Musculoskeletal Infection: The Great Mimickers on Imaging
Abstract
1. Introduction
2. Spine and Sacroiliac Joints
2.1. Non-Infectious Discitis and Spondylodiscitis
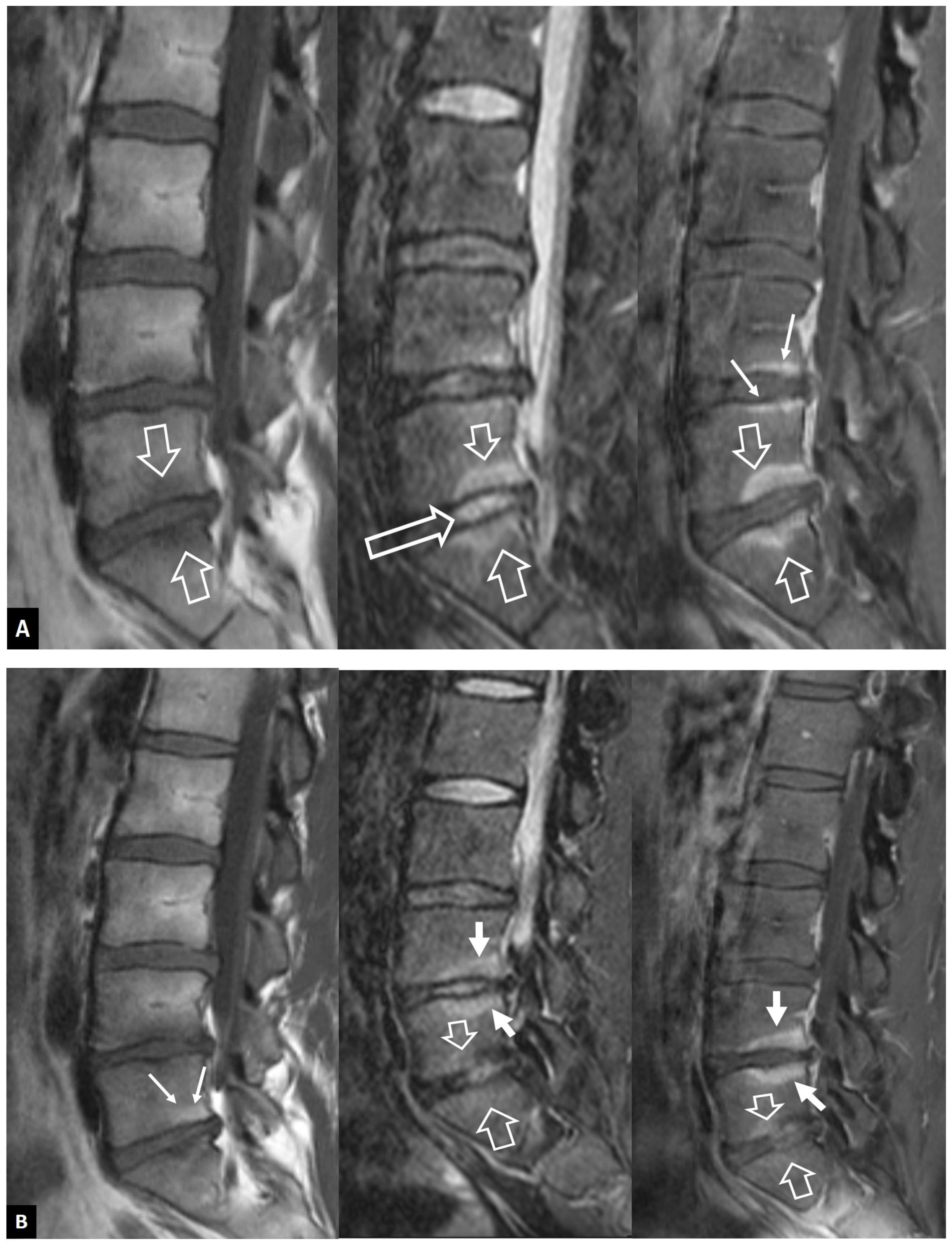
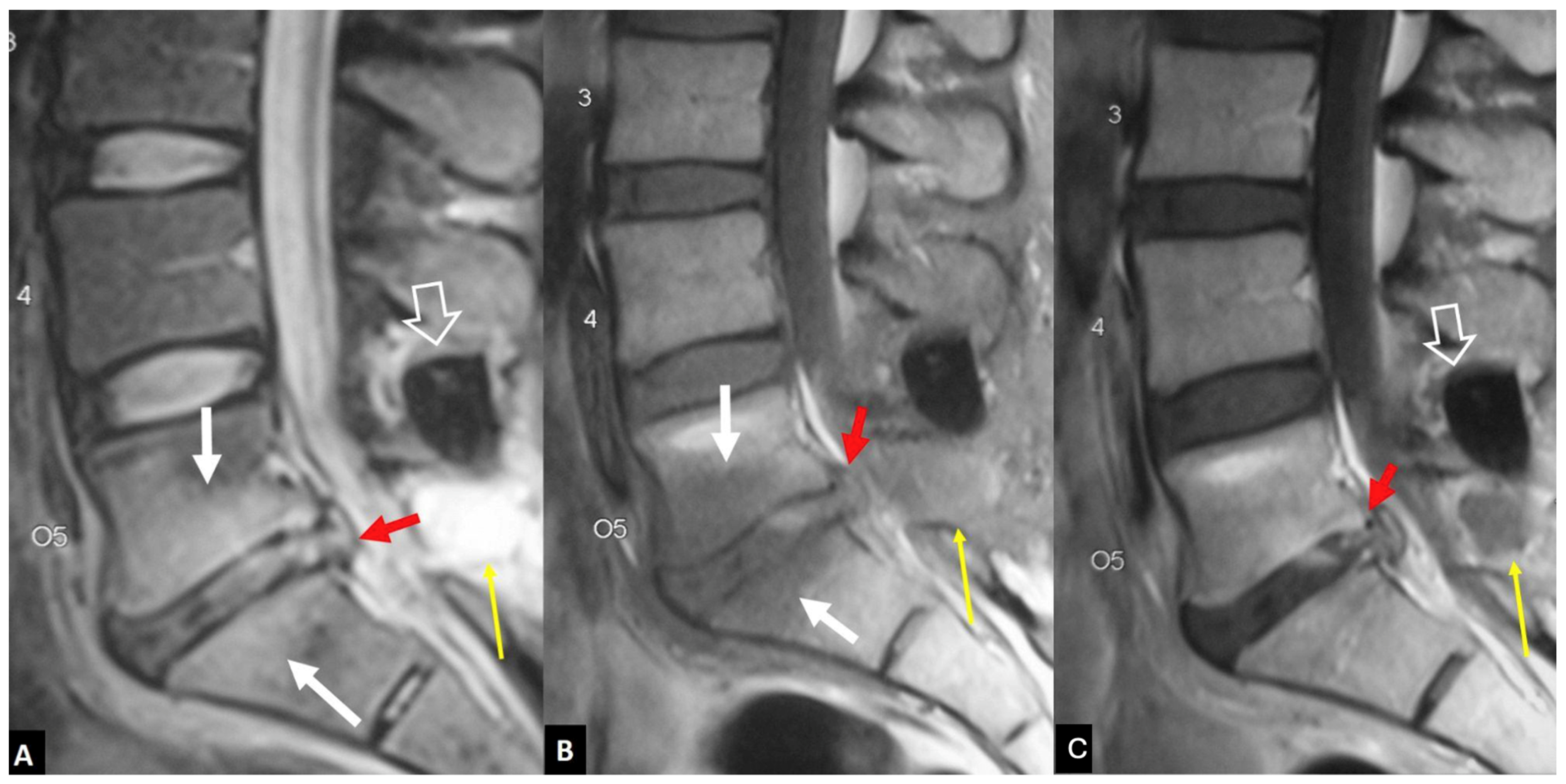
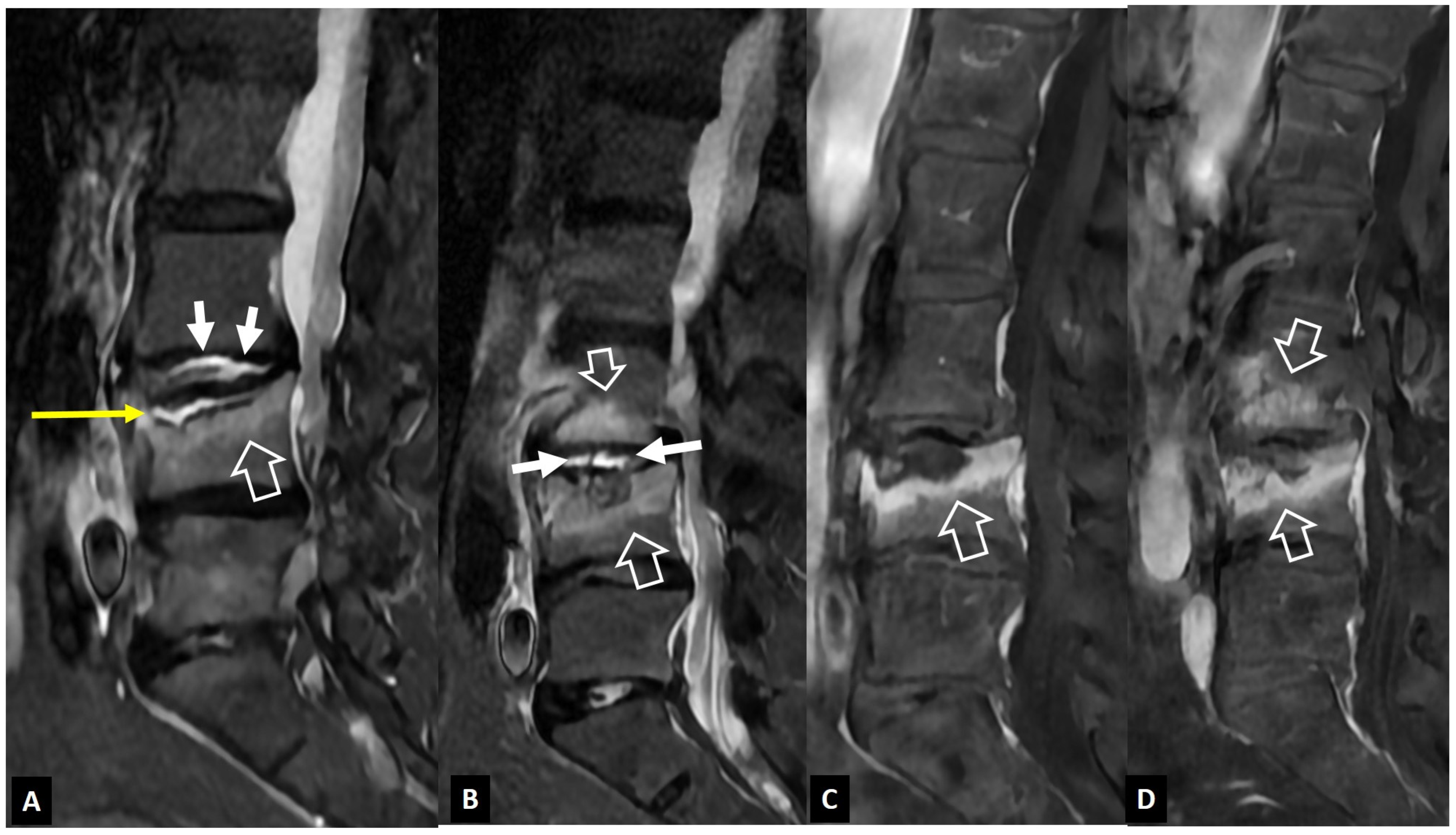
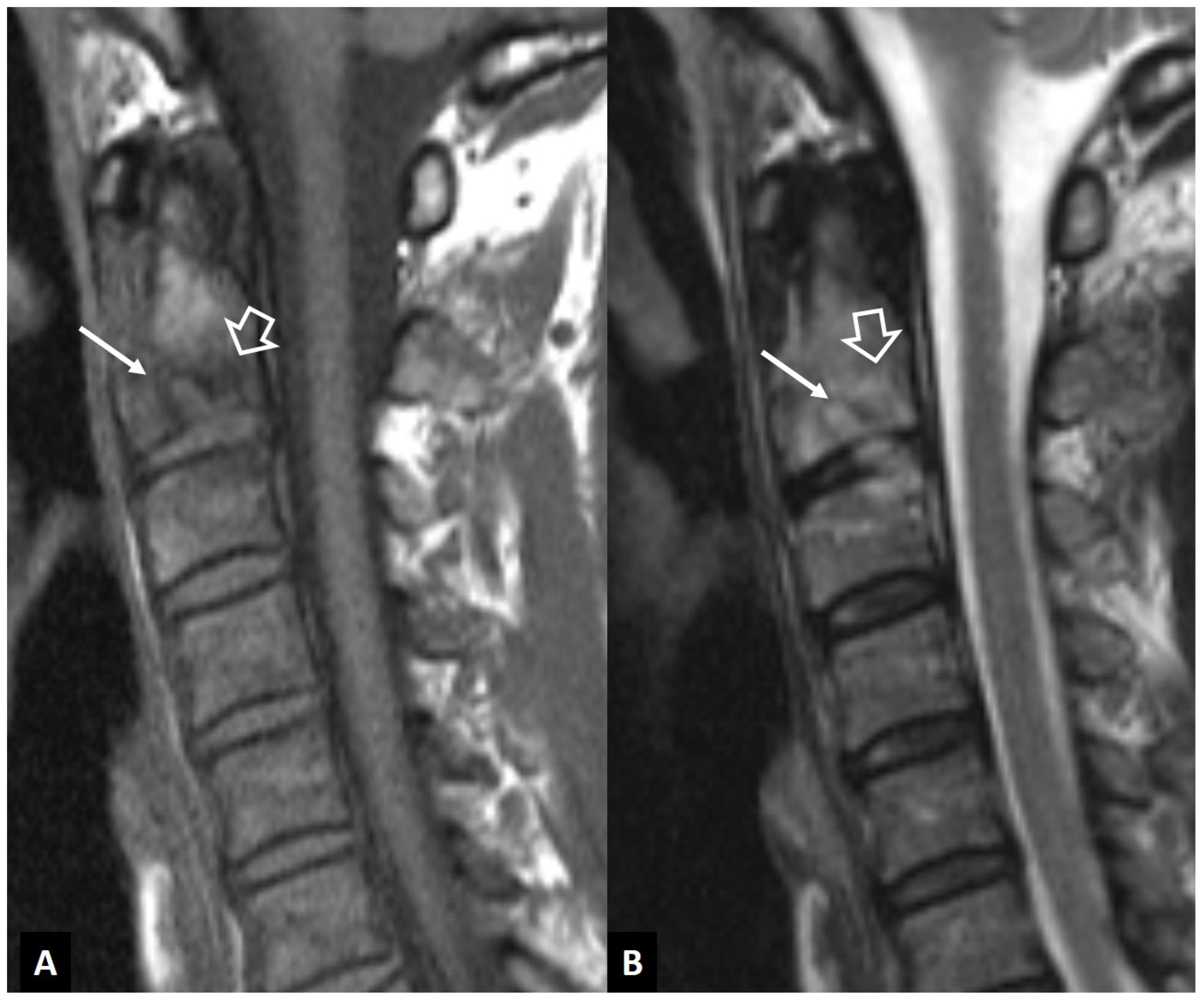
2.1.1. MODIC I Changes
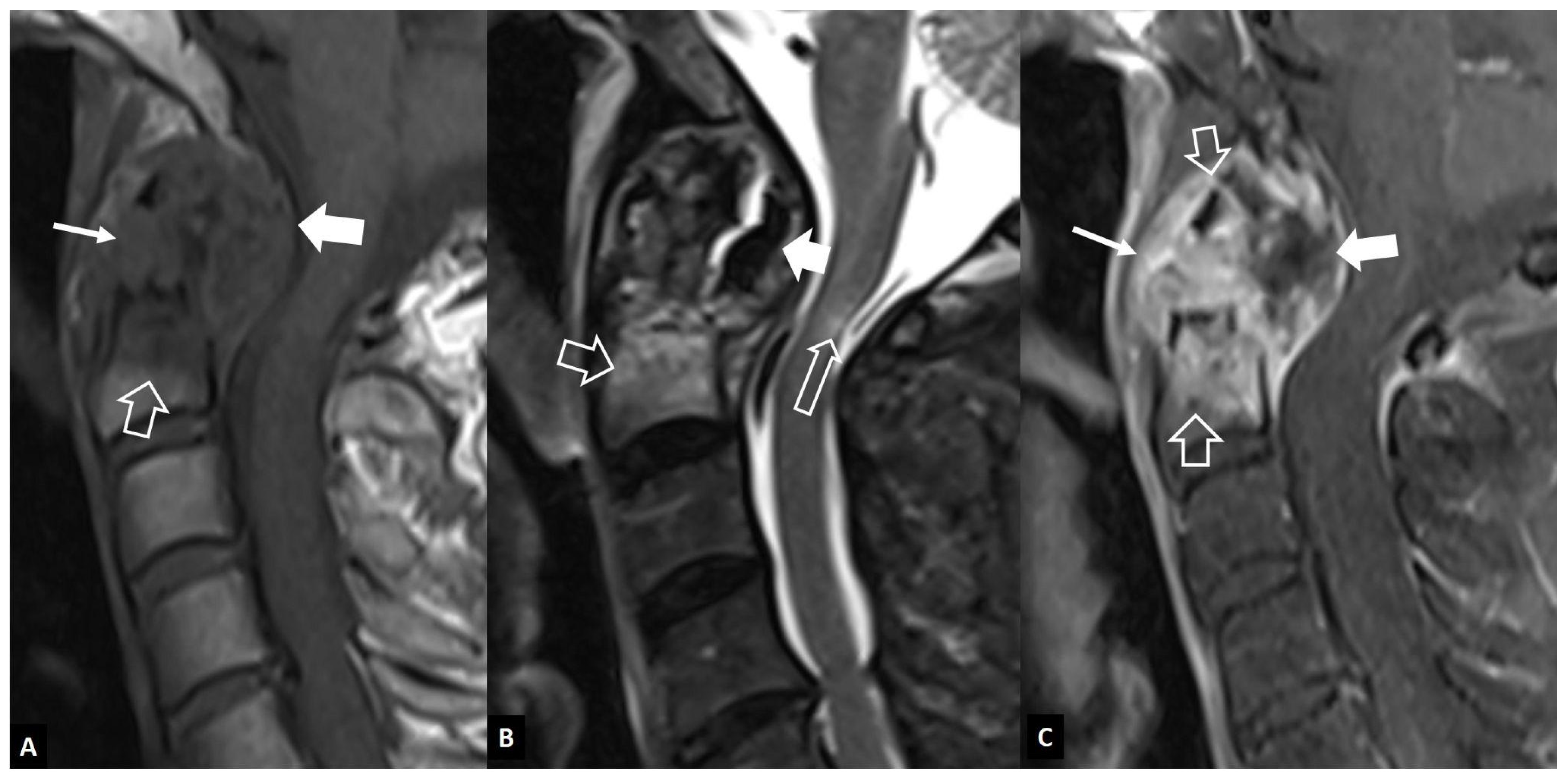
2.1.2. Aseptic Spondylodiscitis
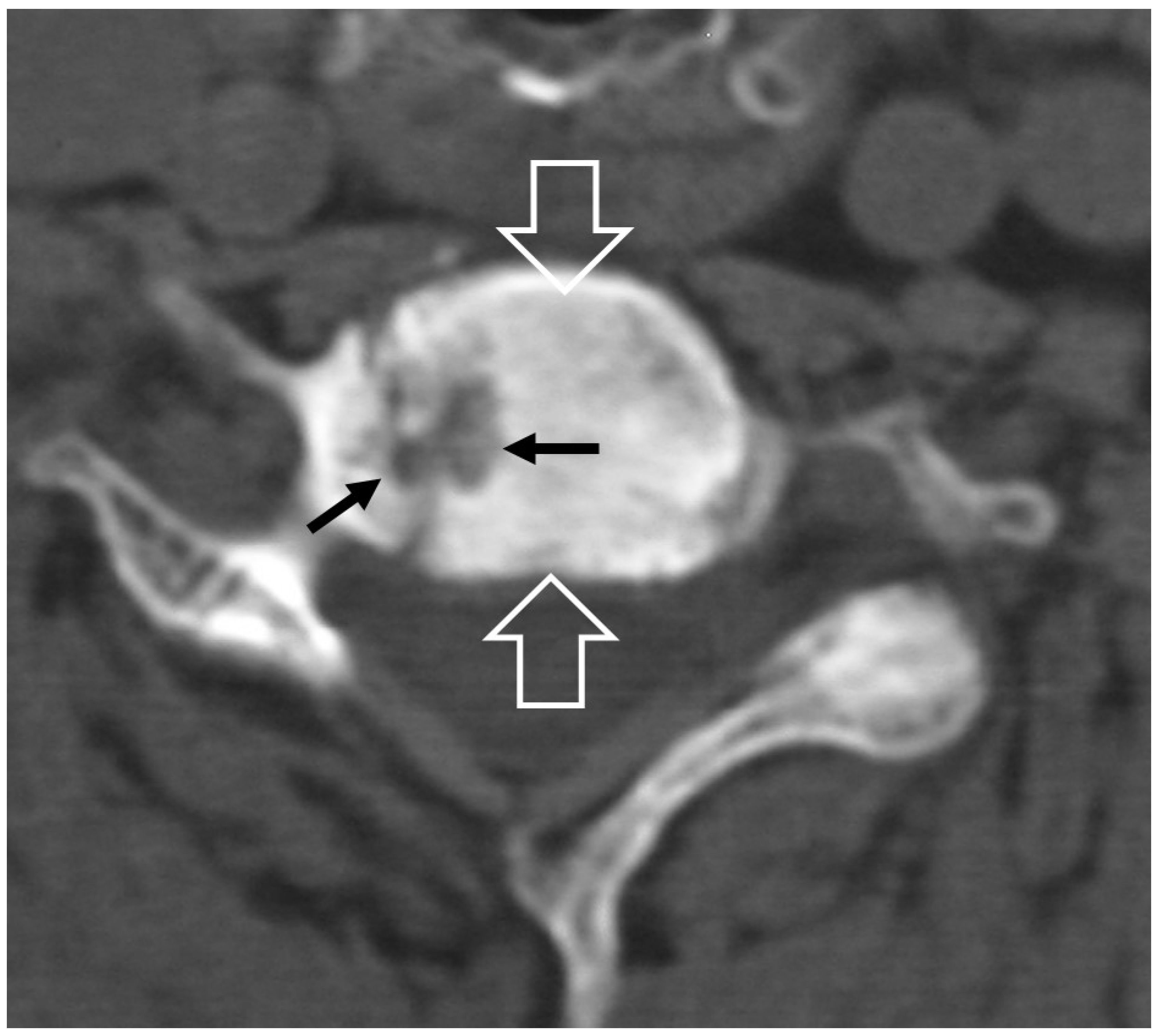
2.1.3. SAPHO Syndrome
2.1.4. Destructive Spondyloarthropathy
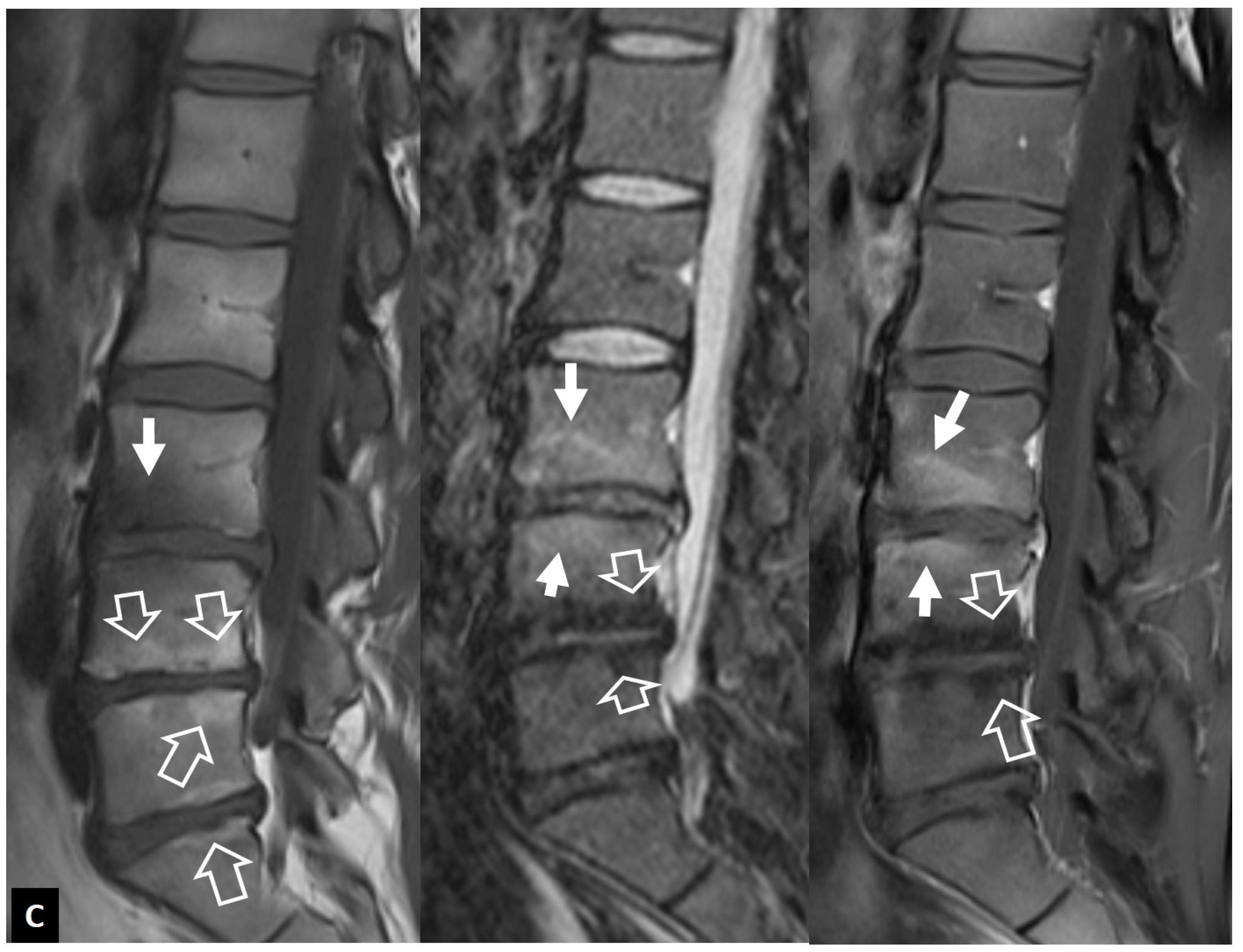
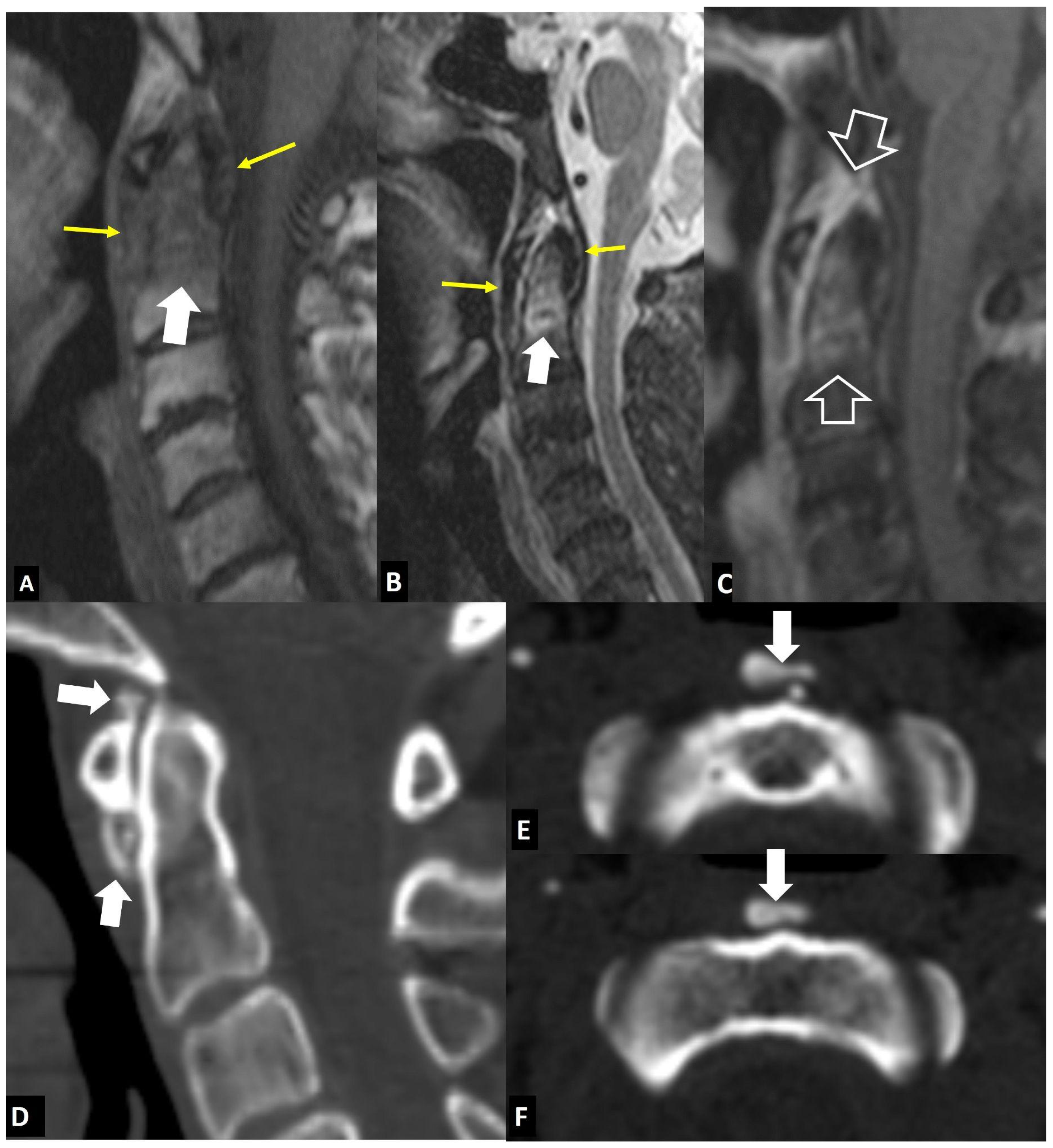
2.1.5. Crystal Deposition
2.1.6. Post-Operative Aseptic Discitis
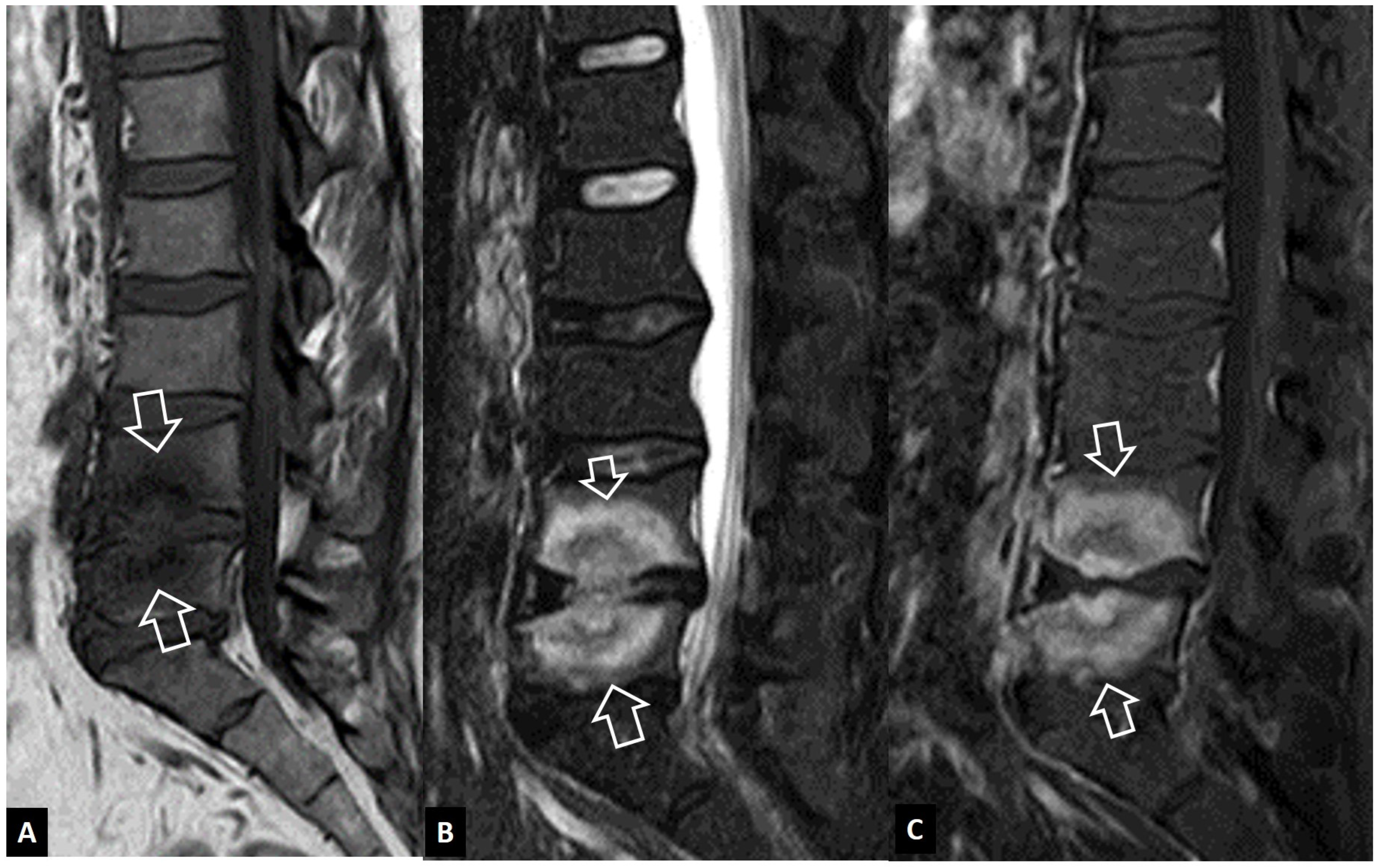
2.1.7. Trauma
2.1.8. Rheumatoid Arthritis
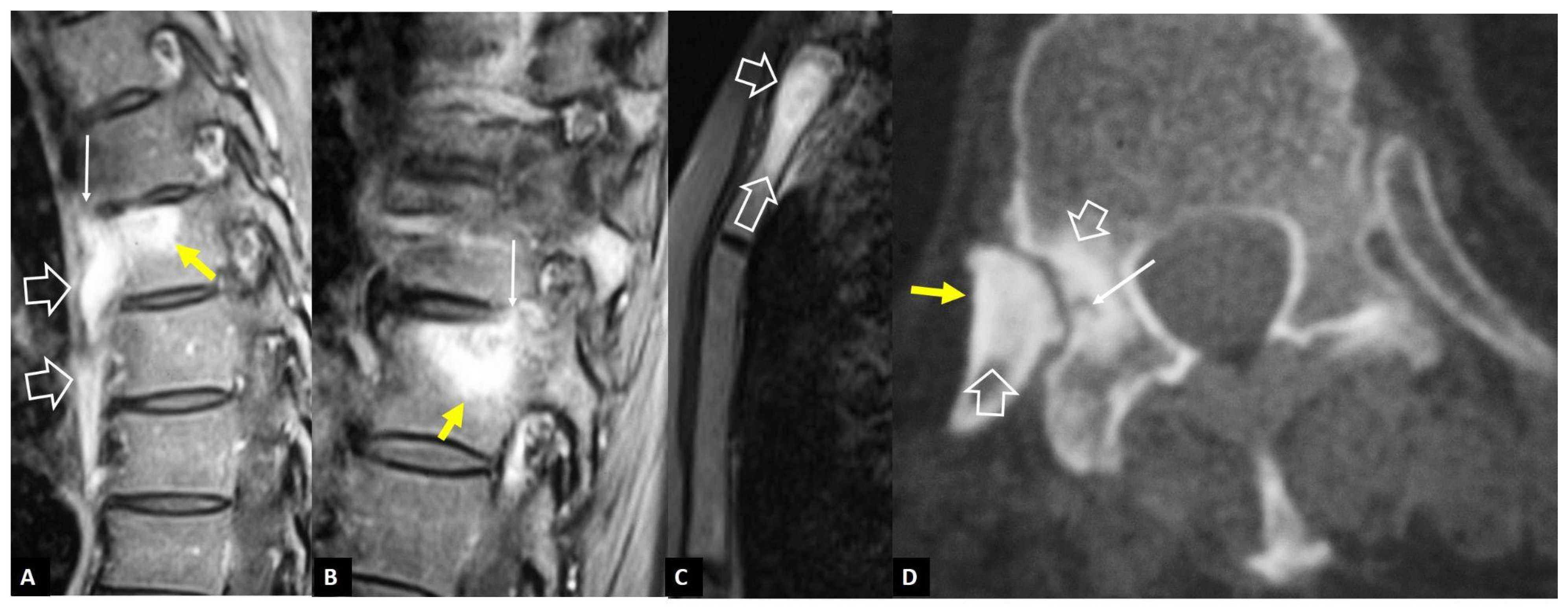
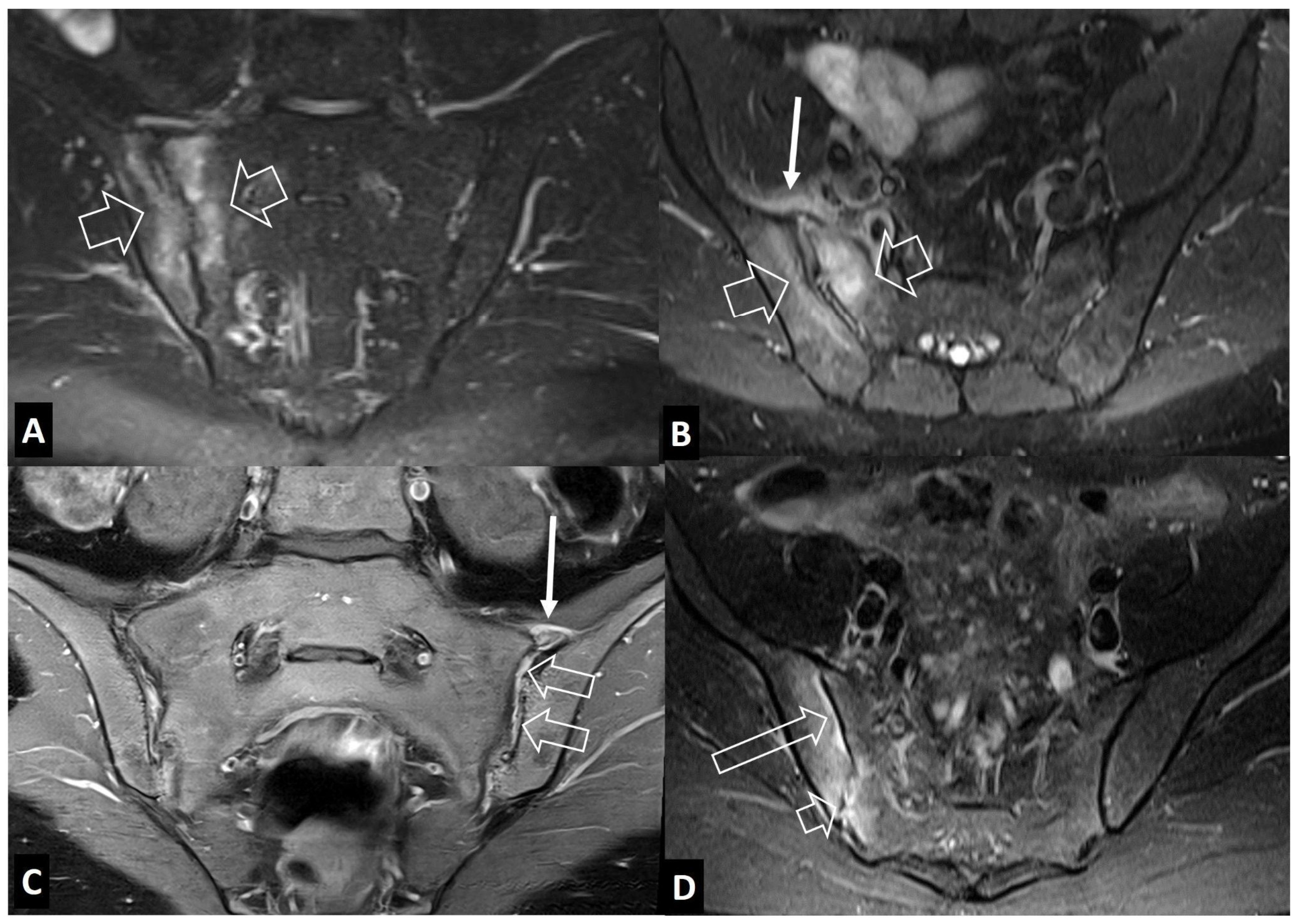
2.2. Non-Infectious Sacroiliitis
3. Long Bones
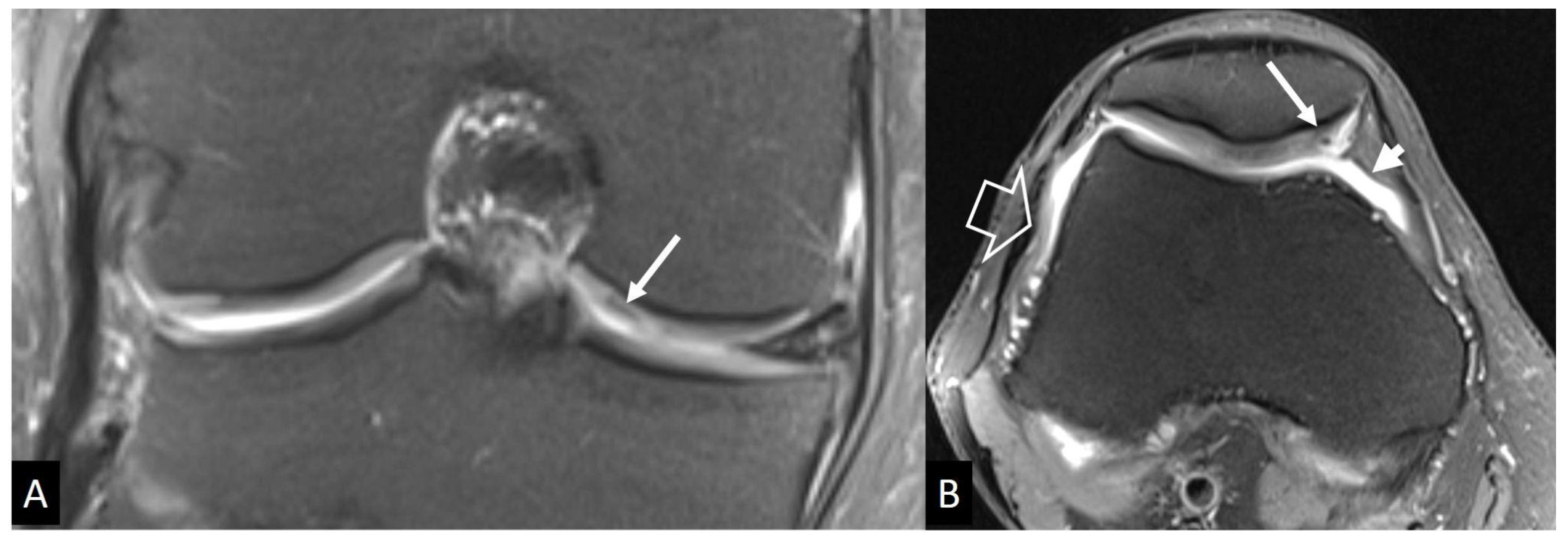
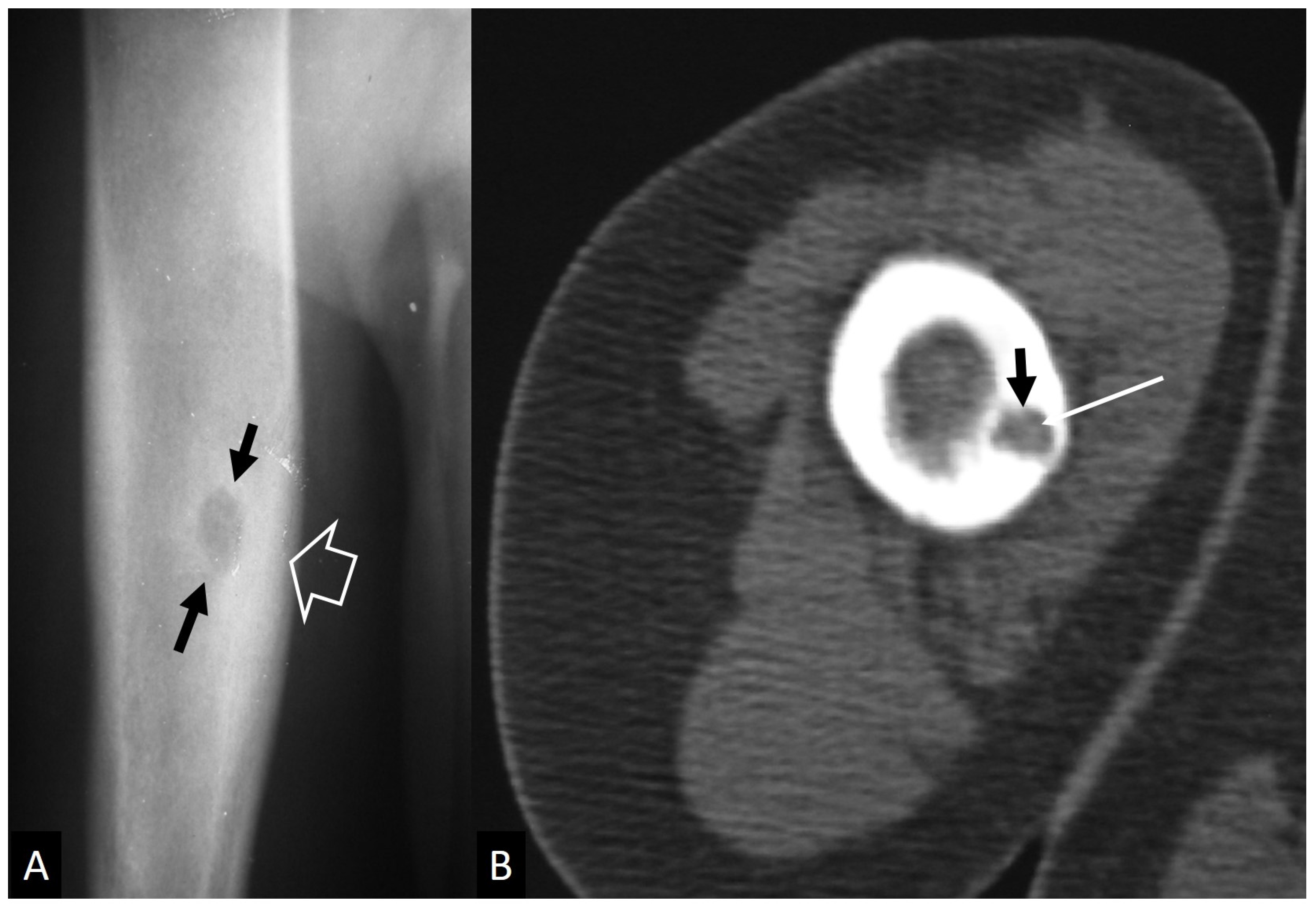
3.1. Stress Injuries
3.2. Neoplasms
3.2.1. Ewing’s Sarcoma
3.2.2. Lymphoma
3.3. Radiation Osteitis–Iatrogenic
4. Peripheral Joints
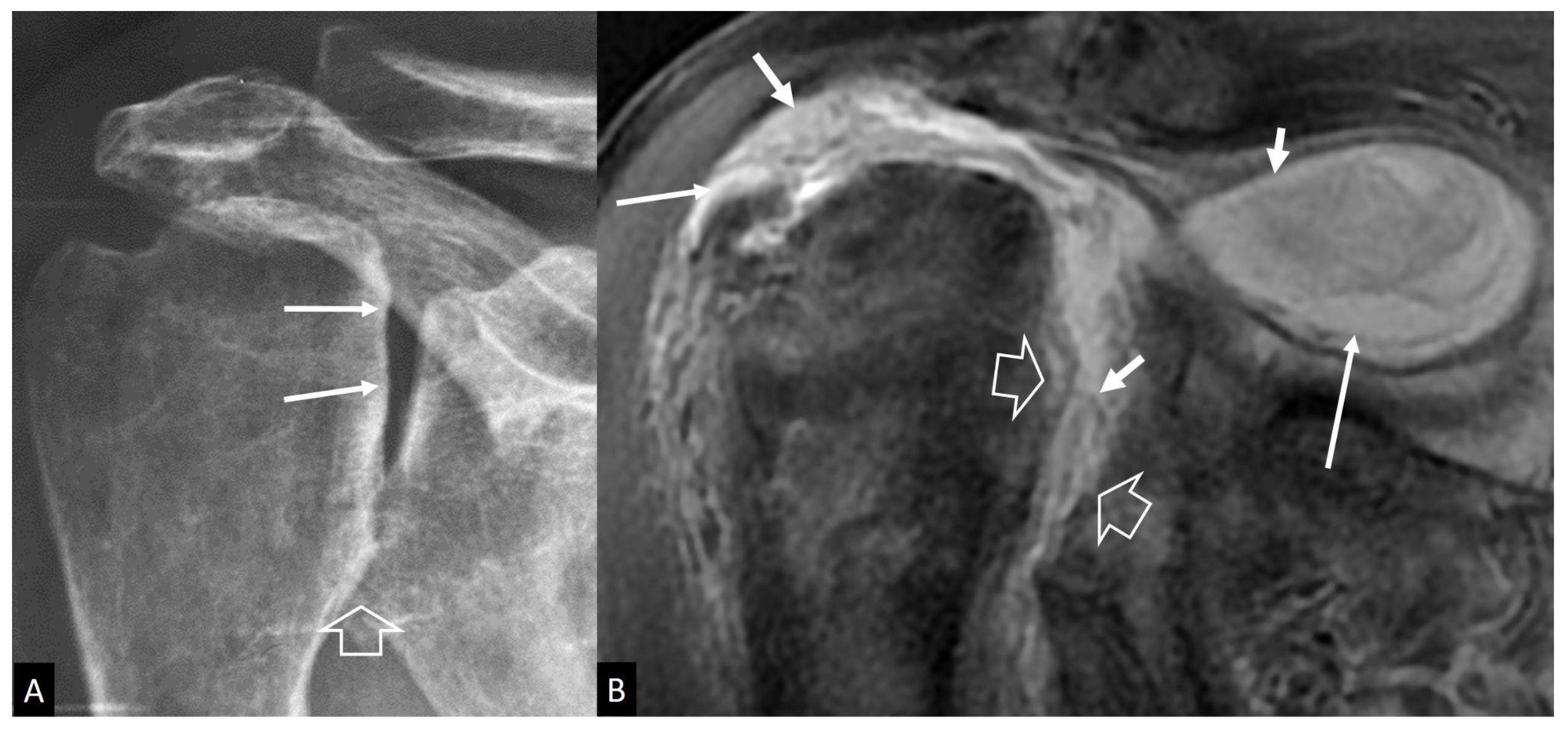
4.1. Imaging Features of Septic Arthritis
4.2. Imaging Features of Septic Arthritis Mimickers
4.2.1. Inflammatory Arthritis
4.2.2. Neuropathic Arthropathy
4.2.3. Crystal-Induced Arthropathies
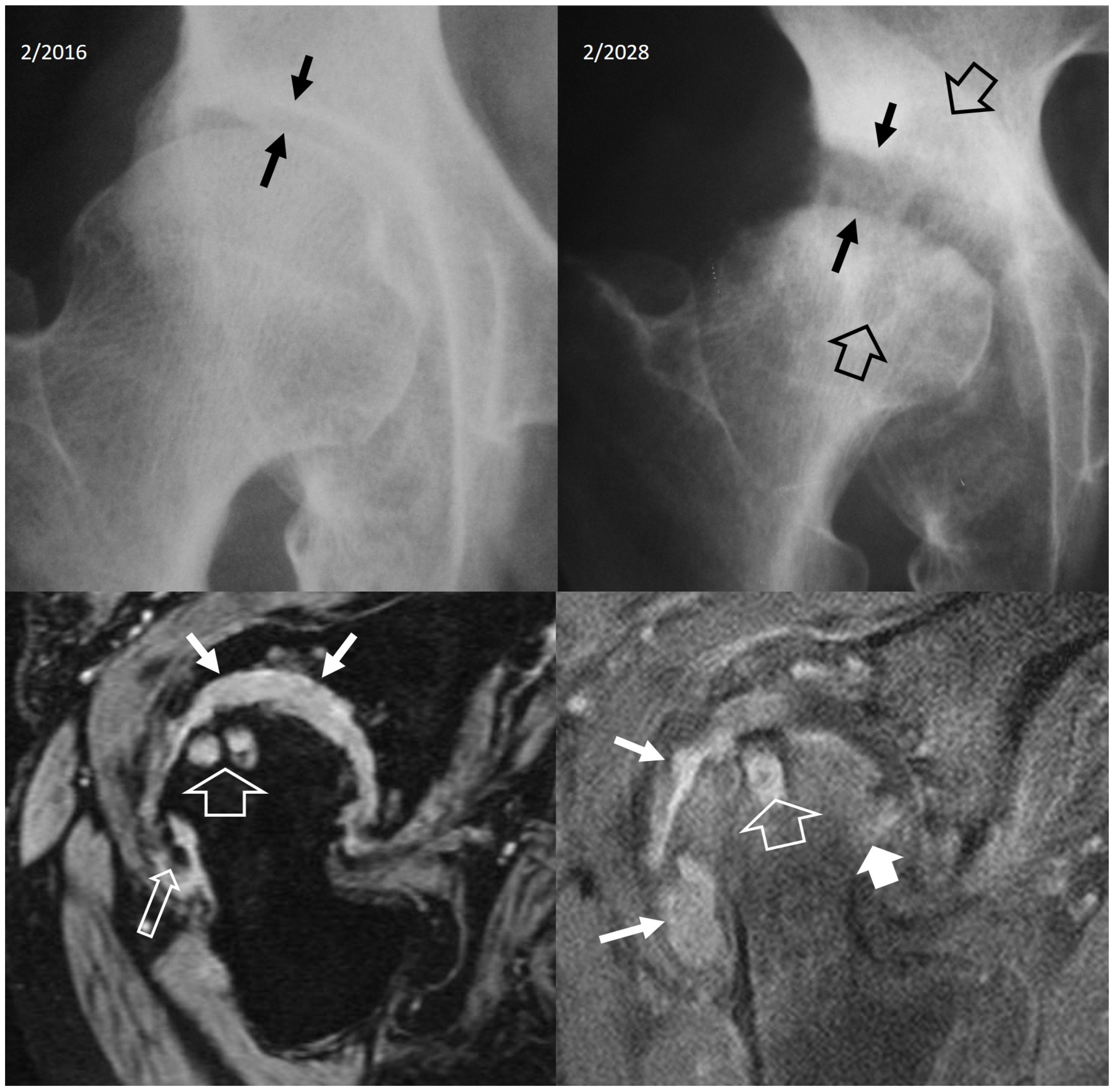
4.2.4. Rapidly Destructive Osteoarthritis of the Hip
4.2.5. Neoplasms
5. Muscles and Soft Tissues
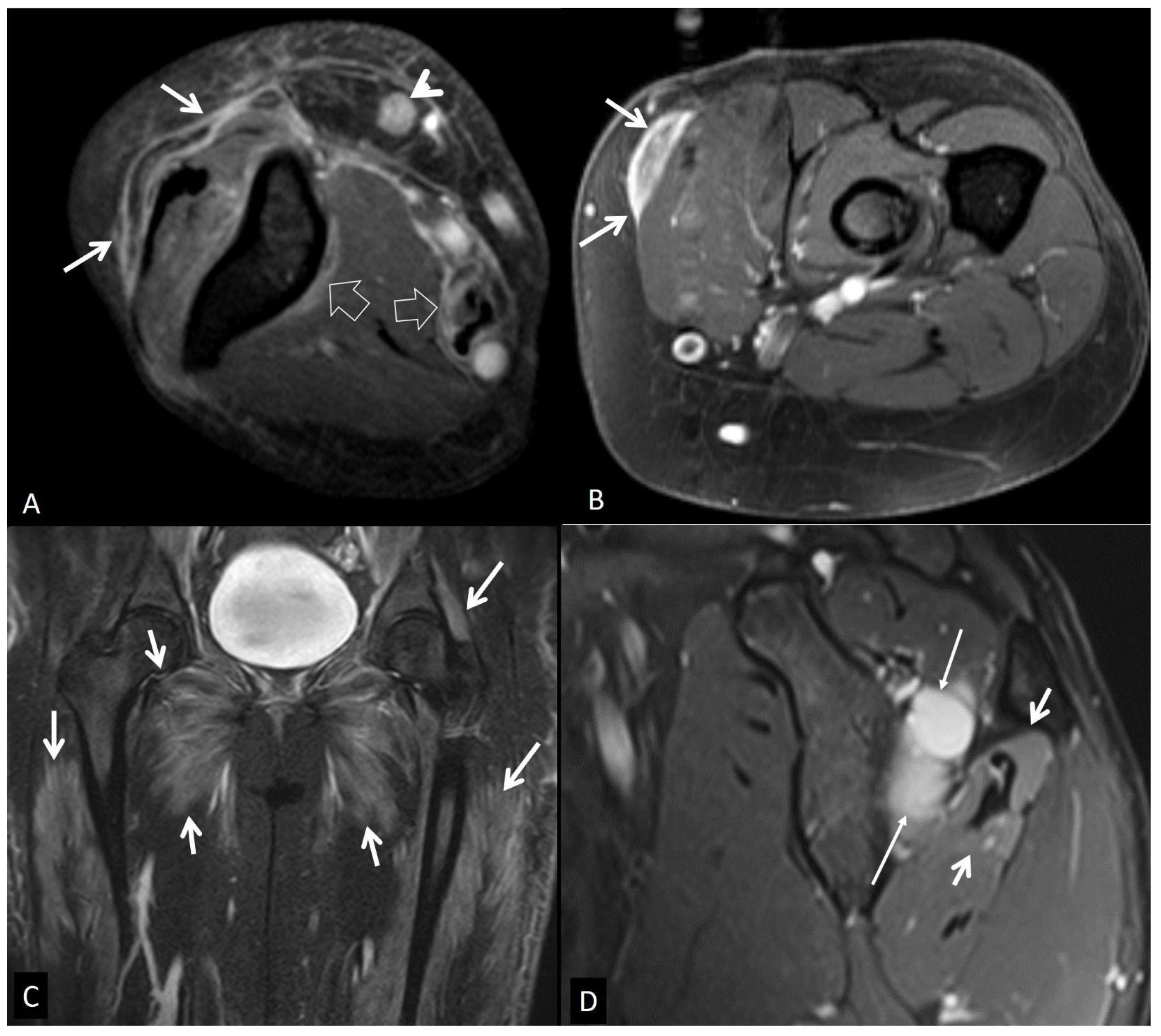
5.1. Non-Infectious Subcutaneous Edema
5.2. Non-Infectious Fasciitis
5.2.1. Eosinophilic Fasciitis
5.2.2. Paraneoplastic Fasciitis
5.2.3. Nodular Fasciitis
5.2.4. Inflammatory Myopathies
5.3. Muscle Denervation
5.4. Traumatic Soft Tissue Injury
5.4.1. Muscle Contusion
5.4.2. Muscle Strain
5.4.3. Delayed Onset Muscle Soreness
5.4.4. Rhabdomyolysis
5.4.5. Myositis Ossificans
5.5. Neoplasm and Post-Therapy Soft-Tissue Changes
5.5.1. Neoplasms
5.5.2. Post-Therapy Soft-Tissue Changes
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shih, Y.C.; Thacker, M.M. Infection Mimics: Differential Diagnoses of Musculoskeletal Infections. In Pediatric Musculoskeletal Infections: Principles & Practice; Belthur, M.V., Ranade, A.S., Herman, M.J., Fernandes, J.A., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 69–93. [Google Scholar]
- Kang, H.J.; Choi, H.Y.; Park, J.S.; Park, S.Y.; Jin, W.; Ryu, K.N. Lesions that mimic musculoskeletal infection: A pictorial essay. Korean J. Radiol. 2018, 78, 200. [Google Scholar] [CrossRef]
- Lim, W.; Barras, C.D.; Zadow, S. Radiologic Mimics of Osteomyelitis and Septic Arthritis: A Pictorial Essay. Radiol. Res. Pract. 2021, 2021, 9912257. [Google Scholar] [CrossRef] [PubMed]
- Crombé, A.; Fadli, D.; Clinca, R.; Reverchon, G.; Cevolani, L.; Girolami, M.; Hauger, O.; Matcuk, G.R.; Spinnato, P. Imaging of Spondylodiscitis: A Comprehensive Updated Review-Multimodality Imaging Findings, Differential Diagnosis, and Specific Microorganisms Detection. Microorganisms 2024, 12, 893. [Google Scholar] [CrossRef] [PubMed]
