Challenges in Contemporary Spine Surgery: A Comprehensive Review of Surgical, Technological, and Patient-Specific Issues
Abstract
:1. Introduction
2. Surgical Challenges
2.1. Complexity of the Spinal Anatomy
2.2. Variability in Patient Anatomy
2.3. Technical Complexity of Modern Approaches
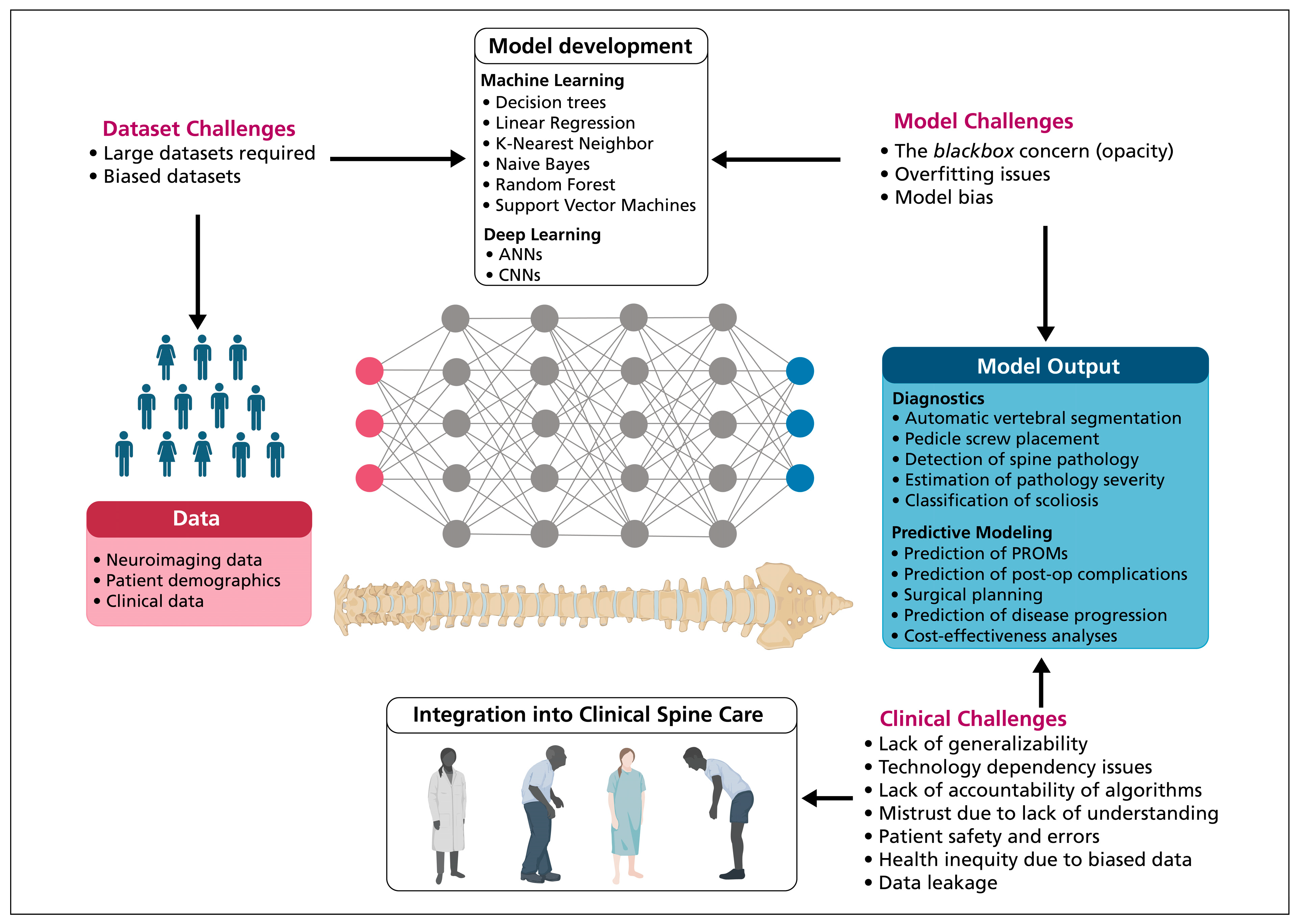
3. Technological Challenges
3.1. Artificial Intelligence Systems
3.2. Visualization Systems
4. Patient-Specific Challenges
4.1. Complications of Spine Surgery
4.2. Patient Outcome Metrics
4.3. Patient Selection
5. Future Directions
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Badhiwala, J.H.; Ahuja, C.S.; Fehlings, M.G. Time is spine: A review of translational advances in spinal cord injury. J. Neurosurg. Spine 2018, 30, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Yamout, T.; Orosz, L.D.; Good, C.R.; Jazini, E.; Allen, B.; Gum, J.L. Technological Advances in Spine Surgery Navigation, Robotics, and Augmented Reality. Orthop. Clin. N. Am. 2023, 54, 237–246. [Google Scholar] [CrossRef]
- Offiah, C.E.; Day, E. The craniocervical junction: Embryology, anatomy, biomechanics and imaging in blunt trauma. Insights Imaging 2017, 8, 29–47. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Lv, S.; Ye, F.; Lin, Q. Imaging anatomy and variation of vertebral artery and bone structure at craniocervical junction. Eur. Spine J. 2009, 18, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Sivaganesan, A.; Kim, H.J. A Review of Indications, Surgical Technique, and Outcomes for the Cervical Pedicle Subtraction Osteotomy. J. Am. Acad. Orthop. Surg. 2022, 30, e295–e300. [Google Scholar] [CrossRef]
- Balestrino, A.; Gondar, R.; Jannelli, G.; Zona, G.; Tessitore, E. Surgical challenges in posterior cervicothoracic junction instrumentation. Neurosurg. Rev. 2021, 44, 3447–3458. [Google Scholar] [CrossRef] [PubMed]
- Oppenheimer, J.H.; DeCastro, I.; McDonnell, D.E. Minimally invasive spine technology and minimally invasive spine surgery: A historical review. Neurosurg. Focus 2009, 27, E9. [Google Scholar] [CrossRef]
- Mobbs, R.J.; Sivabalan, P.; Li, J. Technique, challenges and indications for percutaneous pedicle screw fixation. J. Clin. Neurosci. 2011, 18, 741–749. [Google Scholar] [CrossRef]
- Patgaonkar, P.; Datar, G.; Agrawal, U.; Palanikumar, C.; Agrawal, A.; Goyal, V.; Patel, V. Suprailiac versus transiliac approach in transforaminal endoscopic discectomy at L5-S1: A new surgical classification of L5—Iliac crest relationship and guidelines for approach. J. Spine Surg. 2020, 6, S145–S154. [Google Scholar] [CrossRef]
- Epstein, N.E. Evaluation of varied surgical approaches used in the management of 170 far-lateral lumbar disc herniations: Indications and results. J. Neurosurg. 1995, 83, 648–656. [Google Scholar] [CrossRef]
- Epstein, N.E. Different surgical approaches to far lateral lumbar disc herniations. J. Spinal Disord. 1995, 8, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Klimo, P.; Rao, G.; Brockmeyer, D. Congenital Anomalies of the Cervical Spine. Neurosurg. Clin. N. Am. 2007, 18, 463–478. [Google Scholar] [CrossRef] [PubMed]
- Joaquim, A.F.; Barcelos, A.C.E.S.; Daniel, J.W. Role of Atlas Assimilation in the Context of Craniocervical Junction Anomalies. World Neurosurg. 2021, 151, 201–208. [Google Scholar] [CrossRef]
- Smoker, W.R.K.; Khanna, G. Imaging the craniocervical junction. Child’s Nerv. Syst. 2008, 24, 1123–1145. [Google Scholar] [CrossRef]
- Chun, D.H.; Yoon, D.H.; Kim, K.N.; Yi, S.; Shin, D.A.; Ha, Y. Biomechanical Comparison of Four Different Atlantoaxial Posterior Fixation Constructs in Adults. Spine 2018, 43, E891–E897. [Google Scholar] [CrossRef] [PubMed]
- Wigh, R.E. The Thoracolumbar and Lumbosacral Transitional Junctions. Spine 1980, 5, 215–222. [Google Scholar] [CrossRef]
- Konin, G.; Walz, D. Lumbosacral Transitional Vertebrae: Classification, Imaging Findings, and Clinical Relevance. Am. J. Neuroradiol. 2010, 31, 1778–1786. [Google Scholar] [CrossRef]
- Park, S.K.; Park, J.G.; Kim, B.S.; Huh, J.D.; Kang, H. Thoracolumbar junction: Morphologic characteristics, various variants and significance. Br. J. Radiol. 2016, 89, 20150784. [Google Scholar] [CrossRef] [PubMed]
- Davran, R.; Bayarogullari, H.; Atci, N.; Kayali, A.; Ozturk, F.; Burakgazi, G. Congenital abnormalities of the ribs: Evaluation with multidetector computed tomography. J. Pak. Med. Assoc. 2017, 67, 178–186. [Google Scholar]
- Spadliński, Ł.; Cecot, T.; Majos, A.; Stefańczyk, L.; Pietruszewska, W.; Wysiadecki, G.; Topol, M.; Polguj, M. The Epidemiological, Morphological, and Clinical Aspects of the Cervical Ribs in Humans. BioMed Res. Int. 2016, 2016, 8034613. [Google Scholar] [CrossRef]
- Aly, I.; Chapman, J.R.; Oskouian, R.J.; Loukas, M.; Tubbs, R.S. Lumbar ribs: A comprehensive review. Child’s Nerv. Syst. 2016, 32, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Halalmeh, D.R.; Sandio, A.; Tubbs, R.S.; Moisi, M.D. Anatomical Variations That Can Lead to Spine Surgery at the Wrong Level: Part I, Cervical Spine. Cureus 2020, 12, e8667. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Guinto, F.C.; Madewell, J.E.; Swischuk, L.E.; David, R. The vertebral body: Radiographic configurations in various congenital and acquired disorders. RadioGraphics 1988, 8, 455–485. [Google Scholar] [CrossRef] [PubMed]
- Mellado, J.M.; Larrosa, R.; Martín, J.; Yanguas, N.; Solanas, S.; Cozcolluela, M.R. MDCT of Variations and Anomalies of the Neural Arch and Its Processes: Part 1—Pedicles, Pars Interarticularis, Laminae, and Spinous Process. Am. J. Roentgenol. 2011, 197, W104–W113. [Google Scholar] [CrossRef]
- Lewandrowski, K.-U.; Telfeian, A.E.; Hellinger, S.; León, J.F.R.; de Carvalho, P.S.T.; Ramos, M.R.; Kim, H.S.; Hanson, D.W.; Salari, N.; Yeung, A. Difficulties, Challenges, and the Learning Curve of Avoiding Complications in Lumbar Endoscopic Spine Surgery. Int. J. Spine Surg. 2021, 15 (Suppl. S3), S21–S37. [Google Scholar] [CrossRef]
- Scrofani, R.; De Simone, M.; Migliorini, F.; Amoroso, E.; Maffulli, N.; Narciso, N.; Iaconetta, G. Spontaneous Resolution of Symptomatic Synovial Cysts of the Lumbar Spine: A Comprehensive Review with Two Illustrative Cases. Medicina 2024, 60, 1115. [Google Scholar] [CrossRef]
