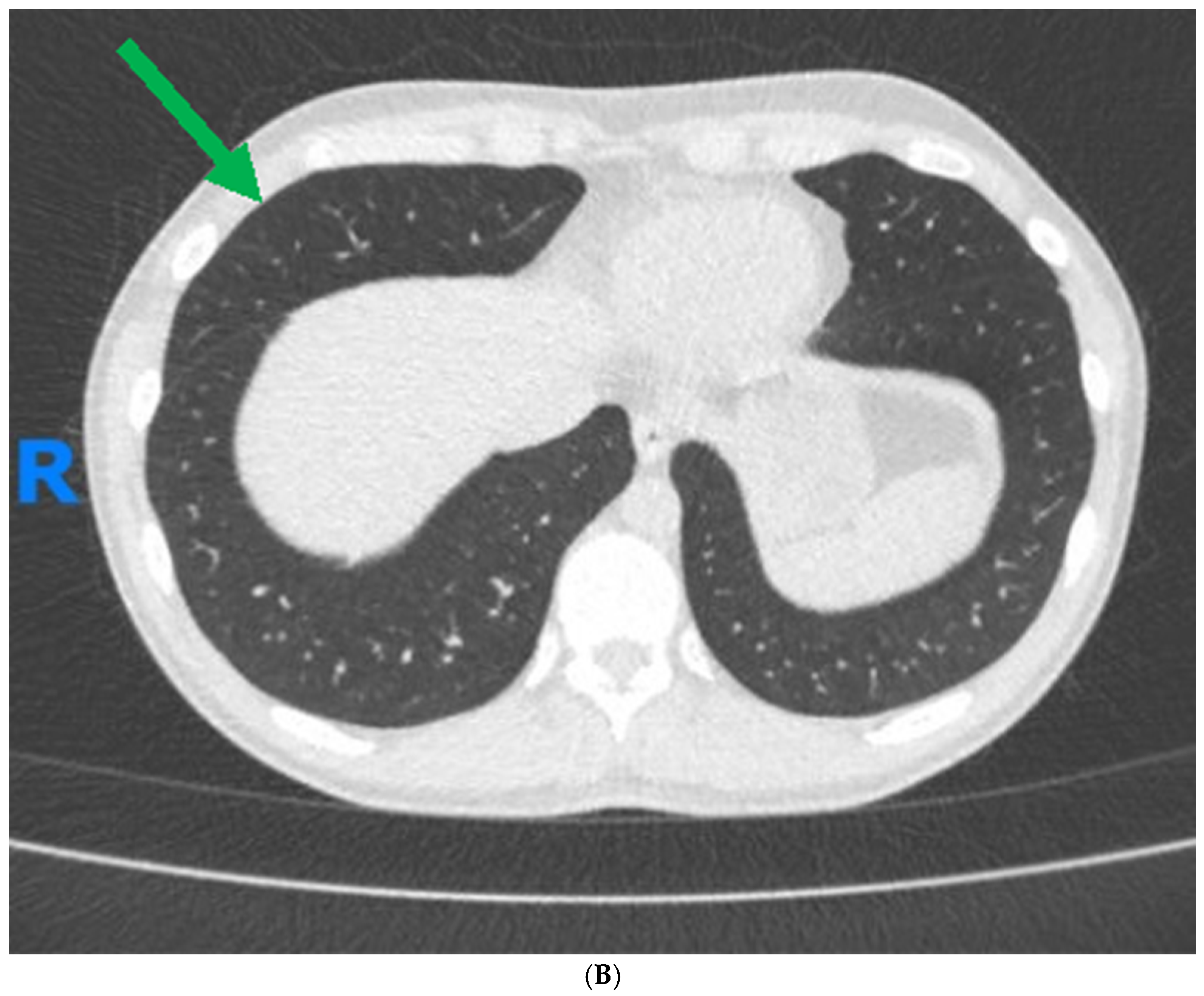
1. Case Report
2. Introduction
3. Clinical Presentation of Pulmonary Involvement in Patients with IBD
4. Pathophysiology of Pulmonary Involvement in IBD
4.1. Immune-Mediated Inflammation
4.2. Genetic Predisposition
4.3. Microbial Translocation and the Gut–Lung Axis
4.4. Environmental Factors
4.5. Drug-Induced Pulmonary Toxicity
4.6. Vascular Involvement
4.7. Enteric-Pulmonary Fistulas
5. Available Diagnostic Modalities and the Challenges Associated with Diagnosis of Pulmonary EIMs
6. Management of Pulmonary Manifestations in Patients with IBD
6.1. Pharmacological Treatment Options
6.2. Non-Pharmacologic Interventions
7. Future Directions for Research on Pulmonary Manifestations of IBD
8. Conclusions
Top of Form
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Barreiro-de Acosta, M.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P.; et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J. Crohn’s Colitis 2017, 11, 649–670, Erratum in J. Crohn’s Colitis 2023, 17, 149. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, I.; Kagouridis, K.; Papiris, S. Patterns of airway involvement in inflammatory bowel diseases. World J. Gastrointest. Pathophysiol. 2014, 5, 560. [Google Scholar] [CrossRef]
- Brassard, P.; Vutcovici, M.; Ernst, P.; Patenaude, V.; Sewitch, M.; Suissa, S.; Bitton, A. Increased incidence of inflammatory bowel disease in Québec residents with airway diseases. Eur. Respir. J. 2014, 45, 962–968. [Google Scholar] [CrossRef]
- Jochmann, A.; Trachsel, D.; Hammer, J. Inflammatory bowel disease and the lung in pediatric patients. Breathe 2021, 17, 200269. [Google Scholar] [CrossRef]
- Rogler, G.; Singh, A.; Kavanaugh, A.; Rubin, D.T. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology 2021, 161, 1118–1132. [Google Scholar] [CrossRef]
- Kelly, M.G.; Frizelle, F.A.; Thornley, P.T.; Beckert, L.; Epton, M.; Lynch, A.C. Inflammatory bowel disease and the lung: Is there a link between surgery and bronchiectasis? Int. J. Color. Dis. 2006, 21, 754–757. [Google Scholar] [CrossRef]
- Desai, D.; Patil, S.; Udwadia, Z.; Maheshwari, S.; Abraham, P.; Joshi, A. Pulmonary manifestations in inflammatory bowel disease: A prospective study. Indian J. Gastroenterol. Off. J. Indian Soc. Gastroenterol. 2011, 30, 225–228. [Google Scholar] [CrossRef]
- Pemmasani, G.; Loftus, E.V.; Tremaine, W.J. Prevalence of Pulmonary Diseases in Association with Inflammatory Bowel Disease. Dig. Dis. Sci. 2022, 67, 5187–5194. [Google Scholar] [CrossRef]
- Black, H.; Mendoza, M.; Murin, S. Thoracic manifestations of inflammatory bowel disease. Chest 2007, 131, 524–532. [Google Scholar] [CrossRef]
- Larsen, S.; Bendtzen, K.; Nielsen, O.H. Extraintestinal manifestations of inflammatory bowel disease: Epidemiology, diagnosis, and management. Ann. Med. 2010, 42, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Wang, L.; Lu, D. Pulmonary manifestations of inflammatory bowel disease. World J. Gastroenterol. 2014, 20, 13501. [Google Scholar] [CrossRef] [PubMed]
- Halling, M.; Kjeldsen, J.; Knudsen, T.; Nielsen, J.; Hansen, L. Patients with inflammatory bowel disease have increased risk of autoimmune and inflammatory diseases. World J. Gastroenterol. 2017, 23, 6137–6146. [Google Scholar] [CrossRef] [PubMed]
- Kröner, P.T.; Lee, A.; Farraye, F.A. Respiratory tract manifestations of inflammatory bowel disease. Inflamm. Bowel. Dis. 2021, 27, 563–574. [Google Scholar] [CrossRef]