- Modic, M.T.; Steinberg, P.M.; Ross, J.S.; Masaryk, T.J.; Carter, J.R. Degenerative disk disease: Assessment of changes in vertebral body marrow with MR imaging. Radiology 1988, 166, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.G.; Watson, R.W.G.; McCormack, D.; Dowling, F.E.; Walsh, M.G.; Fitzpatrick, J.M. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J. Bone Jt. Surg. Br. 2002, 84, 196–201. [Google Scholar] [CrossRef]
- Albert, H.B.; Lambert, P.; Rollason, J.; Sorensen, J.S.; Worthington, T.; Pedersen, M.B.; Nørgaard, H.S.; Vernallis, A.; Busch, F.; Manniche, C.; et al. Does nuclear tissue infected with bacteria following disc herniations lead to Modic changes in the adjacent vertebrae? Eur. Spine J. 2013, 22, 690–696. [Google Scholar] [CrossRef]
- Teichtahl, A.J.; Urquhart, D.M.; Wang, Y.; Wluka, A.E.; O’Sullivan, R.; Jones, G.; Cicuttini, F.M. Modic changes in the lumbar spine and their association with body composition, fat distribution and intervertebral disc height—A 3.0 T-MRI study. BMC Musculoskelet. Disord. 2016, 17, 92–98. [Google Scholar] [CrossRef]
- Schwarz-Nemec, U.; Friedrich, K.M.; Stihsen, C.; Schwarz, F.K.; Trattnig, S.; Weber, M.; Grohs, J.G.; Nemec, S.F. Vertebral Bone Marrow and Endplate Assessment on MR Imaging for the Differentiation of Modic Type 1 Endplate Changes and Infectious Spondylodiscitis. J. Clin. Med. Res. 2020, 9, 826. [Google Scholar] [CrossRef]
- Oztekin, O.; Calli, C.; Kitis, O.; Adibelli, Z.H.; Eren, C.S.; Apaydin, M.; Zileli, M.; Yurtseven, T. Reliability of diffusion weighted MR imaging in differentiating degenerative and infectious end plate changes. Radiol. Oncol. 2010, 44, 97–102. [Google Scholar] [CrossRef]
- Andersson, O. Rontgenbilden vid spondylarthritis ankylopoetica. Nord. Med. Tidskr. 1937, 14, 2000–2002. [Google Scholar]
- Hunter, T. The spinal complications of ankylosing spondylitis. Semin. Arthritis Rheum. 1989, 19, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Shin, K.; Song, Y.; Lee, S.; Kim, T.-H. Andersson lesions of whole spine magnetic resonance imaging compared with plain radiography in ankylosing spondylitis. Rheumatol. Int. 2016, 36, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.N.; Rehman, A.; Hensor, E.M.A.; Marzo-Ortega, H.; Emery, P.; McGonagle, D. Evaluation of the diagnostic utility of spinal magnetic resonance imaging in axial spondylarthritis. Arthritis Rheum. 2009, 60, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Kabasakal, Y.; Garrett, S.L.; Calin, A. The epidemiology of spondylodiscitis in ankylosing spondylitis—A controlled study. Br. J. Rheumatol. 1996, 35, 660–663. [Google Scholar] [CrossRef]
- Madsen, K.B.; Jurik, A.G. MRI grading method for active and chronic spinal changes in spondyloarthritis. Clin. Radiol. 2010, 65, 6–14. [Google Scholar] [CrossRef]