- De Simone, M.; Zoia, C.; Choucha, A.; Kong, D.-S.; De Maria, L. The Transorbital Approach: A Comprehensive Review of Targets, Surgical Techniques, and Multiportal Variants. J. Clin. Med. 2024, 13, 2712. [Google Scholar] [CrossRef] [PubMed]
- Ransom, N.A.; Gollogly, S.; Lewandrowski, K.-U.; Yeung, A. Navigating the learning curve of spinal endoscopy as an established traditionally trained spine surgeon. J. Spine Surg. 2020, 6, S197–S207. [Google Scholar] [CrossRef]
- Mayo, B.C.; Massel, D.H.; Bohl, D.D.; Long, W.W.; Modi, K.D.; Singh, K. Anterior Cervical Discectomy and Fusion: The Surgical Learning Curve. Spine 2016, 41, 1580–1585. [Google Scholar] [CrossRef]
- Judy, B.F.; Menta, A.; Pak, H.L.; Azad, T.D.; Witham, T.F. Augmented Reality and Virtual Reality in Spine Surgery: A Comprehensive Review. Neurosurg. Clin. N. Am. 2024, 35, 207–216. [Google Scholar] [CrossRef]
- Park, S.-M.; Shen, F.; Kim, H.-J.; Kim, H.; Chang, B.-S.; Lee, C.-K.; Yeom, J.S. How Many Screws Are Necessary to Be Considered an Experienced Surgeon for Freehand Placement of Thoracolumbar Pedicle Screws?: Analysis Using the Cumulative Summation Test for Learning Curve. World Neurosurg. 2018, 118, e550–e556. [Google Scholar] [CrossRef] [PubMed]
- Sclafani, J.A.; Kim, C.W. Complications associated with the initial learning curve of minimally invasive spine surgery: A systematic review. Clin. Orthop. Relat. Res. 2014, 472, 1711–1717. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.; Monteiro, P.; Silva, P.A.; Vaz, R. Learning curve and complications of minimally invasive transforaminal lumbar interbody fusion. Neurosurg. Focus 2013, 35, E7. [Google Scholar] [CrossRef]
- Doherty, P.; Welch, A.; Tharpe, J.; Moore, C.; Ferry, C. Transforaminal Lumbar Interbody Fusion with Rigid Interspinous Process Fixation: A Learning Curve Analysis of a Surgeon Team’s First 74 Cases. Cureus 2017, 9, e1290. [Google Scholar] [CrossRef]
- Staartjes, V.E.; de Wispelaere, M.P.; Miedema, J.; Schröder, M.L. Recurrent Lumbar Disc Herniation After Tubular Microdiscectomy: Analysis of Learning Curve Progression. World Neurosurg. 2017, 107, 28–34. [Google Scholar] [CrossRef]
- Lee, K.H.; Yeo, W.; Soeharno, H.; Yue, W.M. Learning curve of a complex surgical technique: Minimally invasive transforaminal lumbar interbody fusion (MIS TLIF). J. Spinal Disord. Tech. 2014, 27, E234–E240. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Merrill, R.K.; Overley, S.C.; Leven, D.M.; Meaike, J.J.; Vaishnav, A.; Gang, C.; Qureshi, S.A. Radiation Exposure in Minimally Invasive Transforaminal Lumbar Interbody Fusion: The Effect of the Learning Curve. Int. J. Spine Surg. 2019, 13, 39–45. [Google Scholar] [CrossRef]
- Yerramneni, V.K.; Kanala, R.R.; Yerragunta, T.; Kolpakawar, S.; Kumar, K.S.V.; Suman, A. Minimally invasive transforaminal lumbar interbody fusion: Technical tips, learning curve, short-term clinical outcome, and brief review. J. Craniovertebr. Junction Spine 2021, 12, 387–392. [Google Scholar] [CrossRef]
- Martín-Láez, R.; Martínez-Agüeros, J.Á.; Suárez-Fernández, D.; Montiaga-Núñez, F.; Vázquez-Barquero, A. Complications of endoscopic microdiscectomy using the EASYGO! system: Is there any difference with conventional discectomy during the learning-curve period? Acta Neurochir. 2012, 154, 1023–1032. [Google Scholar] [CrossRef]
- Jain, S.; Merchant, Z.; Kire, N.; Patel, J.; Patel, A.; Kundnani, V. Learning Curve of Microendoscopic Discectomy in Single-Level Prolapsed Intervertebral Disc in 120 Patients. Glob. Spine J. 2020, 10, 571–577. [Google Scholar] [CrossRef]
- Kim, J.-E.; Yoo, H.-S.; Choi, D.-J.; Hwang, J.-H.; Park, E.J.; Chung, S. Learning Curve and Clinical Outcome of Biportal Endoscopic-Assisted Lumbar Interbody Fusion. BioMed Res. Int. 2020, 2020, 8815432. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Ye, J.; Li, T.; Yu, Y.; Fan, X. Evaluation of the learning curve and complications in unilateral biportal endoscopic transforaminal lumbar interbody fusion: Cumulative sum analysis and risk-adjusted cumulative sum analysis. J. Orthop. Surg. Res. 2024, 19, 194. [Google Scholar] [CrossRef]
- Sun, B.; Shi, C.; Xu, Z.; Wu, H.; Zhang, Y.; Chen, Y.; Wu, X.-D.; Yuan, W. Learning Curve for Percutaneous Endoscopic Lumbar Diskectomy in Bi-needle Technique Using Cumulative Summation Test for Learning Curve. World Neurosurg. 2019, 129, e586–e593. [Google Scholar] [CrossRef]
- Son, S.; Ahn, Y.; Lee, S.G.; Kim, W.K. Learning curve of percutaneous endoscopic interlaminar lumbar discectomy versus open lumbar microdiscectomy at the L5–S1 level. PLoS ONE 2020, 15, e0236296. [Google Scholar] [CrossRef]
- Pennington, Z.; Judy, B.F.; Zakaria, H.M.; Lakomkin, N.; Mikula, A.L.; Elder, B.D.; Theodore, N. Learning curves in robot-assisted spine surgery: A systematic review and proposal of application to residency curricula. Neurosurg. Focus 2022, 52, E3. [Google Scholar] [CrossRef] [PubMed]
- Akazawa, T.; Torii, Y.; Ueno, J.; Umehara, T.; Iinuma, M.; Yoshida, A.; Tomochika, K.; Ohtori, S.; Niki, H. Learning curves for robotic-assisted spine surgery: An analysis of the time taken for screw insertion, robot setting, registration, and fluoroscopy. Eur. J. Orthop. Surg. Traumatol. 2024, 34, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Tong, Y.; Harris, L.; Owusu-Sarpong, S.; Goldstein, J. Proficiency Development and Learning Curve in Robot-Assisted Spine Surgery Using the ExcelsiusGPS® System: Experience from a Single Institution. Glob. Spine J. 2024; online first. [Google Scholar] [CrossRef]
- Siddiqui, M.I.; Wallace, D.J.; Salazar, L.M.; Vardiman, A.B. Robot-Assisted Pedicle Screw Placement: Learning Curve Experience. World Neurosurg. 2019, 130, e417–e422. [Google Scholar] [CrossRef]
- Kam, J.K.T.; Gan, C.; Dimou, S.; Awad, M.; Kavar, B.; Nair, G.; Morokoff, A. Learning Curve for Robot-Assisted Percutaneous Pedicle Screw Placement in Thoracolumbar Surgery. Asian Spine J. 2019, 13, 920–927. [Google Scholar] [CrossRef]
- Jiang, K.; Hersh, A.M.; Bhimreddy, M.; Weber-Levine, C.; Davidar, A.D.; Menta, A.K.; Routkevitch, D.; Alomari, S.; Judy, B.F.; Lubelski, D.; et al. Learning Curves for Robot-Assisted Pedicle Screw Placement: Analysis of Operative Time for 234 Cases. Oper. Neurosurg. 2023, 25, 482–488. [Google Scholar] [CrossRef]