- Storch, I.; Sachar, D.; Katz, S. Pulmonary manifestations of inflammatory bowel disease. Inflamm. Bowel. Dis. 2003, 9, 104–115. [Google Scholar] [CrossRef]
- Harbord, M.; Annese, V.; Vavricka, S.R.; Allez, M.; Acosta, M.B.-D.; Borberg, K.; Burisch, J.; De Vos, M.; De Vries, A.-M.; Dick, A.D.; et al. The First European Evidence-based Consensus on Extra-intestinal Manifestations in Inflammatory Bowel Disease. J. Crohn’s Colitis 2016, 10, 239–254. [Google Scholar] [CrossRef]
- Ott, C.; Schölmerich, J. Extraintestinal manifestations and complications in IBD. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 585–595. [Google Scholar] [CrossRef]
- Keir, H.R.; Chalmers, J.D. Pathophysiology of Bronchiectasis. Semin. Respir. Crit. Care Med. 2021, 42, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Mudawi, D.; Heyes, K.; Hastings, R.; Rivera-Ortega, P.; Chaudhuri, N. An update on interstitial lung disease. Br. J. Hosp. Med. 2021, 82, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Eliadou, E.; Moleiro, J.; Ribaldone, D.G.; Astegiano, M.; Rothfuss, K.; Taxonera, C.; Ghalim, F.; Carbonnel, F.; Verstockt, B.; Festa, S.; et al. Interstitial and Granulomatous Lung Disease in Inflammatory Bowel Disease Patients. J. Crohn’s Colitis 2020, 14, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Drakopanagiotakis, F.; Paschalaki, K.; Abu-Hijleh, M.; Aswad, B.; Karagianidis, N.; Kastanakis, E.; Braman, S.; Polychronopoulos, V. Cryptogenic and secondary organizing pneumonia: Clinical presentation, radiographic findings, treatment response, and prognosis. Chest 2011, 139, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, C.A.M.; Gabbiadini, R.; Dal Buono, A.; Quadarella, A.; De Marco, A.; Repici, A.; Bezzio, C.; Simonetta, E.; Aliberti, S.; Armuzzi, A. Lung Involvement in Inflammatory Bowel Diseases: Shared Pathways and Unwanted Connections. J. Clin. Med. 2023, 12, 6419. [Google Scholar] [CrossRef] [PubMed]
- Veloso Fernando Tavarela, M.D.; Carvalho Joäo, M.D.; Magro Fernando, M.D. Immune-Related Systemic Manifestations of Inflammatory Bowel Disease: A Prospective Study of 792 Patients. J. Clin. Gastroenterol. 1996, 23, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Massart, A.; Hunt, D.P. Pulmonary Manifestations of Inflammatory Bowel Disease. Am. J. Med. 2020, 133, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Keely, S.; Talley, N.; Hansbro, P. Pulmonary-intestinal cross-talk in mucosal inflammatory disease. Mucosal. Immunol. 2012, 5, 7–18. [Google Scholar] [CrossRef]
- Kuenzig, M.E.; Bishay, K.; Leigh, R.; Kaplan, G.G.; Benchimol, E.I.; Crowdscreen, S.R.; Review Team. Co-occurrence of Asthma and the Inflammatory Bowel Diseases: A Systematic Review and Meta-analysis. Clin. Transl. Gastroenterol. 2018, 9, 188. [Google Scholar] [CrossRef]
- Chao, K.L.; Kulakova, L.; Herzberg, O. Gene polymorphism linked to increased asthma and IBD risk alters gasdermin-B structure, a sulfatide and phosphoinositide binding protein. Proc. Natl. Acad. Sci. USA 2017, 114, E1128–E1137. [Google Scholar] [CrossRef]
- Zergham, A.; Sekhon, A.; Mebasher, A.; Tserenpil, G.; Malik, B. Inflammatory bowel disease and obstructive pulmonary disease: A two-way association? Cureus 2020, 12, e6836. [Google Scholar] [CrossRef]
- Vutcovici, M.; Brassard, P.; Bitton, A. Inflammatory bowel disease and airway diseases. World J. Gastroenterol. 2016, 22, 7735. [Google Scholar] [CrossRef]