- Park, Y.-S.; Kim, J.-H.; Ryu, J.-A.; Kim, T.-H. The Andersson lesion in ankylosing spondylitis: Distinguishing between the inflammatory and traumatic subtypes. J. Bone Jt. Surg. Br. 2011, 93, 961–966. [Google Scholar] [CrossRef]
- Sieper, J.; Rudwaleit, M.; Baraliakos, X.; Brandt, J.; Braun, J.; Burgos-Vargas, R.; Dougados, M.; Hermann, K.G.; Landewé, R.; Maksymowych, W. The Assessment of SpondyloArthritis International Society (ASAS) Handbook: A guide to assess spondyloarthritis. Ann. Rheum. Dis. 2009, 68, ii1–ii44. [Google Scholar] [CrossRef]
- Benhamou, C.L.; Chamot, A.M.; Kahn, M.F. Synovitis-acne-pustulosis hyperostosis-osteomyelitis syndrome (SAPHO). A new syndrome among the spondyloarthropathies? Clin. Exp. Rheumatol. 1988, 6, 109–112. [Google Scholar]
- Kuntz, D.; Naveau, B.; Bardin, T.; Drueke, T.; Treves, R.; Dryll, A. Destructive spondylarthropathy in hemodialyzed patients. A new syndrome. Arthritis Rheum. 1984, 27, 369–375. [Google Scholar] [CrossRef]
- Theodorou, D.J.; Theodorou, S.J.; Resnick, D. Imaging in dialysis spondyloarthropathy. Semin. Dial. 2002, 15, 290–296. [Google Scholar] [CrossRef]
- Ring, D.; Vaccaro, A.R.; Scuderi, G.; Pathria, M.N.; Garfin, S.R. Acute calcific retropharyngeal tendinitis. Clinical presentation and pathological characterization. J. Bone Jt. Surg. Am. 1994, 76, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Offiah, C.E.; Hall, E. Acute calcific tendinitis of the longus colli muscle: Spectrum of CT appearances and anatomical correlation. Br. J. Radiol. 2009, 82, e117–e121. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, A.K.; Ryan, L.M. Calcium Pyrophosphate Deposition Disease. N. Engl. J. Med. 2016, 374, 2575–2584. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, C.S., 3rd; Casden, A.M.; Abdelwahab, I.F. Calcium pyrophosphate deposition disease mimicking infection in the lumbar spine. Orthopedics 1999, 22, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Bouvet, J.P.; le Parc, J.M.; Michalski, B.; Benlahrache, C.; Auquier, L. Acute neck pain due to calcifications surrounding the odontoid process: The crowned dens syndrome. Arthritis Rheum. 1985, 28, 1417–1420. [Google Scholar] [CrossRef]
- Ye, C.; Shi, M.; Xie, D.; Wu, H.; Chen, Q.; Yang, L. A rare case of intervertebral disc calcification combined with ossification of the posterior longitudinal ligament in a child: A case report and literature review. BMC Musculoskelet. Disord. 2024, 25, 118. [Google Scholar] [CrossRef]
- Van Goethem, J.W.; Van de Kelft, E.; Biltjes, I.G.; van Hasselt, B.A.; van den Hauwe, L.; Parizel, P.M.; De Schepper, A.M. MRI after successful lumbar discectomy. Neuroradiology 1996, 38 (Suppl. S1), S90–S96. [Google Scholar] [CrossRef]
- Shafaie, F.F.; Bundschuh, C.; Jinkins, J.R. The posttherapeutic lumbosacral spine. In Posttherapeutic Neurodiagnostic Imaging; Lippincott-Raven: Philadelphia, PA, USA, 1997; pp. 223–243. [Google Scholar]
- Pawar, A.Y.; Biswas, S.K. Postoperative spine infections. Asian Spine J. 2016, 10, 176–183. [Google Scholar] [CrossRef]