- Haider, G.; Shah, V.; Lopez, I.; Wagner, K.E.; Stienen, M.N.; Veeravagu, A. Experience with the utilization of new-generation shared-control robotic system for spinal instrumentation. J. Neurosurg. Sci. 2024; online first. [Google Scholar] [CrossRef]
- Lonner, B.S.; Scharf, C.; Antonacci, D.; Goldstein, Y.; Panagopoulos, G. The learning curve associated with thoracoscopic spinal instrumentation. Spine 2005, 30, 2835–2840. [Google Scholar] [CrossRef]
- Ghogawala, Z.; Dunbar, M.; Essa, I. Artificial Intelligence for the Treatment of Lumbar Spondylolisthesis. Neurosurg. Clin. N. Am. 2019, 30, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, B.S.; Mazmudar, A.; Driscoll, C.; Svet, M.; Goergen, J.; Kelsten, M.; Shlobin, N.A.; Kesavabhotla, K.; Smith, Z.A.; Dahdaleh, N.S. Using artificial intelligence (AI) to predict postoperative surgical site infection: A retrospective cohort of 4046 posterior spinal fusions. Clin. Neurol. Neurosurg. 2020, 192, 105718. [Google Scholar] [CrossRef] [PubMed]
- Khan, O.; Badhiwala, J.H.; Grasso, G.; Fehlings, M.G. Use of Machine Learning and Artificial Intelligence to Drive Personalized Medicine Approaches for Spine Care. World Neurosurg. 2020, 140, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Arvind, V.; Kim, J.S.; Oermann, E.K.; Kaji, D.; Cho, S.K. Predicting Surgical Complications in Adult Patients Undergoing Anterior Cervical Discectomy and Fusion Using Machine Learning. Neurospine 2018, 15, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S. Sparse SVM for Sufficient Data Reduction. IEEE Trans. Pattern Anal. Mach. Intell. 2022, 44, 5560–5571. [Google Scholar] [CrossRef] [PubMed]
- Karandikar, P.; Massaad, E.; Hadzipasic, M.; Kiapour, A.; Joshi, R.S.; Shankar, G.M.; Shin, J.H. Machine Learning Applications of Surgical Imaging for the Diagnosis and Treatment of Spine Disorders: Current State of the Art. Neurosurgery 2022, 90, 372–382. [Google Scholar] [CrossRef]
- Watson, D.S.; Krutzinna, J.; Bruce, I.N.; Griffiths, C.E.; McInnes, I.B.; Barnes, M.R.; Floridi, L. Clinical applications of machine learning algorithms: Beyond the black box. BMJ 2019, 364, l886. [Google Scholar] [CrossRef]
- Staartjes, V.E.; Stienen, M.N. Data Mining in Spine Surgery: Leveraging Electronic Health Records for Machine Learning and Clinical Research. Neurospine 2019, 16, 654–656. [Google Scholar] [CrossRef]
- Challen, R.; Denny, J.; Pitt, M.; Gompels, L.; Edwards, T.; Tsaneva-Atanasova, K. Artificial intelligence, bias and clinical safety. BMJ Qual. Saf. 2019, 28, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Siller, S.; Zoellner, C.; Fuetsch, M.; Trabold, R.; Tonn, J.-C.; Zausinger, S. A high-definition 3D exoscope as an alternative to the operating microscope in spinal microsurgery. J. Neurosurg. Spine 2020, 33, 705–714. [Google Scholar] [CrossRef]
- Zulbaran-Rojas, A.; Rouzi, M.D.; Zahiri, M.; Ouattas, A.; Walter, C.M.; Nguyen, H.; Bidadi, S.; Najafi, B.; Lemole, G.M. Objective assessment of postural ergonomics in neurosurgery: Integrating wearable technology in the operating room. J. Neurosurg. Spine 2024, 41, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.-J.; Zhao, T.-B.; Dai, J.; Wang, Q.-L.; Zhang, Q.-Z.; Cao, J.-Y.; Liu, X.-F. Clinical comparison of three-dimensional exoscope vs. operative microscope in transforaminal lumbar interbody fusion: A retrospective case-control study. Front. Surg. 2022, 9, 926329. [Google Scholar] [CrossRef] [PubMed]
- Glassman, S.D.; Hamill, C.L.; Bridwell, K.H.; Schwab, F.J.; Dimar, J.R.; Lowe, T.G. The Impact of Perioperative Complications on Clinical Outcome in Adult Deformity Surgery. Spine 2007, 32, 2764–2770. [Google Scholar] [CrossRef]
- Badiee, R.K.; Mayer, R.; Pennicooke, B.; Chou, D.; Mummaneni, P.V.; Tan, L.A. Complications following posterior cervical decompression and fusion: A review of incidence, risk factors, and prevention strategies. J. Spine Surg. 2020, 6, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Barbanti-Brodano, G.; Griffoni, C.; Halme, J.; Tedesco, G.; Terzi, S.; Bandiera, S.; Ghermandi, R.; Evangelisti, G.; Girolami, M.; Pipola, V.; et al. Spinal surgery complications: An unsolved problem—Is the World Health Organization Safety Surgical Checklist an useful tool to reduce them? Eur. Spine J. 2020, 29, 927–936. [Google Scholar] [CrossRef]
- Yearley, A.G.; Chalif, J.I.; Chalif, E.J.; Zaidi, H.A. The Relationship Among Surgeon Experience, Complications, and Radiographic Outcomes in Spine Deformity Surgery: The Experience of a Junior Surgeon. World Neurosurg. 2022, 168, e399–e407. [Google Scholar] [CrossRef]
- Park, H.-J.; Kim, S.-K.; Lee, S.-C.; Kim, W.; Han, S.; Kang, S.-S. Dural Tears in Percutaneous Biportal Endoscopic Spine Surgery: Anatomical Location and Management. World Neurosurg. 2020, 136, e578–e585. [Google Scholar] [CrossRef]
- O’neill, K.R.; Neuman, B.J.; Peters, C.; Riew, K.D. Risk factors for dural tears in the cervical spine. Spine 2014, 39, E1015–E1020. [Google Scholar] [CrossRef]
- Smorgick, Y.; Baker, K.C.; Herkowitz, H.; Montgomery, D.; Badve, S.A.; Bachison, C.; Ericksen, S.; Fischgrund, J.S. Predisposing factors for dural tear in patients undergoing lumbar spine surgery. J. Neurosurg. Spine 2015, 22, 483–486. [Google Scholar] [CrossRef]
- Khan, M.H.; Rihn, J.; Steele, G.; Davis, R.; Donaldson, W.F.; Kang, J.D.; Lee, J.Y. Postoperative management protocol for incidental dural tears during degenerative lumbar spine surgery: A review of 3183 consecutive degenerative lumbar cases. Spine 2006, 31, 2609–2613. [Google Scholar] [CrossRef]
- McDonald, C.L.; Alsoof, D.; Glueck, J.; Osorio, C.; Stone, B.; McCluskey, L.; Diebo, B.G.; Daniels, A.H.; Basques, B.A. Adjacent Segment Disease After Spinal Fusion. JBJS Rev. 2023, 11, e23. [Google Scholar] [CrossRef] [PubMed]
- Hilibrand, A.S.; Carlson, G.D.; Palumbo, M.A.; Jones, P.K.; Bohlman, H.H. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J. Bone Jt. Surg. 1999, 81, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Sears, W.R.; Sergides, I.G.; Kazemi, N.; Smith, M.; White, G.J.; Osburg, B. Incidence and prevalence of surgery at segments adjacent to a previous posterior lumbar arthrodesis. Spine J. 2011, 11, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Radcliff, K.; Curry, P.; Hilibrand, A.; Kepler, C.; Lurie, J.; Zhao, W.; Albert, T.J.; Weinstein, J. Risk for Adjacent Segment and Same Segment Reoperation After Surgery for Lumbar Stenosis. Spine 2013, 38, 531–539. [Google Scholar] [CrossRef]