- Ekbom, A.; Brandt, L.; Granath, F.; Löfdahl, C.G.; Egesten, A. Increased risk of both ulcerative colitis and Crohn’s disease in a population suffering from COPD. Lung 2008, 186, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Raftery, A.L.; Tsantikos, E.; Harris, N.L.; Hibbs, M.L. Links Between Inflammatory Bowel Disease and Chronic Obstructive Pulmonary Disease. Front. Immunol. 2020, 11, 2144. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.U.; Kim, J.D.; Park, C.S. Gene-Environment Interactions in Asthma: Genetic and Epigenetic Effects. Yonsei Med. J. 2015, 56, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, S.; Panza, E.; Vecchia, C.; Parazzini, F.; Decarli, A.; Porro, G. Nonspecific inflammatory bowel disease and smoking. Am. J. Epidemiol. 1987, 125, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Mahid, S.; Minor, K.; Soto, R.; Hornung, C.; Galandiuk, S. Smoking and inflammatory bowel disease: A meta-analysis. Mayo Clin. Proc. 2006, 81, 1462–1471. [Google Scholar] [CrossRef]
- Cosnes, J.; Carbonnel, F.; Beaugerie, L.; Quintrec, Y.; Gendre, J. Effects of cigarette smoking on the long-term course of Crohn’s disease. Gastroenterology 1996, 110, 424–431. [Google Scholar] [CrossRef]
- Lindberg, E.; Järnerot, G.; Huitfeldt, B. Smoking in Crohn’s disease: Effect on localisation and clinical course. Gut 1992, 33, 779–782. [Google Scholar] [CrossRef]
- Fricker, M.; Goggins, B.; Mateer, S.; Jones, B.; Kim, R.; Gellatly, S.; Jarnicki, A.; Powell, N.; Oliver, B.; Radford-Smith, G.; et al. Chronic cigarette smoke exposure induces systemic hypoxia that drives intestinal dysfunction. JCI Insight 2018, 3, 3. [Google Scholar] [CrossRef]
- Severs, M.; Erp, S.; Valk, M.; Mangen, M.; Fidder, H.; Have, M.; Bodegraven, A.; Jong, D.; Woude, C.; Romberg-Camps, M.; et al. Smoking is Associated With Extra-intestinal Manifestations in Inflammatory Bowel Disease. J. Crohn’s Colitis 2016, 10, 455–461. [Google Scholar] [CrossRef]
- Rothfuss, K.S.; Stange, E.F.; Herrlinger, K.R. Extraintestinal manifestations and complications in inflammatory bowel diseases. World J. Gastroenterol. WJG 2006, 12, 4819. [Google Scholar] [CrossRef]
- Spagnolo, P.; Bonniaud, P.; Rossi, G.; Sverzellati, N.; Cottin, V. Drug-induced interstitial lung disease. Eur. Respir. J. 2022, 60, 2102776. [Google Scholar] [CrossRef]
- Hill, A.T.; Sullivan, A.L.; Chalmers, J.D.; De Soyza, A.; Elborn, S.J.; Floto, A.R.; Grillo, L.; Gruffydd-Jones, K.; Harvey, A.; Haworth, C.S.; et al. British Thoracic Society Guideline for bronchiectasis in adults. Thorax 2019, 74 (Suppl. S1), 1–69. [Google Scholar] [CrossRef] [PubMed]
- Basseri, B.; Enayati, P.; Marchevsky, A.; Papadakis, K.A. Pulmonary manifestations of inflammatory bowel disease: Case presentations and review. J. Crohn’s Colitis 2010, 4, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Parry, S.D.; Barbatzas, C.; Peel, E.; Barton, J. Sulphasalazine and lung toxicity. Eur. Respir. J. 2002, 19, 756–764. [Google Scholar] [CrossRef]
- Lateef, O.; Shakoor, N.; Balk, R. Methotrexate pulmonary toxicity. Expert Opin. Drug Saf. 2005, 4, 723–730. [Google Scholar] [CrossRef]
- Hamed, K.; Dighriri, I.; Baomar, A.; Alharthy, B.; Alenazi, F.; Alali, G.; Alenazy, R.; Alhumaidi, N.; Alhulayfi, D.; Alotaibi, Y.; et al. Overview of Methotrexate Toxicity: A Comprehensive Literature Review. Cureus 2022, 14, e29518. [Google Scholar] [CrossRef]
- Keane, J.; Gershon, S.; Wise, R.P.; Mirabile-Levens, E.; Kasznica, J.; Schwieterman, W.D.; Siegel, J.N.; Braun, M.M. Tuberculosis associated with infliximab, a tumor necrosis factor α–neutralizing agent. N. Engl. J. Med. 2001, 345, 1098–1104. [Google Scholar] [CrossRef]