- Kumar, Y.; Gupta, N.; Chhabra, A.; Fukuda, T.; Soni, N.; Hayashi, D. Magnetic resonance imaging of bacterial and tuberculous spondylodiscitis with associated complications and non-infectious spinal pathology mimicking infections: A pictorial review. BMC Musculoskelet. Disord. 2017, 18, 244. [Google Scholar] [CrossRef]
- Kumaran, S.P.; Thippeswamy, P.B.; Reddy, B.N.; Neelakantan, S.; Viswamitra, S. An Institutional review of tuberculosis spine mimics on MR imaging: Cases of Mistaken Identity. Neurol. India 2019, 67, 1408–1418. [Google Scholar] [CrossRef]
- Joaquim, A.F.; Appenzeller, S. Cervical spine involvement in rheumatoid arthritis—A systematic review. Autoimmun. Rev. 2014, 13, 1195–1202. [Google Scholar] [CrossRef]
- Interligator, S.; Le Bozec, A.; Cluzel, G.; Devilder, M.; Ghaouche, J.; Guenoun, D.; Fleury, A.; Petit Lemaire, F.; Carlier, R.Y.; Valente, C.; et al. Infectious sacroiliitis: MRI- and CT-based assessment of disease extent, complications, and anatomic correlation. Skeletal Radiol. 2023, 53, 2247–2262. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Hong, S.H.; Kim, J.Y.; Yoo, H.J.; Choi, J.-Y.; Yi, M.; Kang, H.S. Unilateral sacroiliitis: Differential diagnosis between infectious sacroiliitis and spondyloarthritis based on MRI findings. AJR Am. J. Roentgenol. 2015, 205, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, M.E. The “gray cortex”: An early sign of stress fracture. Skelet. Radiol. 1995, 24, 201–203. [Google Scholar] [CrossRef] [PubMed]
- Berger, F.H.; de Jonge, M.C.; Maas, M. Stress fractures in the lower extremity. The importance of increasing awareness amongst radiologists. Eur. J. Radiol. 2007, 62, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.A.; Hegedus, E.J.; Lenchik, L.; Kuhn, K.J.; Santiago, L.; Smoliga, J.M. Diagnostic Accuracy of Various Imaging Modalities for Suspected Lower Extremity Stress Fractures: A Systematic Review with Evidence-Based Recommendations for Clinical Practice. Am. J. Sports Med. 2016, 44, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Sadigh, S.; Mankad, K.; Kapse, N.; Rajeswaran, G. The imaging of osteomyelitis. Quant. Imaging Med. Surg. 2016, 6, 184–198. [Google Scholar] [CrossRef]
- Henninger, B.; Glodny, B.; Rudisch, A.; Trieb, T.; Loizides, A.; Putzer, D.; Judmaier, W.; Schocke, M.F. Ewing sarcoma versus osteomyelitis: Differential diagnosis with magnetic resonance imaging. Skelet. Radiol. 2013, 42, 1097–1104. [Google Scholar] [CrossRef]
- McCarville, M.B.; Chen, J.Y.; Coleman, J.L.; Li, Y.; Li, X.; Adderson, E.E.; Neel, M.D.; Gold, R.E.; Kaufman, R.A. Distinguishing Osteomyelitis from Ewing Sarcoma on Radiography and MRI. AJR Am. J. Roentgenol. 2015, 205, 640–650, quiz 651. [Google Scholar] [CrossRef]
- Guermazi, A.; Brice, P.; de Kerviler, E.E.; Fermé, C.; Hennequin, C.; Meignin, V.; Frija, J. Extranodal Hodgkin disease: Spectrum of disease. Radiographics 2001, 21, 161–179. [Google Scholar] [CrossRef]