- Pressman, E.; Liaw, D.; Monsour, M.; Wang, C.P.; Gassie, K.; Alikhani, P. Factors associated with hardware failure after lateral thoracolumbar fusions–A ten year case series. Clin. Neurol. Neurosurg. 2023, 224, 107564. [Google Scholar] [CrossRef]
- Amankulor, N.M.; Xu, R.; Iorgulescu, J.B.; Chapman, T.; Reiner, A.S.; Riedel, E.; Lis, E.; Yamada, Y.; Bilsky, M.; Laufer, I. The incidence and patterns of hardware failure after separation surgery in patients with spinal metastatic tumors. Spine J. 2014, 14, 1850–1859. [Google Scholar] [CrossRef]
- Smith, J.S.; Klineberg, E.; Lafage, V.; Shaffrey, C.I.; Schwab, F.; Lafage, R.; Hostin, R.; Mundis, G.M., Jr.; Errico, T.J.; Kim, H.J.; et al. Prospective multicenter assessment of perioperative and minimum 2-year postoperative complication rates associated with adult spinal deformity surgery. J. Neurosurg. Spine 2016, 25, 1–14. [Google Scholar] [CrossRef]
- Bernhardt, M.; Bridwell, K.H. Segmental analysis of the sagittal plane alignment of the normal thoracic and lumbar spines and thoracolumbar junction. Spine 1989, 14, 717–721. [Google Scholar] [CrossRef]
- Lowe, T.G.; Lenke, L.; Betz, R.; Newton, P.; Clements, D.; Haher, T.; Crawford, A.; Letko, L.; Wilson, L.A. Distal junctional kyphosis of adolescent idiopathic thoracic curves following anterior or posterior instrumented fusion: Incidence, risk factors, and prevention. Spine 2006, 31, 299–302. [Google Scholar] [CrossRef]
- Lee, J.; Park, Y.-S. Proximal Junctional Kyphosis: Diagnosis, Pathogenesis, and Treatment. Asian Spine J. 2016, 10, 593–600. [Google Scholar] [CrossRef]
- Passias, P.G.; Vasquez-Montes, D.; Poorman, G.W.; Protopsaltis, T.; Horn, S.R.; Bortz, C.A.; Segreto, F.; Diebo, B.; Ames, C.; Smith, J.; et al. Predictive model for distal junctional kyphosis after cervical deformity surgery. Spine J. 2018, 18, 2187–2194. [Google Scholar] [CrossRef] [PubMed]
- Herzog, R.J.; Marcotte, P.J. Assessment of spinal fusion: Critical evaluation of imaging techniques. Spine 1996, 21, 1114–1118. [Google Scholar] [CrossRef] [PubMed]
- Leven, D.; Cho, S.K. Pseudarthrosis of the Cervical Spine: Risk Factors, Diagnosis and Management. Asian Spine J. 2016, 10, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Hofler, R.C.; Swong, K.; Martin, B.; Wemhoff, M.; Jones, G.A. Risk of Pseudoarthrosis After Spinal Fusion: Analysis from the Healthcare Cost and Utilization Project. World Neurosurg. 2018, 120, e194–e202. [Google Scholar] [CrossRef]
- Wang, T.; Ding, W. Risk factors for adjacent segment degeneration after posterior lumbar fusion surgery in treatment for degenerative lumbar disorders: A meta-analysis. J. Orthop. Surg. Res. 2020, 15, 582. [Google Scholar] [CrossRef]
- Patel, R.V.; Yearley, A.G.; Isaac, H.; Chalif, E.J.; Chalif, J.I.; Zaidi, H.A. Advances and Evolving Challenges in Spinal Deformity Surgery. J. Clin. Med. 2023, 12, 6386. [Google Scholar] [CrossRef]
- Xia, Y.; Xu, R.; Kosztowski, T.A.; Ramhmdani, S.; Ahmed, A.K.; Lo, S.-F.L.; Bydon, A. Reoperation for Proximal Adjacent Segment Pathology in Posterior Cervical Fusion Constructs that Fuse to C2 vs C3. Neurosurgery 2019, 85, E520–E526. [Google Scholar] [CrossRef]
- DePasse, J.M.; Durand, W.; Eltorai, A.E.; Palumbo, M.A.; Daniels, A.H. Timing of complications following posterior cervical fusion. J. Orthop. 2018, 15, 522–526. [Google Scholar] [CrossRef]
- Katsevman, G.A.; Daffner, S.D.; Brandmeir, N.J.; Emery, S.E.; France, J.C.; Sedney, C.L. Complexities of spine surgery in obese patient populations: A narrative review. Spine J. 2020, 20, 501–511. [Google Scholar] [CrossRef]
- Lee, J.J.; Odeh, K.I.; Holcombe, S.A.; Patel, R.D.; Wang, S.C.; Goulet, J.A.; Graziano, G.P. Fat Thickness as a Risk Factor for Infection in Lumbar Spine Surgery. Orthopedics 2016, 39, e1124–e1128. [Google Scholar] [CrossRef]
- Aleem, I.S.; Tan, L.A.; Nassr, A.; Riew, K.D. Surgical Site Infection Prevention Following Spine Surgery. Glob. Spine J. 2020, 10 (Suppl. S1), 92S–98S. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-T.; Lee, D.-H.; Cho, D.-C.; Sung, J.-K.; Kim, Y.-B. Preoperative Risk Factors for Recurrent Lumbar Disk Herniation in L5–S1. J. Spinal Disord. Tech. 2015, 28, E571–E577. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Han, Z.; Liu, J.; Yu, L.; Yu, X. Risk Factors for Recurrent Lumbar Disc Herniation: A Systematic Review and Meta-Analysis. Medicine 2016, 95, e2378. [Google Scholar] [CrossRef] [PubMed]
- Hlubek, R.J.; Mundis, G.M. Treatment for Recurrent Lumbar Disc Herniation. Curr. Rev. Musculoskelet. Med. 2017, 10, 517–520. [Google Scholar] [CrossRef]
- Papadakis, M.; Aggeliki, L.; Papadopoulos, E.C.; Girardi, F.P. Common surgical complications in degenerative spinal surgery. World J. Orthop. 2013, 4, 62–66. [Google Scholar] [CrossRef]
- Ghobrial, G.M.; Williams, K.A.; Arnold, P.; Fehlings, M.; Harrop, J.S. Iatrogenic neurologic deficit after lumbar spine surgery: A review. Clin. Neurol. Neurosurg. 2015, 139, 76–80. [Google Scholar] [CrossRef]
- Cheah, J.; Zhang, A.L.; Tay, B. Intraoperative Use of Neuromonitoring in Multilevel Thoracolumbar Spine Instrumentation and the Effects on Postoperative Neurological Injuries. Clin. Spine Surg. 2017, 30, 321–327. [Google Scholar] [CrossRef]
- Liu, H.; Wu, J.; Tang, Y.; Li, H.; Wang, W.; Li, C.; Zhou, Y. Percutaneous placement of lumbar pedicle screws via intraoperative CT image–based augmented reality–guided technology. J. Neurosurg. Spine 2019, 32, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Lykissas, M.G.; Aichmair, A.; Hughes, A.P.; Sama, A.A.; Lebl, D.R.; Taher, F.; Du, J.Y.; Cammisa, F.P.; Girardi, F.P. Nerve injury after lateral lumbar interbody fusion: A review of 919 treated levels with identification of risk factors. Spine J. 2014, 14, 749–758. [Google Scholar] [CrossRef]
- Mobbs, R.J. From the Subjective to the Objective era of outcomes analysis: How the tools we use to measure outcomes must change to be reflective of the pathologies we treat in spinal surgery. J. Spine Surg. 2021, 7, 456–457. [Google Scholar] [CrossRef]