- Blonski, W.; Lichtenstein, G.R. Safety of biologic therapy. Inflamm. Bowel Dis. 2007, 13, 769–796. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, D.; Privitera, G.; Schepis, T.; Larosa, L.; Onali, S.; Scaldaferri, F.; Gasbarrini, A.; Caprioli, F.; Armuzzi, A. Drug-Related Pneumonitis in Patients Receiving Vedolizumab Therapy for Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2022, 20, e1483–e1487. [Google Scholar] [CrossRef]
- D’Amico, F.; Parigi, T.L.; Bonovas, S.; Peyrin-Biroulet, L.; Danese, S. Long-term safety of approved biologics for ulcerative colitis. Expert Opin. Drug Saf. 2020, 19, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Miravitlles, M.; Auladell-Rispau, A.; Monteagudo, M.; Vázquez-Niebla, J.C.; Mohammed, J.; Nuñez, A.; Urrútia, G. Systematic review on long-term adverse effects of inhaled corticosteroids in the treatment of COPD. Eur. Respir. Rev. 2021, 30, 210075. [Google Scholar] [CrossRef]
- Sy, A.; Khalidi, N.; Dehghan, N.; Barra, L.; Carette, S.; Cuthbertson, D.; Hoffman, G.S.; Koening, C.L.; Langford, C.A.; McAlear, C.; et al. Vasculitis in patients with inflammatory bowel diseases: A study of 32 patients and systematic review of the literature. Semin. Arthritis Rheum. 2016, 45, 475–482. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kruschewski, M.; Buhr, H.J. The vasculitis in IBD is associated with the degree of inflammation. Dig. Dis. Sci. 2010, 55, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, C.; Abitbol, V.; El Karoui, K.; Bourrier, A.; Paule, R.; Vuitton, L.; Maurier, F.; Laharie, D.; Fuméry, M.; Agard, C.; et al. IgA vasculitis in patients with inflammatory bowel disease: New insights into the role of TNF-α blockers. Rheumatology 2022, 61, 1957–1965. [Google Scholar] [CrossRef] [PubMed]
- Sokumbi, O.; Wetter, D.A.; Makol, A.; Warrington, K.J. Vasculitis associated with tumor necrosis factor-α inhibitors. Mayo Clin. Proc. 2012, 87, 739–745. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Blanchard, J.F.; Houston, D.S.; Wajda, A. The incidence of deep venous thrombosis and pulmonary embolism among patients with inflammatory bowel disease: A population-based cohort study. Thromb. Haemost. 2001, 85, 430–434. [Google Scholar] [PubMed]
- Giannotta, M.; Tapete, G.; Emmi, G.; Silvestri, E.; Milla, M. Thrombosis in inflammatory bowel diseases: What’s the link? Thromb. J. 2015, 13, 1–9. [Google Scholar] [CrossRef]
- Chung, W.; Lin, C.; Hsu, W.; Kao, C. Inflammatory bowel disease increases the risks of deep vein thrombosis and pulmonary embolism in the hospitalized patients: A nationwide cohort study. Thromb. Res. 2015, 135, 492–496. [Google Scholar] [CrossRef]
- Zezos, P.; Kouklakis, G.; Saibil, F. Inflammatory bowel disease and thromboembolism. World J. Gastroenterol. 2014, 20, 13863–13878. [Google Scholar] [CrossRef] [PubMed]
- Irving, P.; Pasi, K.; Rampton, D. Thrombosis and inflammatory bowel disease. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2005, 3, 617–628. [Google Scholar] [CrossRef]
- Schwartz, D.; Loftus, E.; Tremaine, W.; Panaccione, R.; Harmsen, W.; Zinsmeister, A.; Sandborn, W. The natural history of fistulizing Crohn’s disease in Olmsted County, Minnesota. Gastroenterology 2002, 122, 875–880. [Google Scholar] [CrossRef]