- Majeed, A.; Chan, O.; Okolo, O.; Shponka, V.; Georgescu, A.; Persky, D. Hodgkin Lymphoma Mimicking Osteomyelitis. Case Rep. Oncol. 2017, 10, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Mika, J.; Schleicher, I.; Gerlach, U.; Adler, C.-P.; Uhl, M.; Knoeller, S.M. Primary bone lymphomas thought to be osteomyelitis urgently demand a rapid diagnosis in bone pathology. Anticancer Res. 2012, 32, 4905–4912. [Google Scholar] [PubMed]
- Mulligan, M.E.; McRae, G.A.; Murphey, M.D. Imaging features of primary lymphoma of bone. AJR Am. J. Roentgenol. 1999, 173, 1691–1697. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Shirkhoda, A.; Tehranzadeh, J.; Armin, A.R.; Irwin, R.; Les, K. Primary bone lymphoma: Radiographic-MR imaging correlation. Radiographics 2003, 23, 1371–1383, discussion 1384–1387. [Google Scholar] [CrossRef]
- Mulligan, M.E.; Kransdorf, M.J. Sequestra in primary lymphoma of bone: Prevalence and radiologic features. AJR Am. J. Roentgenol. 1993, 160, 1245–1248. [Google Scholar] [CrossRef]
- Phal, P.M.; Myall, R.W.T.; Assael, L.A.; Weissman, J.L. Imaging findings of bisphosphonate-associated osteonecrosis of the jaws. AJNR Am. J. Neuroradiol. 2007, 28, 1139–1145. [Google Scholar] [CrossRef]
- Ross, J.J. Septic arthritis of native joints. Infect. Dis. Clin. N. Am. 2017, 31, 203–218. [Google Scholar] [CrossRef]
- Graif, M.; Schweitzer, M.E.; Deely, D.; Matteucci, T. The septic versus nonseptic inflamed joint: MRI characteristics. Skelet. Radiol. 1999, 28, 616–620. [Google Scholar] [CrossRef]
- Pierce, J.L.; Perry, M.T.; Wessell, D.E.; Lenchik, L.; Ahlawat, S.; Baker, J.C.; Banks, J.; Caracciolo, J.T.; DeGeorge, K.C.; Demertzis, J.L.; et al. ACR Appropriateness Criteria® Suspected Osteomyelitis, Septic Arthritis, or Soft Tissue Infection (Excluding Spine and Diabetic Foot): 2022 Update. J. Am. Coll. Radiol. 2022, 19, S473–S487. [Google Scholar] [CrossRef]
- Simpfendorfer, C.S. Radiologic approach to musculoskeletal infections. Infect. Dis. Clin. N. Am. 2017, 31, 299–324. [Google Scholar] [CrossRef]
- Hansford, B.G.; Stacy, G.S. Musculoskeletal aspiration procedures. Semin. Interv. Radiol. 2012, 29, 270–285. [Google Scholar]
- Montgomery, N.I.; Rosenfeld, S. Pediatric osteoarticular infection update. J. Pediatr. Orthop. 2015, 35, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Karchevsky, M.; Schweitzer, M.E.; Morrison, W.B.; Parellada, J.A. MRI findings of septic arthritis and associated osteomyelitis in adults. AJR Am. J. Roentgenol. 2004, 182, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Kim, S.M.; Ahn, J.M.; Chung, H.W.; Shin, M.J.; Kang, H.S. Tuberculous versus pyogenic arthritis: MR imaging evaluation. Radiology 2001, 218, 848–853. [Google Scholar] [CrossRef]
- Colebatch, A.N.; Edwards, C.J.; Østergaard, M.; van der Heijde, D.; Balint, P.V.; D’Agostino, M.A.; Forslind, K.; Grassi, W.; Haavardsholm, E.A.; Haugeberg, G.; et al. EULAR recommendations for the use of imaging of the joints in the clinical management of rheumatoid arthritis. Ann. Rheum. Dis. 2013, 72, 804–814. [Google Scholar] [CrossRef]