- Akcay, S.; Koc, A.M.; Eskut, N.; Koskderelioglu, A. Horner’s Syndrome and Vertebral Artery Occlusion Concomitant with Brachial Plexus Injury in a Patient with Anterior Approach Cervical Disc Herniation Surgery. Cureus 2021, 13, e17810. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.; Sharma, V.; Kumar, M.; Thakur, L. A systematic review of risk factors and adverse outcomes associated with anterior cervical discectomy and fusion surgery over the past decade. J. Craniovertebr. Junction Spine 2024, 15, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.T.; Park, D.K.; Lee, M.J.; Kim, S.W.; An, H.S. Anatomical variations of the vertebral artery segment in the lower cervical spine: Analysis by three-dimensional computed tomography angiography. Spine 2008, 33, 2422–2426. [Google Scholar] [CrossRef]
- Yang, J.J.; Kim, H.-J.; Lee, J.B.; Park, S. Preoperative Radiographic Simulation for Partial Uncinate Process Resection during Anterior Cervical Discectomy and Fusion to Achieve Adequate Foraminal Decompression and Prevention of Vertebral Artery Injury. Asian Spine J. 2023, 17, 1024–1034. [Google Scholar] [CrossRef]
- Novegno, F.; Granaroli, P.; Ciccoritti, L.; Lunardi, P.; Fraioli, M.F. Chylous fistula: Management of a rare complication following right anterior cervical spine approach. Eur. Spine J. 2019, 28 (Suppl. S2), 61–67. [Google Scholar] [CrossRef]
- Grevitt, M.; Khazim, R.; Webb, J.; Mulholland, R.; Shepperd, J. The short form-36 health survey questionnaire in spine surgery. J. Bone Jt. Surg. Br. 1997, 79, 48–52. [Google Scholar] [CrossRef]
- The EuroQol Group. EuroQol—A new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [Google Scholar] [CrossRef]
- BenDebba, M.; Heller, J.; Ducker, T.B.; Eisinger, J.M. Cervical spine outcomes questionnaire: Its development and psychometric properties. Spine 2002, 27, 2116; discussion 2124. [Google Scholar] [CrossRef] [PubMed]
- Yonenobu, K.; Abumi, K.; Nagata, K.; Taketomi, E.; Ueyama, K. Interobserver and intraobserver reliability of the japanese orthopaedic association scoring system for evaluation of cervical compression myelopathy. Spine 2001, 26, 1890–1894; discussion 1895. [Google Scholar] [CrossRef]
- Vernon, H. The Neck Disability Index: State-of-the-art, 1991–2008. J. Manip. Physiol. Ther. 2008, 31, 491–502. [Google Scholar] [CrossRef]
- Fairbank, J.C.; Couper, J.; Davies, J.B.; O’Brien, J.P. The Oswestry low back pain disability questionnaire. Physiotherapy 1980, 66, 271–273. [Google Scholar] [PubMed]
- Roland, M.; Fairbank, J. The Roland–Morris Disability Questionnaire and the Oswestry Disability Questionnaire. Spine 2000, 25, 3115–3124. [Google Scholar] [CrossRef]
- Haher, T.R.; Gorup, J.M.; Shin, T.M.; Homel, P.; Merola, A.A.; Grogan, D.P.; Pugh, L.; Lowe, T.G.; Murray, M. Results of the Scoliosis Research Society instrument for evaluation of surgical outcome in adolescent idiopathic scoliosis. A multicenter study of 244 patients. Spine 1999, 24, 1435–1440. [Google Scholar] [CrossRef]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Zanoli, G.; Strömqvist, B.; Jönsson, B. Visual analog scales for interpretation of back and leg pain intensity in patients operated for degenerative lumbar spine disorders. Spine 2001, 26, 2375–2380. [Google Scholar] [CrossRef]
- Hawker, G.A.; Mian, S.; Kendzerska, T.; French, M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthr. Care Res. 2011, 63 (Suppl. S11), S240–S252. [Google Scholar] [CrossRef]
- Mueller, B.; Carreon, L.Y.; Glassman, S.D. Comparison of the EuroQOL-5D with the Oswestry Disability Index, back and leg pain scores in patients with degenerative lumbar spine pathology. Spine 2013, 38, 757–761. [Google Scholar] [CrossRef]
- Lee, C.-P.; Fu, T.-S.; Liu, C.-Y.; Hung, C.-I. Psychometric evaluation of the Oswestry Disability Index in patients with chronic low back pain: Factor and Mokken analyses. Health Qual. Life Outcomes 2017, 15, 192. [Google Scholar] [CrossRef]
- Koç, M.; Bayar, B.; Bayar, K. A Comparison of Back Pain Functional Scale with Roland Morris Disability Questionnaire, Oswestry Disability Index, and Short Form 36-Health Survey. Spine 2018, 43, 877–882. [Google Scholar] [CrossRef]
- Singh, V.; Mitra, S. Autonomic variability, depression and the disability paradox in spinal cord injury. Spinal Cord Ser. Cases 2022, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.A.; Schwartz, C.E. Patient-reported outcomes in spine surgery: Past, current, and future directions. J. Neurosurg. Spine 2019, 31, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Bean, J.F.; Ölveczky, D.D.; Kiely, D.K.; LaRose, S.I.; Jette, A.M. Performance-based versus patient-reported physical function: What are the underlying predictors? Phys. Ther. 2011, 91, 1804–1811. [Google Scholar] [CrossRef]
- Taylor, A.M.; Phillips, K.; Patel, K.V.; Turk, D.C.; Dworkin, R.H.; Beaton, D.; Clauw, D.J.; Gignac, M.A.; Markman, J.D.; Williams, D.A.; et al. Assessment of physical function and participation in chronic pain clinical trials: IMMPACT/OMERACT recommendations. Pain 2016, 157, 1836–1850. [Google Scholar] [CrossRef] [PubMed]
- Chiarotto, A.; Ostelo, R.W.; Boers, M.; Terwee, C.B. A systematic review highlights the need to investigate the content validity of patient-reported outcome measures for physical functioning in patients with low back pain. J. Clin. Epidemiol. 2018, 95, 73–93. [Google Scholar] [CrossRef]
- Brodke, D.S.; Goz, V.; Lawrence, B.D.; Spiker, W.R.; Neese, A.; Hung, M. Oswestry Disability Index: A psychometric analysis with 1,610 patients. Spine J. 2017, 17, 321–327. [Google Scholar] [CrossRef]
- Wibault, J.; Öberg, B.; Dedering, Å.; Löfgren, H.; Zsigmond, P.; Persson, L.; Peolsson, A. Individual factors associated with neck disability in patients with cervical radiculopathy scheduled for surgery: A study on physical impairments, psychosocial factors, and life style habits. Eur. Spine J. 2014, 23, 599–605. [Google Scholar] [CrossRef]
- Beighley, A.; Zhang, A.; Huang, B.; Carr, C.; Mathkour, M.; Werner, C.; Scullen, T.; Kilgore, M.D.; Maulucci, C.M.; Dallapiazza, R.F.; et al. Patient-reported outcome measures in spine surgery: A systematic review. J. Craniovertebr. Junction Spine 2022, 13, 378–389. [Google Scholar] [CrossRef]