- Karmy-Jones, R.; Chagpar, A.; Vallieres, E.; Hamilton, S. Colobronchial fistula due to Crohn’s disease. Ann. Thorac. Surg. 1995, 60, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Gumbo, T.; Rice, T.W.; Mawhorter, S. Rice, and Steven Mawhorter. Recurrent pneumonia from an ileobronchial fistula complicating Crohn’s disease. J. Clin. Gastroenterol. 2001, 32, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Ho, I.K.; Guarino, D.P.; Pertsovskiy, Y.; Cerulli, M.A. Infliximab treatment of an esophagobronchial fistula in a patient with extensive Crohn’s disease of the esophagus. J. Clin. Gastroenterol. 2002, 34, 488–489. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Periwal, P.; Jain, A.; Jain, S.; Yadav, S.; Jain, A.; Chawla, A. Iatrogenic fecopneumothorax: A rare cause of hydropneumothorax. J. Med. Soc. 2018, 32, 66–68. [Google Scholar] [CrossRef]
- Markogiannakis, H.; Theodorou, D.; Tzertzemelis, D.; Dardamanis, D.; Toutouzas, K.; Misthos, P.; Katsaragakis, S. Fecopneumothorax: A rare complication of esophagectomy. Ann. Thorac. Surg. 2007, 84, 651–652. [Google Scholar] [CrossRef]
- Seelig, M.; Klingler, P.; Scho¨nleben, K. Tension fecopneumothorax due to colonic perforation in a diaphragmatic hernia. Chest 1999, 115, 288–291. [Google Scholar] [CrossRef]
- Zhao, J.; Ma, N.; Zhao, Z.; Lei, J.; Lu, Q.; Tian, F.; Zhou, Y.; Han, Y.; Li, X. Colobronchial fistula: The pathogenesis, clinical presentations, diagnosis and treatment. J. Thorac. Dis. 2017, 9, 187–193. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Su, C.G.; Judge, T.A.; Lichtenstein, G.R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol. Clin. 2002, 31, 307–327. [Google Scholar] [CrossRef]
- Perluk, T.M.; Friedman Regev, I.; Freund, O.; Kleinhendler, E.; Hershko, T.; Ben-Ami, S.; Bar-Shai, A.; Unterman, A. Importance of physician history taking in complementing patient-reported interstitial lung disease questionnaire. BMC Pulm. Med. 2022, 22, 489. [Google Scholar] [CrossRef]
- Ellrichmann, M.; Bethge, J.; Boesenkoetter, J.; Conrad, C.; Noth, R.; Bahmer, T.; Nikolaus, S.; Aden, K.; Zeissig, S.; Schreiber, S. Subclinical Pulmonary Involvement in Active IBD Responds to Biologic Therapy. J. Crohn’s Colitis 2021, 15, 1339–1345. [Google Scholar] [CrossRef]
- Songür, N.; Songür, Y.; Tüzün, M.; Doğan, I.; Tüzün, D.; Ensari, A.; Hekimoglu, B. Pulmonary function tests and high-resolution CT in the detection of pulmonary involvement in inflammatory bowel disease. J. Clin. Gastroenterol. 2003, 37, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Mahadeva, R.; Walsh, G.; Flower, C.D.; Shneerson, J.M. Clinical and radiological characteristics of lung disease in inflammatory bowel disease. Eur. Respir. J. 2000, 15, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.S.; Burakoff, R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol. Hepatol. 2011, 7, 235–241. [Google Scholar]
- Cozzi, D.; Moroni, C.; Addeo, G.; Danti, G.; Lanzetta, M.M.; Cavigli, E.; Falchini, M.; Marra, F.; Piccolo, C.L.; Brunese, L.; et al. Radiological patterns of lung involvement in inflammatory bowel disease. Gastroenterol. Res. Pract. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Maglione, M.; Aksamit, T.; Santamaria, F. Paediatric and adult bronchiectasis: Specific management with coexisting asthma, COPD, rheumatological disease and inflammatory bowel disease. Respirology 2019, 24, 1063–1072. [Google Scholar] [CrossRef]