- Weishaupt, D.; Schweitzer, M.E. MR imaging of septic arthritis and rheumatoid arthritis of the shoulder. Magn. Reson. Imaging Clin. N. Am. 2004, 12, 111–124. [Google Scholar] [CrossRef]
- McQueen, F.M.; Gao, A.; Ostergaard, M.; King, A.; Shalley, G.; Robinson, E.; Doyle, A.; Clark, B.; Dalbeth, N. High-grade MRI bone oedema is common within the surgical field in rheumatoid arthritis patients undergoing joint replacement and is associated with osteitis in subchondral bone. Ann. Rheum. Dis. 2007, 66, 1581–1587. [Google Scholar] [CrossRef]
- Boutry, N.; Morel, M.; Flipo, R.-M.; Demondion, X.; Cotten, A. Early rheumatoid arthritis: A review of MRI and sonographic findings. AJR Am. J. Roentgenol. 2007, 189, 1502–1509. [Google Scholar] [CrossRef]
- Amrami, K.K. Imaging of the seronegative spondyloarthopathies. Radiol. Clin. N. Am. 2012, 50, 841–854. [Google Scholar] [CrossRef]
- Jones, E.A.; Manaster, B.J.; May, D.A.; Disler, D.G. Neuropathic osteoarthropathy: Diagnostic dilemmas and differential diagnosis. Radiographics 2000, 20, S279–S293. [Google Scholar] [CrossRef]
- Ahmadi, M.E.; Morrison, W.B.; Carrino, J.A.; Schweitzer, M.E.; Raikin, S.M.; Ledermann, H.P. Neuropathic arthropathy of the foot with and without superimposed osteomyelitis: MR Imaging Characteristics. Radiology 2006, 238, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, A.J.; DiPreta, J.A. Classifications in brief: Eichenholtz classification of Charcot arthropathy. Clin. Orthop. Relat. Res. 2015, 473, 1168–1171. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, H.R. Crystal-induced arthritis: An overview. Am. J. Med. 1996, 100, 46S–52S. [Google Scholar] [CrossRef]
- Abhishek, A. Calcium pyrophosphate deposition disease: A review of epidemiologic findings. Curr. Opin. Rheumatol. 2016, 28, 133–139. [Google Scholar] [CrossRef]
- Rosales-Alexander, J.L.; Balsalobre Aznar, J.; Magro-Checa, C. Calcium pyrophosphate crystal deposition disease: Diagnosis and treatment. Open Access Rheumatol. 2014, 6, 39–47. [Google Scholar] [CrossRef]
- Mandl, P.; D’Agostino, M.A.; Navarro-Compán, V.; Geßl, I.; Sakellariou, G.; Abhishek, A.; Becce, F.; Dalbeth, N.; Ea, H.K.; Filippucci, E.; et al. 2023 EULAR recommendations on imaging in diagnosis and management of crystal-induced arthropathies in clinical practice. Ann. Rheum. Dis. 2024, 83, 752–759. [Google Scholar] [CrossRef]
- Frediani, B.; Filippou, G.; Falsetti, P.; Lorenzini, S.; Baldi, F.; Acciai, C.; Siagkri, C.; Marotto, D.; Galeazzi, M.; Marcolongo, R. Diagnosis of calcium pyrophosphate dihydrate crystal deposition disease: Ultrasonographic criteria proposed. Ann. Rheum. Dis. 2005, 64, 638–640. [Google Scholar] [CrossRef]
- Buckens, C.F.; Terra, M.P.; Maas, M. Computed Tomography and MR Imaging in crystalline-induced arthropathies. Radiol. Clin. N. Am. 2017, 55, 1023–1034. [Google Scholar] [CrossRef]