- Stadhouder, A.; Buckens, C.F.M.; Holtslag, H.R.; Öner, F.C. Are existing outcome instruments suitable for assessment of spinal trauma patients? J. Neurosurg. Spine 2010, 13, 638–647. [Google Scholar] [CrossRef]
- Anderson, D.B.; Mathieson, S.; Eyles, J.; Maher, C.G.; Van Gelder, J.M.; Tomkins-Lane, C.C.; Ammendolia, C.; Bella, V.; Ferreira, M.L. Measurement properties of walking outcome measures for neurogenic claudication: A systematic review and meta analysis. Spine J. 2019, 19, 1378–1396. [Google Scholar] [CrossRef]
- Stienen, M.N.; Rezaii, P.G.; Ho, A.L.; Veeravagu, A.; Zygourakis, C.C.; Tomkins-Lane, C.; Park, J.; Ratliff, J.K.; Desai, A.M. Objective activity tracking in spine surgery: A prospective feasibility study with a low-cost consumer grade wearable accelerometer. Sci. Rep. 2020, 10, 4939. [Google Scholar] [CrossRef] [PubMed]
- Chakravorty, A.; Mobbs, R.J.; Anderson, D.B.; Rooke, K.; Phan, K.; Yoong, N.; Maharaj, M.; Choy, W.J. The role of wearable devices and objective gait analysis for the assessment and monitoring of patients with lumbar spinal stenosis: Systematic review. BMC Musculoskelet. Disord. 2019, 20, 288. [Google Scholar] [CrossRef] [PubMed]
- Mobbs, R.J.; Fonseka, R.D.; Natarajan, P. Wearable sensor technology in spine care. J. Spine Surg. 2022, 8, 84–86. [Google Scholar] [CrossRef] [PubMed]
- Siccoli, A.; de Wispelaere, M.P.; Schröder, M.L.; Staartjes, V.E. Machine learning–based preoperative predictive analytics for lumbar spinal stenosis. Neurosurg. Focus 2019, 46, E5. [Google Scholar] [CrossRef] [PubMed]
- Janssen, M.; Garcia, R.; Miller, L.; Reed, W.; Zigler, J.; Ferko, N.; Hollmann, S. Challenges and Solutions for Lumbar Total Disc Replacement Implantation. Spine 2017, 42 (Suppl. S24), S108–S111. [Google Scholar] [CrossRef]
- Tezuka, F.; Sakai, T.; Abe, M.; Yamashita, K.; Takata, Y.; Higashino, K.; Chikawa, T.; Nagamachi, A.; Sairyo, K. Anatomical considerations of the iliac crest on percutaneous endoscopic discectomy using a transforaminal approach. Spine J. 2017, 17, 1875–1880. [Google Scholar] [CrossRef]
- Dowling, Á.; Lewandrowski, K.-U.; da Silva, F.H.P.; Parra, J.A.A.; Portillo, D.M.; Giménez, Y.C.P. Patient selection protocols for endoscopic transforaminal, interlaminar, and translaminar decompression of lumbar spinal stenosis. J. Spine Surg. 2020, 6 (Suppl. S1), S120–S132. [Google Scholar] [CrossRef]
- Lewandrowski, K.-U. Retrospective analysis of accuracy and positive predictive value of preoperative lumbar MRI grading after successful outcome following outpatient endoscopic decompression for lumbar foraminal and lateral recess stenosis. Clin. Neurol. Neurosurg. 2019, 179, 74–80. [Google Scholar] [CrossRef]
- Mummaneni, P.V.; Shaffrey, C.I.; Lenke, L.G.; Park, P.; Wang, M.Y.; La Marca, F.; Smith, J.S.; Mundis, G.M.; Okonkwo, D.O.; Moal, B.; et al. The minimally invasive spinal deformity surgery algorithm: A reproducible rational framework for decision making in minimally invasive spinal deformity surgery. Neurosurg. Focus 2014, 36, E6. [Google Scholar] [CrossRef]
- Mummaneni, P.V.; Park, P.; Shaffrey, C.I.; Wang, M.Y.; Uribe, J.S.; Fessler, R.G.; Chou, D.; Kanter, A.S.; Okonkwo, D.O.; Mundis, G.M.; et al. The MISDEF2 algorithm: An updated algorithm for patient selection in minimally invasive deformity surgery. J. Neurosurg. Spine 2019, 32, 221–228. [Google Scholar] [CrossRef]
- Patgaonkar, P.; Goyal, V.; Patel, P.; Dhole, K.; Ravi, A.; Patel, V.; Borole, P. An algorithm for selection of full endoscopic approach for symptomatic nerve root decompression. N. Am. Spine Soc. J. 2023, 15, 100244. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.N.; Lurie, J.D.; Tosteson, T.D.; Hanscom, B.; Tosteson, A.N.; Blood, E.A.; Birkmeyer, N.J.; Hilibrand, A.S.; Herkowitz, H.; Cammisa, F.P.; et al. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N. Engl. J. Med. 2007, 356, 2257–2270. [Google Scholar] [CrossRef] [PubMed]
- Kristof, R.A.; Aliashkevich, A.F.; Schuster, M.; Meyer, B.; Urbach, H.; Schramm, J. Degenerative lumbar spondylolisthesis—Induced radicular compression: Nonfusion-related decompression in selected patients without hypermobility on flexion—Extension radiographs. J. Neurosurg. 2002, 97 (Suppl. S3), 281–286. [Google Scholar] [CrossRef] [PubMed]
- Austevoll, I.M.; Hermansen, E.; Fagerland, M.W.; Storheim, K.; Brox, J.I.; Solberg, T.; Rekeland, F.; Franssen, E.; Weber, C.; Brisby, H.; et al. Decompression with or without Fusion in Degenerative Lumbar Spondylolisthesis. N. Engl. J. Med. 2021, 385, 526–538. [Google Scholar] [CrossRef]
- Gadjradj, P.S.; Basilious, M.; Goldberg, J.L.; Sommer, F.; Navarro-Ramirez, R.; Mykolajtchuk, C.; Ng, A.Z.; Medary, B.; Hussain, I.; Härtl, R. Decompression alone versus decompression with fusion in patients with lumbar spinal stenosis with degenerative spondylolisthesis: A systematic review and meta-analysis. Eur. Spine J. 2023, 32, 1054–1067. [Google Scholar] [CrossRef]
- Sastry, R.A.; Chen, J.-S.; Shao, B.; Weil, R.J.; Chang, K.-E.; Maynard, K.; Syed, S.H.; Sullivan, P.L.Z.; Camara, J.Q.; Niu, T.; et al. Patterns in Decompression and Fusion Procedures for Patients with Lumbar Stenosis After Major Clinical Trial Results, 2016 to 2019. JAMA Netw. Open 2023, 6, e2326357. [Google Scholar] [CrossRef]
- Tozawa, K.; Matsubayashi, Y.; Kato, S.; Doi, T.; Taniguchi, Y.; Kumanomido, Y.; Higashikawa, A.; Yosihida, Y.; Kawamura, N.; Sasaki, K.; et al. Surgical outcomes between posterior decompression alone and posterior decompression with fusion surgery among patients with Meyerding grade 2 degenerative spondylolisthesis: A multicenter cohort study. BMC Musculoskelet. Disord. 2022, 23, 902. [Google Scholar] [CrossRef]
- Huang, M.; Buchholz, A.; Goyal, A.; Bisson, E.; Ghogawala, Z.; Potts, E.; Knightly, J.; Coric, D.; Asher, A.; Foley, K.; et al. Impact of surgeon and hospital factors on surgical decision-making for grade 1 degenerative lumbar spondylolisthesis: A Quality Outcomes Database analysis. J. Neurosurg. Spine 2021, 34, 768–778. [Google Scholar] [CrossRef]
- Rosenberg, N.J. Degenerative spondylolisthesis. Predisposing factors. J. Bone Jt. Surg. 1975, 57, 467–474. [Google Scholar] [CrossRef]