- Chuah, C.S.; Noble, C.; Leitch, A. Case of steroid-resistant Crohn’s-associated bronchiolitis in the setting of quiescent gastrointestinal disease treated with infliximab. BMJ Case Rep. 2018, 11, e226934. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghosh, S.; Deshwal, H.; Haraf, R.; Raju, S.; Saeedan, M.B.; Sarkar, P.; Gildea, T.; Farver, C.F.; Mehta, A.C. Pulmonary Manifestations of Inflammatory Bowel Disease and Treatment Strategies. CHEST Pulm. 2023, 1, 100018. [Google Scholar] [CrossRef]
- Hayek, A.J.; Pfanner, T.P.; White, H.D. Inflammatory bowel disease of the lung: The role of infliximab? Respir. Med. Case Rep. 2015, 15, 85–88. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Pedersen, N.; Duricova, D.; Munkholm, P. Pulmonary Crohn’s disease: A rare extra-intestinal manifestation treated with infliximab. J. Crohn’s Colitis 2009, 3, 207–211. [Google Scholar] [CrossRef]
- Camus, P.H.; Colby, T.V. The lung in inflammatory bowel disease. Eur. Respir. J. 2000, 15, 5–10. [Google Scholar] [CrossRef]
- Schleiermacher, D.; Hoffmann, J.C. Pulmonary abnormalities in inflammatory bowel disease. J. Crohn’s Colitis 2007, 1, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Vavricka, S.R.; Gubler, M.; Gantenbein, C.; Spoerri, M.; Froehlich, F.; Seibold, F.; Protic, M.; Michetti, P.; Straumann, A.; Fournier, N.; et al. Anti-TNF treatment for extraintestinal manifestations of inflammatory bowel disease in the Swiss IBD Cohort Study. Inflamm. Bowel Dis. 2017, 23, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Romano, C.; Cardile, S. Pulmonary implications in inflammatory bowel disease: Not a rare event. Expert Opin. Drug Saf. 2016, 15, 1001–1002. [Google Scholar] [CrossRef] [PubMed]
- Taveras, N.T.; Martinez, A.R.; Kumar, R.; Jamil, A.; Kumar, B. Pulmonary manifestations of inflammatory bowel disease. Cureus 2021, 13, e14216. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.; Jayaram, L.; Karalus, N.; Eaton, T.; Tong, C.; Hockey, H.; Milne, D.; Fergusson, W.; Tuffery, C.; Sexton, P.; et al. Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): A randomised, double-blind, placebo-controlled trial. Lancet 2012, 380, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Montesi, S.B.; Silver, R.M.; Hossain, T.; Macrea, M.; Herman, D.; Barnes, H.; Adegunsoye, A.; Azuma, A.; Chung, L.; et al. Treatment of Systemic Sclerosis-associated Interstitial Lung Disease: Evidence-based Recommendations. An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2024, 209, 137–152. [Google Scholar] [CrossRef]
- Berezne, A.; Ranque, B.; Valeyre, D.; Brauner, M.; Allanore, Y.; Launay, D.; Mouthon, L. Therapeutic strategy combining intravenous cyclophosphamide followed by oral azathioprine to treat worsening interstitial lung disease associated with systemic sclerosis: A retrospective multicenter open-label study. J. Rheumatol. 2008, 35, 1064–1072. [Google Scholar]
- Iudici, M.; Cuomo, G.; Vettori, S.; Bocchino, M.; Zamparelli, A.; Cappabianca, S.; Valentini, G. Low-dose pulse cyclophosphamide in interstitial lung disease associated with systemic sclerosis (SSc-ILD): Efficacy of maintenance immunosuppression in responders and non-responders. Semin. Arthritis Rheum. 2015, 44, 437–444. [Google Scholar] [CrossRef]
- Tashkin, D.; Roth, M.; Clements, P.; Furst, D.; Khanna, D.; Kleerup, E.; Goldin, J.; Arriola, E.; Volkmann, E.; Kafaja, S.; et al. Mycophenolate Mofetil versus Oral Cyclophosphamide in Scleroderma-related Interstitial Lung Disease: Scleroderma Lung Study II (SLS-II), a double-blind, parallel group, randomised controlled trial. The Lancet. Respir. Med. 2016, 4, 708–719. [Google Scholar] [CrossRef]