- Gersing, A.S.; Schwaiger, B.J.; Heilmeier, U.; Joseph, G.B.; Facchetti, L.; Kretzschmar, M.; Lynch, J.A.; McCulloch, C.E.; Nevitt, M.C.; Steinbach, L.S.; et al. Evaluation of Chondrocalcinosis and Associated Knee Joint Degeneration Using MR Imaging: Data from the Osteoarthritis Initiative. Eur. Radiol. 2017, 27, 2497–2506. [Google Scholar] [CrossRef]
- Lequesne, M. Rapid destructive coxarthritis. Rhumatologie 1970, 22, 51–63. [Google Scholar]
- Flemming, D.J.; Gustas-French, C.N. Rapidly progressive osteoarthritis: A Review of the clinical and radiologic presentation. Curr. Rheumatol. Rep. 2017, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Boutry, N.; Paul, C.; Leroy, X.; Fredoux, D.; Migaud, H.; Cotton, A. Rapidly destructive osteoarthritis of the hip: MR imaging findings. AJR Am. J. Roentgenol. 2002, 179, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Zazgyva, A.; Gurzu, S.; Gergely, I.; Jung, I.; Roman, C.O.; Pop, T.S. Clinico-radiological diagnosis and grading of rapidly progressive osteoarthritis of the hip. Medicine 2017, 96, e6395. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.W.; Hong, S.H.; Choi, J.-Y.; Koh, Y.H.; Lee, J.W.; Choi, J.-A.; Kang, H.S. Radiologic diagnosis of osteoid osteoma: From simple to challenging findings. Radiographics 2010, 30, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Bedoya, M.A.; Iwasaka-Neder, J.; Tsai, A.; Bixby, S.D. Intra-articular osteoid osteomas: Imaging manifestations and mimics. Radiographics 2024, 44, e230208. [Google Scholar] [CrossRef]
- Bohndorf, K. Infection of the appendicular skeleton. Eur. Radiol. 2004, 14 (Suppl. S3), E53–E63. [Google Scholar] [CrossRef]
- Davies, A.M.; Grimer, R. The penumbra sign in subacute osteomyelitis. Eur. Radiol. 2005, 15, 1268–1270. [Google Scholar] [CrossRef]
- Hayeri, M.R.; Pouya, Z.; Shehata, M.L.; Teytelboym, O.M.; Huang, B.K. Soft-Tissue infections and their imaging mimics: From cellulitis to necrotizing fasciitis. RadioGraphics 2016, 36, 1888–1910. [Google Scholar] [CrossRef]
- Yu, J.S.; Habib, P. MR imaging of urgent inflammatory and infectious conditions affecting the soft tissues of the musculoskeletal system. Emerg. Radiol. 2009, 16, 267–276. [Google Scholar] [CrossRef]
- Baumann, F.; Brühlmann, P.; Andreisek, G.; Michel, B.A.; Marincek, B.; Weishaupt, D. MRI for diagnosis and monitoring of patients with eosinophilic fasciitis. AJR Am. J. Roentgenol. 2005, 184, 169–174. [Google Scholar] [CrossRef]
- Gaeta, M.; Mileto, A.; Musumeci, O. MRI findings of neutrophilic fasciitis in a patient with acute febrile neutrophilic dermatosis (Sweet’s syndrome). Skeletal Radiol. 2011, 40, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Leung, L.Y.; Shu, S.J.; Chan, A.C.; Chan, M.K.; Chan, C.H. Nodular fasciitis: MRI appearance and literature review. Skeletal Radiol. 2002, 31, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; De Schepper, A.; Vanhoenacker, F.; De Raeve, H.; Gielen, J.; Aparisi, F.; Rausin, L.; Somville, J. Nodular fasciitis: Correlation of MRI findings and histopathology. Skeletal Radiol. 