- Dimitriou, D.; Winkler, E.; Weber, S.; Haupt, S.; Betz, M.; Farshad, M. A Simple Preoperative Score Predicting Failure Following Decompression Surgery for Degenerative Lumbar Spinal Stenosis. Spine 2023, 48, 610–616. [Google Scholar] [CrossRef]
- Leone, A.; Guglielmi, G.; Cassar-Pullicino, V.N.; Bonomo, L. Lumbar intervertebral instability: A review. Radiology 2007, 245, 62–77. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, C.; Curran, J.; Benzel, E.C.; Potter, R.; Magge, S.N.; Harrington, J.F.; Coumans, J.-V.; Ghogawala, Z. Radiographic predictors of delayed instability following decompression without fusion for degenerative Grade I lumbar spondylolisthesis. J. Neurosurg. Spine 2013, 18, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Yagi, M.; Machida, M.; Yasuda, A.; Konomi, T.; Miyake, A.; Fujiyoshi, K.; Kaneko, S.; Takemitsu, M.; Yato, Y.; et al. Reoperation rate and risk factors of elective spinal surgery for degenerative spondylolisthesis: Minimum 5-year follow-up. Spine J. 2015, 15, 1536–1544. [Google Scholar] [CrossRef]
- Eismont, F.J.; Norton, R.P.; Hirsch, B.P. Surgical management of lumbar degenerative spondylolisthesis. J. Am. Acad. Orthop. Surg. 2014, 22, 203–213. [Google Scholar] [CrossRef]
- Försth, P.; Ólafsson, G.; Carlsson, T.; Frost, A.; Borgström, F.; Fritzell, P.; Öhagen, P.; Michaëlsson, K.; Sandén, B. A Randomized, Controlled Trial of Fusion Surgery for Lumbar Spinal Stenosis. N. Engl. J. Med. 2016, 374, 1413–1423. [Google Scholar] [CrossRef]
- Ghogawala, Z.; Dziura, J.; Butler, W.E.; Dai, F.; Terrin, N.; Magge, S.N.; Coumans, J.-V.C.; Harrington, J.F.; Amin-Hanjani, S.; Schwartz, J.S.; et al. Laminectomy plus Fusion versus Laminectomy Alone for Lumbar Spondylolisthesis. N. Engl. J. Med. 2016, 374, 1424–1434. [Google Scholar] [CrossRef] [PubMed]
- Inose, H.; Kato, T.; Yuasa, M.; Yamada, T.; Maehara, H.; Hirai, T.; Yoshii, T.; Kawabata, S.; Okawa, A. Comparison of Decompression, Decompression Plus Fusion, and Decompression Plus Stabilization for Degenerative Spondylolisthesis: A Prospective, Randomized Study. Clin. Spine Surg. 2018, 31, E347–E352. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.-P.; Chen, H.-L.; Cheng, H.-B. Prevalence of adjacent segment degeneration after spine surgery: A systematic review and meta-analysis. Spine 2013, 38, 597–608. [Google Scholar] [CrossRef]
- Machado, G.C.; Ferreira, P.H.; Harris, I.A.; Pinheiro, M.B.; Koes, B.W.; van Tulder, M.; Rzewuska, M.; Maher, C.G.; Ferreira, M.L. Effectiveness of surgery for lumbar spinal stenosis: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0122800. [Google Scholar] [CrossRef]
- Mourad, R.; Kolisnyk, S.; Baiun, Y.; Falk, A.; Yuriy, T.; Valerii, F.; Kopeev, A.; Suldina, O.; Pospelov, A.; Kim, J.; et al. Performance of hybrid artificial intelligence in determining candidacy for lumbar stenosis surgery. Eur. Spine J. 2022, 31, 2149–2155. [Google Scholar] [CrossRef]
- Bratton, R.L. Assessment and management of acute low back pain. Am. Fam. Physician 1999, 60, 2299–2308. [Google Scholar] [PubMed]
- Kalidindi, K.K.V.; Sharma, J.K.; Jagadeesh, N.H.; Sath, S.; Chhabra, H.S. Robotic spine surgery: A review of the present status. J. Med. Eng. Technol. 2020, 44, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Canseco, J.A.; Karamian, B.A.; Lambrechts, M.J.; Issa, T.Z.; Conaway, W.; Minetos, P.D.; Bowles, D.; Alexander, T.; Sherman, M.; Schroeder, G.D.; et al. Risk stratification of patients undergoing outpatient lumbar decompression surgery. Spine J. 2023, 23, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Passias, P.G.; Ahmad, W.; Oh, C.; Imbo, B.; Naessig, S.; Pierce, K.; Lafage, V.; Lafage, R.; Hamilton, D.K.; Protopsaltis, T.S.; et al. Development of Risk Stratification Predictive Models for Cervical Deformity Surgery. Neurosurgery 2022, 91, 928–935. [Google Scholar] [CrossRef]
- Bcharah, G.; Gupta, N.; Panico, N.; Winspear, S.; Bagley, A.; Turnow, M.; D’Amico, R.; Ukachukwu, A.-E.K. Innovations in Spine Surgery: A Narrative Review of Current Integrative Technologies. World Neurosurg. 2024, 184, 127–136. [Google Scholar] [CrossRef]

| Anatomical Region | Complexities | Variabilities | Surgical Challenges | Recommendations |
|---|---|---|---|---|
| Craniocervical junction (CCJ) | Houses spinal cord, cranial nerves IX–XII, and vertebral artery. | Atlantooccipital assimilation, basilar invagination, high-riding vertebral arteries. | Limited landmarks and visualization; close proximity to critical structures. | Thorough preoperative imaging; mastery of local anatomy. |
| Cervical spine | Instrumentation near the vertebral artery. | Anatomical variants and previous surgeries. | Low margin of error due to close apposition to vertebral artery. | Tailored surgical approaches; posterior subtraction osteotomies often performed at C7. |
| Cervicothoracic junction (CTJ) | Transitional area with thin and convergent pedicles. | Variations in arthrodesis extent requirements. | Specific techniques needed to prevent failure and adjacent segment diseases. | Careful consideration in instrumentation technique. |
| Thoracic spine | Smaller pedicles compared to the lumbar spine. | Rib variants may lead to wrong entry points. | Increased risk of spinal cord injury with instrumentation. | Caution with percutaneous screws, attention to anatomical variations. |
| Lumbosacral junction (L5-S1) | Challenges in surgical access and visualization. | Large sacral ala, high-riding ilium, lateral-lying facet joints. | Complications with nerve root trauma from blind removal of disc fragments. | Larger surgical corridors; minimize blind technique use. |
| Type of Complication | Incidence Rate | Relevance |
|---|---|---|
| Complications related to Instrumentation | ||
| Dural tears | 1–17%, higher in revision surgeries | Instrumentation complications increase with age, previous surgeries, and poor bone quality. |
| ASD | 2.9% (cervical), 2.5% (lumbar) | |
| Hardware failure | 2.8–32% | |
| Junctional kyphosis | 5% to 46% (PJK) and 7.1% to 24% (DKJ) | |
| Pseudoarthrosis | 5 to 35% (lumbar) | |
| Non-Instrumentation Complications | ||
| Surgical site infections | Up to 10% | Proper patient selection and advanced imaging help mitigate the risk of these complications. |