- Alharbi, M.G.; Kalra, H.S.; Suri, M.; Soni, N.; Okpaleke, N.; Yadav, S.; Shah, S.; Iqbal, Z.; Hamid, P. Pulmonary Rehabilitation in Management of Chronic Obstructive Pulmonary Disease. Cureus 2021, 13, e18414. [Google Scholar] [CrossRef]
- Carrera, E.; Manzano, R.; Garrido, E. Efficacy of the vaccination in inflammatory bowel disease. World J. Gastroenterol. 2013, 19, 1349–1353. [Google Scholar] [CrossRef] [PubMed]
- Wasan, S.; Baker, S.; Skolnik, P.; Farraye, F. A Practical Guide to Vaccinating the Inflammatory Bowel Disease Patient. Am. J. Gastroenterol. 2010, 105, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Reich, J.; Wasan, S.; Farraye, F. Vaccinating Patients with Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2016, 12, 540–546. [Google Scholar]
- Manser, C.; Maillard, M.; Rogler, G.; Schreiner, P.; Rieder, F.; Bühler, S. Vaccination in Patients with Inflammatory Bowel Diseases. Digestion 2020, 101, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Stobaugh, D.J.; Deepak, P.; Ehrenpreis, E.D. Hospitalizations for vaccine preventable pneumonias in patients with inflammatory bowel disease: A 6-year analysis of the Nationwide Inpatient Sample. Clin. Exp. Gastroenterol. 2013, 6, 43–49. [Google Scholar] [CrossRef]
- Suzuki, A.; Noro, R.; Omori, J.; Terasaki, Y.; Tanaka, T.; Fujita, K.; Takano, N.; Sakurai, Y.; Suga, M.; Hayashi, A.; et al. Pulmonary manifestation of inflammatory bowel disease: Two case reports. Respir. Med. Case Rep. 2023, 45, 101914. [Google Scholar] [CrossRef]
- Perluk, T.; Abu Bandora, E.; Freund, O.; Jacob, T.; Friedman Regev, I.; Kleinhendler, E.; Shteinberg, M.; Bar-Shai, A.; Oestriecher-Kedem, Y. Asymptomatic Dysphagia and Aspiration in Patients with Idiopathic Bronchiectasis. Lung 2024, 202, 189–195. [Google Scholar] [CrossRef]
- Jess, T.; Loftus, E.V.; Harmsen, W.S., Jr.; Zinsmeister, A.R.; Tremaine, W.J.; Melton, L.J., 3rd; Munkholm, P.; Sandborn, W.J. Survival and cause specific mortality in patients with inflammatory bowel disease: A long term outcome study in Olmsted County 2022, Minnesota, 1940–2004. Gut 2006, 55, 1248–1254. [Google Scholar] [CrossRef]
- Macaluso, F.S.; Giuliano, A.; Fries, W.; Viola, A.; Abbruzzese, A.; Cappello, M.; Giuffrida, E.; Carrozza, L.; Privitera, A.C.; Magnano, A.; et al. Severe Activity of Inflammatory Bowel Disease is a Risk Factor for Severe COVID-19. Inflamm. Bowel Dis. 2023, 29, 217–221. [Google Scholar] [CrossRef]
- Talbot, R.W.; Heppell, J.; Dozois, R.R.; Beart, R.W., Jr. Vascular complications of inflammatory bowel disease. Mayo Clin. Proc. 1986, 61, 140–145. [Google Scholar] [CrossRef]
| Type of Drug | Potential Pulmonary Adverse Events |
|---|---|
| 5-Aminosalicylic Acid (5-ASA) and Sulfasalazine | ILDs, hypersensitivity pneumonitis, eosinophilic pneumonia, or nonspecific interstitial pneumonitis [42,43] |
| Methotrexate | Acute or chronic pulmonary toxicity, manifesting as cough and dyspnea [44,45] |
| Advanced therapies | |
| Corticosteroids | Long-term use can lead to opportunistic infections [50] |
| Diagnostic Tool | Findings |
|---|---|
| Pulmonary Function Tests | Obstructive or restrictive patterns. Decreased diffusion capacity in interstitial lung disease. Might be normal in mild/early disease [73] |
| Chest X-ray | Patterns of interstitial lung disease, airway dilation, pleural involvement. Might be non-specific. |
| High-resolution computed tomography | More sensitive than X-ray, can identify features such as bronchial wall thickening, airway dilation, and mucoid impaction that are indicative of bronchiectasis, tree-in-bud patterns, ground-glass opacities, or honeycombing indicative of fibrotic changes [74,75]. |