2002, 31, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Tanboon, J.; Uruha, A.; Stenzel, W.; Nishino, I. Where are we moving in the classification of idiopathic inflammatory myopathies? Curr. Opin. Neurol. 2020, 33, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Bottai, M.; Tjärnlund, A.; Santoni, G.; Werth, V.P.; Pilkington, C.; De Visser, M.; Alfredsson, L.; Amato, A.A.; Barohn, R.J.; Liang, M.H.; et al. EULAR/ACR Classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups: A methodology report. RMD Open 2017, 3, e000507. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, M.J.; Powell, T.; Brennan, D.; Lynch, T.; McCarthy, C.J.; Eustace, S.J. Whole-body MR imaging in the diagnosis of polymyositis. AJR Am. J. Roentgenol. 2002, 179, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Kamath, S.; Venkatanarasimha, N.; Walsh, M.A. MRI appearance of muscle denervation. Skelet. Radiol. 2008, 37, 397–404. [Google Scholar] [CrossRef]
- Petersilge, C.A.; Pathria, M.N.; Gentili, A.; Recht, M.P.; Resnick, D. Denervation hypertrophy of muscle 2008, MR features. J. Comput. Assist. Tomogr. 1995, 19, 596–600. [Google Scholar] [CrossRef]
- Guermazi, A.; Roemer, F.; Robinson, P.; Tol, J.; Regatte, R.; Crema, M. Imaging of muscle injuries in sports medicine: Sports imaging series. Radiology 2017, 282, 646–663. [Google Scholar] [CrossRef]
- Lu, C.H.; Tsang, Y.M.; Yu, C.W.; Wu, M.Z.; Hsu, C.Y.; Shih, T.T. Rhabdomyolysis: Magnetic resonance imaging and computed tomography findings. J. Comput. Assist. Tomogr. 2007, 31, 368–374. [Google Scholar] [CrossRef]
- Beltran, J.; Simon, D.C.; Katz, W.; Weis, L.D. Increased MR signal intensity in skeletal muscle adjacent to malignant tumors: Pathologic correlation and clinical relevance. Radiology 1987, 162 Pt 1, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Garner, H.W.; Kransdorf, M.J.; Bancroft, L.W.; Peterson, J.J.; Berquist, T.H.; Murphey, M.D. Benign and malignant soft-tissue tumors: Posttreatment MR imaging. RadioGraphics 2009, 29, 119–134. [Google Scholar] [CrossRef] [PubMed]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klontzas, M.E.; Vassalou, E.E.; Spanakis, K.; Alpantaki, K.; Karantanas, A.H. Musculoskeletal Infection: The Great Mimickers on Imaging. J. Clin. Med. 2024, 13, 5424. https://doi.org/10.3390/jcm13185424
Klontzas ME, Vassalou EE, Spanakis K, Alpantaki K, Karantanas AH. Musculoskeletal Infection: The Great Mimickers on Imaging. Journal of Clinical Medicine. 2024; 13(18):5424. https://doi.org/10.3390/jcm13185424
Chicago/Turabian StyleKlontzas, Michail E., Evangelia E. Vassalou, Konstantinos Spanakis, Kalliopi Alpantaki, and Apostolos H. Karantanas. 2024. "Musculoskeletal Infection: The Great Mimickers on Imaging" Journal of Clinical Medicine 13, no. 18: 5424. https://doi.org/10.3390/jcm13185424
APA StyleKlontzas, M. E., Vassalou, E. E., Spanakis, K., Alpantaki, K., & Karantanas, A. H. (2024). Musculoskeletal Infection: The Great Mimickers on Imaging. Journal of Clinical Medicine, 13(18), 5424. https://doi.org/10.3390/jcm13185424