| Recurrent disc herniations | 5–18% | |
| Neurological deficits | Up to 6% | |
| Outcome Measure | Description | Number of Items | Score | Indications | Limitations | Recommendations |
|---|---|---|---|---|---|---|
| Generic PROMs | ||||||
| EQ-5D | Measures health in five dimensions (mobility, self-care, usual activities, pain/discomfort, anxiety/depression) across three levels. A total of 3125 health states are defined. | 25 | 0 (death) to 1.0 (perfect health). | Generic measure of health. | Ceiling effects in general population. | Revise levels of severity to increase reliability and reduce ceiling effects. |
| SF-36 | Evaluates health through eight domains, yielding two summary scores: Physical Component Summary (PCS) and Mental Component Summary (MCS). | 36 | No single score is produced. | Generic measure of health. | Subjective criteria and variable patient appraisal affect reliability. | Use alongside spine-specific instruments like ODI or NDI to gain detailed insights into spine-related health impacts. |
| Cervical Spine PROMs | ||||||
| CSOQ | Composite measures include healthcare utilization, symptoms, distress, disability, and pain severity in shoulder/arm and neck. | 35 | 0–100. | Cervical spine surgery. | May not address non-cervical spine issues. | Supplement with additional measures that assess general health or other specific spine areas to provide a comprehensive assessment. |
| mJOA | Assesses cervical myelopathy in four components. Score ranges from 0–18. | – | 0–18; ≥15: mild, 12–14: moderate, <12: severe. | Cervical spondylotic myelopathy. | Requires physician administration and lacks validation. | Combine with objective clinical assessments; validate the scale through extensive clinical trials. |
| NDI | Ten sections covering pain intensity, personal care, lifting, reading, headaches, concentration, work, driving, sleeping, and recreation. | 10 | ≤7 corresponds to a good outcome. | Neck disability. | Significant floor and ceiling effects; influenced by psychosocial factors. | Screen for depressive disorders; consider multidimensional scoring. |
| Lumbar Spine PROMs | ||||||
| ODI | Evaluates interference of back pain with daily activities, sleeping, personal care, social life, sex life, and traveling. | 10 | 0–100; 0–20: minimal disability; 21–44: moderate disability; 41–60, severe disability; 61–80, crippling back pain; 81–100, either bed bound or exaggerating symptoms. | Chronic back pain. | Significant floor/ceiling effects, affected by non-pain factors like depression. | Use alongside other tools like RMDQ to cover different disability ranges and incorporate psychological assessment. |
| RMDQ | Measures activity limitation from lower back pain. | 24 | 0 (no disability) to 24 (maximum disability). | Low back pain. | Large ceiling effect; does not cover psychological or social issues. | Revise to include psychosocial factors and reduce redundancy. |
| Mental Health PROMs | ||||||
| BDI | Assesses depression over emotional and somatic symptoms. | 21 | 0–63; 0–13, no depression or minimal symptoms; 14–19: mild depression; 20–28: moderate depression; 29–63: severe depression. | Depression, particularly in patients with lower back pain. | Primarily focused on depression, may not address other emotional factors. | Ensure comprehensive use in relevant clinical assessments. |
| HADS | Measures mood and emotional disorders using anxiety and depression subscales. | 14 | 0–24; >8 in both subscales indicates clinical anxiety or depression. | Used in various health conditions including spine issues. | Not validated for low back pain. | Use together with functional outcome measures (like ODI or NDI); validate for specific conditions like low back pain. |
| Scoliosis PROMs | ||||||
| SRS-22 | Assesses spinal deformity in 4 domains (function/activity, pain, self-perceived image, and mental health) with 2 additional questions about treatment satisfaction. | 22 | 22–110. Higher scores indicate better quality of life. | Spinal deformity. | Influenced by demographic factors. | Adjust scoring to minimize demographic biases. |
| Pain PROMs | ||||||
| NRS | Rates pain intensity on an 11-point scale. | – | 0 (no pain) to 10 (maximum pain). | Back and leg pain. | Does not reflect pain complexity or variability. | Enhance with tools that capture dynamic aspects of pain. |
| VAS | Simple 100 mm line from no pain to severe pain. | – | 0 (no pain) to 10 (worst imaginable pain). | Back and leg pain. | Subjective and may not quantify pain impact on function. | Incorporate questions regarding pain interference with function to capture a more complete picture of pain’s effects on life quality. |
| Factors Affecting Patient Selection | Examples | Relevance |
|---|---|---|
| Anatomical complexity | Transitional L5/S1 anatomy, pelvic anatomy differences. | Affects choice of endoscopic approaches. |
| Comorbidities | Obesity, diabetes, multilevel disease. | May preclude patients from certain minimally invasive surgeries. |
| Predictive algorithms | MISDEF, FAPDIS, image-based stratification. | Assist in selecting appropriate patients for MISS. |
| Challenges in degenerative spondylolisthesis | Deciding between decompression alone vs. decompression with fusion. | Patient-related factors (age, pain type) affect outcomes. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mensah, E.O.; Chalif, J.I.; Baker, J.G.; Chalif, E.; Biundo, J.; Groff, M.W. Challenges in Contemporary Spine Surgery: A Comprehensive Review of Surgical, Technological, and Patient-Specific Issues. J. Clin. Med. 2024, 13, 5460. https://doi.org/10.3390/jcm13185460
Mensah EO, Chalif JI, Baker JG, Chalif E, Biundo J, Groff MW. Challenges in Contemporary Spine Surgery: A Comprehensive Review of Surgical, Technological, and Patient-Specific Issues. Journal of Clinical Medicine. 2024; 13(18):5460. https://doi.org/10.3390/jcm13185460
Chicago/Turabian StyleMensah, Emmanuel O., Joshua I. Chalif, Jessica G. Baker, Eric Chalif, Jason Biundo, and Michael W. Groff. 2024. "Challenges in Contemporary Spine Surgery: A Comprehensive Review of Surgical, Technological, and Patient-Specific Issues" Journal of Clinical Medicine 13, no. 18: 5460. https://doi.org/10.3390/jcm13185460