| Bronchoscopy | Detection of causative pathogens by bronchial washings. Bronchoalveolar lavage for cell analysis to identify lymphocytosis, eosinophilia, and CD4:CD8 ratio. |
| Lung Biopsy | Can provide definitive histological evidence of pulmonary involvement, revealing patterns of inflammation, fibrosis, or granuloma formation. |
| Aspect | Research Needs |
|---|---|
| Epidemiological Data | Conduct large-scale, longitudinal studies to better understand the prevalence, incidence, and burden of pulmonary manifestations in IBD. |
| Pathophysiological Mechanisms | Elucidate underlying mechanisms linking IBD and pulmonary manifestations, focusing on the gut–lung axis, immune response, genetic predisposition, and environmental factors. |
| Impact of Biological Therapies | Investigate the long-term pulmonary health impact of biologic therapies through post-marketing surveillance studies and registry data to understand respiratory side effects. |
| Diagnostic and Screening Tools | Develop and validate screening tools and biomarkers for the early detection of pulmonary involvement in IBD, with high sensitivity and specificity, to enable earlier interventions and potentially improve outcomes. Standardized protocols for assessing pulmonary involvement in IBD can enhance the reliability and efficacy of the diagnosis. |
| Treatment Efficacy and Safety | Conduct randomized controlled trials to assess the efficacy and safety of treatments for pulmonary manifestations in IBD, focusing on newer pharmacological agents and other treatment modalities. |
| Multidisciplinary Management Approaches | Study multidisciplinary management strategies to develop standardized care pathways for IBD patients with pulmonary manifestations. |
| Quality of Life Assessments | Perform studies that assess the impact of pulmonary manifestations on the quality of life of IBD patients, including patient-reported outcomes. |
| Long-term outcomes | Conduct comprehensive research to gather data on the long-term outcomes of pulmonary manifestations in IBD, identifying more prognostic factors and the impact on overall survival and quality of life. |
| Genetic and Environmental Interactions | Research genetic factors predisposing IBD patients to pulmonary manifestations and the role of environmental exposures for risk stratification and personalized medicine approaches. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herling, A.; Perluk, T.M.; Freund, O.; Maharshak, N.; Cohen, N.A. Pulmonary Manifestations of IBD: Case Report and Review of the Literature. J. Clin. Med. 2024, 13, 5401. https://doi.org/10.3390/jcm13185401
Herling A, Perluk TM, Freund O, Maharshak N, Cohen NA. Pulmonary Manifestations of IBD: Case Report and Review of the Literature. Journal of Clinical Medicine. 2024; 13(18):5401. https://doi.org/10.3390/jcm13185401
Chicago/Turabian StyleHerling, Amit, Tal Moshe Perluk, Ophir Freund, Nitsan Maharshak, and Nathaniel Aviv Cohen. 2024. "Pulmonary Manifestations of IBD: Case Report and Review of the Literature" Journal of Clinical Medicine 13, no. 18: 5401. https://doi.org/10.3390/jcm13185401
APA StyleHerling, A., Perluk, T. M., Freund, O., Maharshak, N., & Cohen, N. A. (2024). Pulmonary Manifestations of IBD: Case Report and Review of the Literature. Journal of Clinical Medicine, 13(18), 5401. https://doi.org/10.3390/jcm13185401







