Management of Otogenic Meningitis: A Proposal for Practical Guidelines from a Multicenter Experience with a Systematic Review
Abstract
1. Introduction
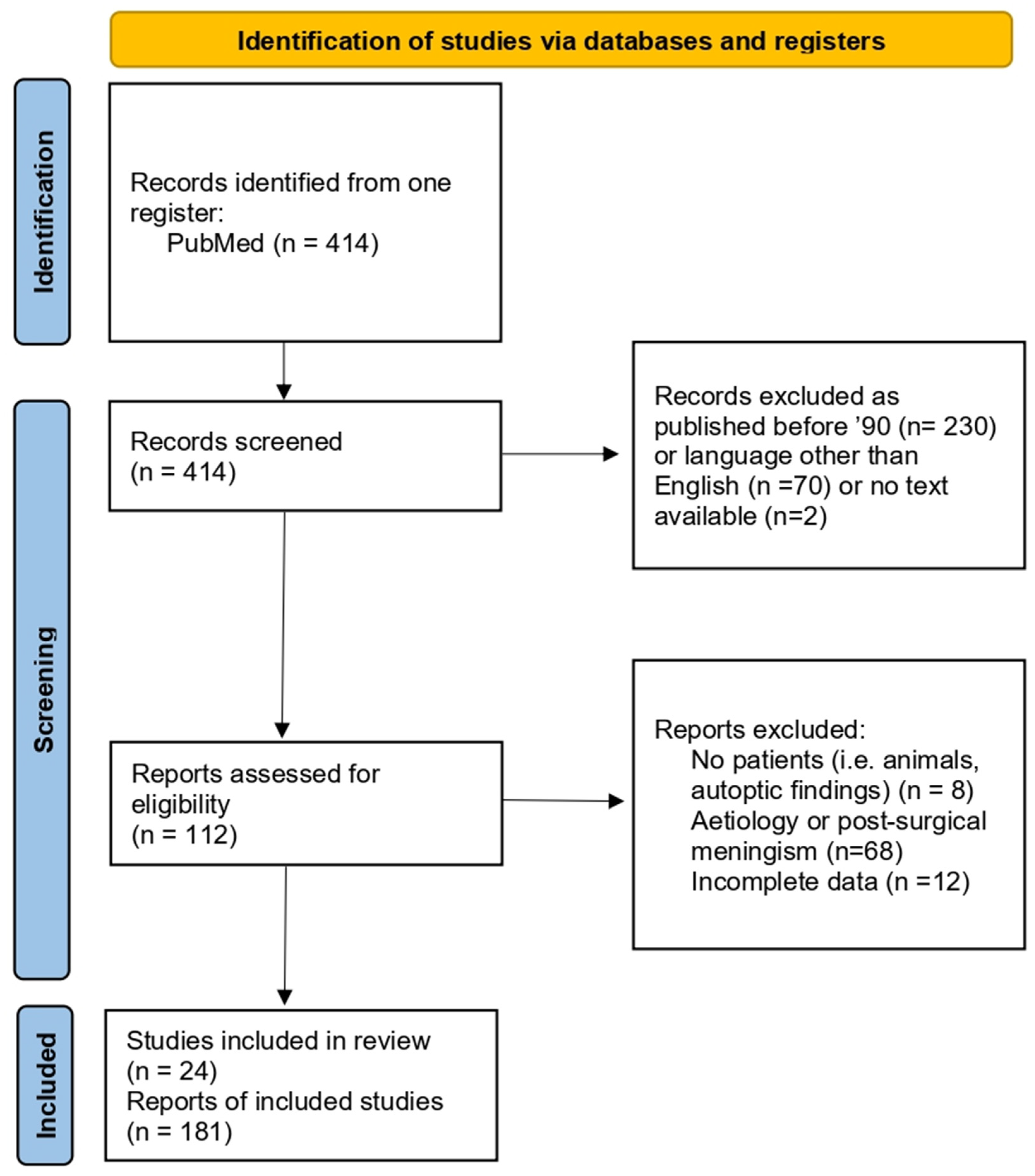
2. Materials and Methods
2.1. Our Surgical Experience in Otogenic Meningitis
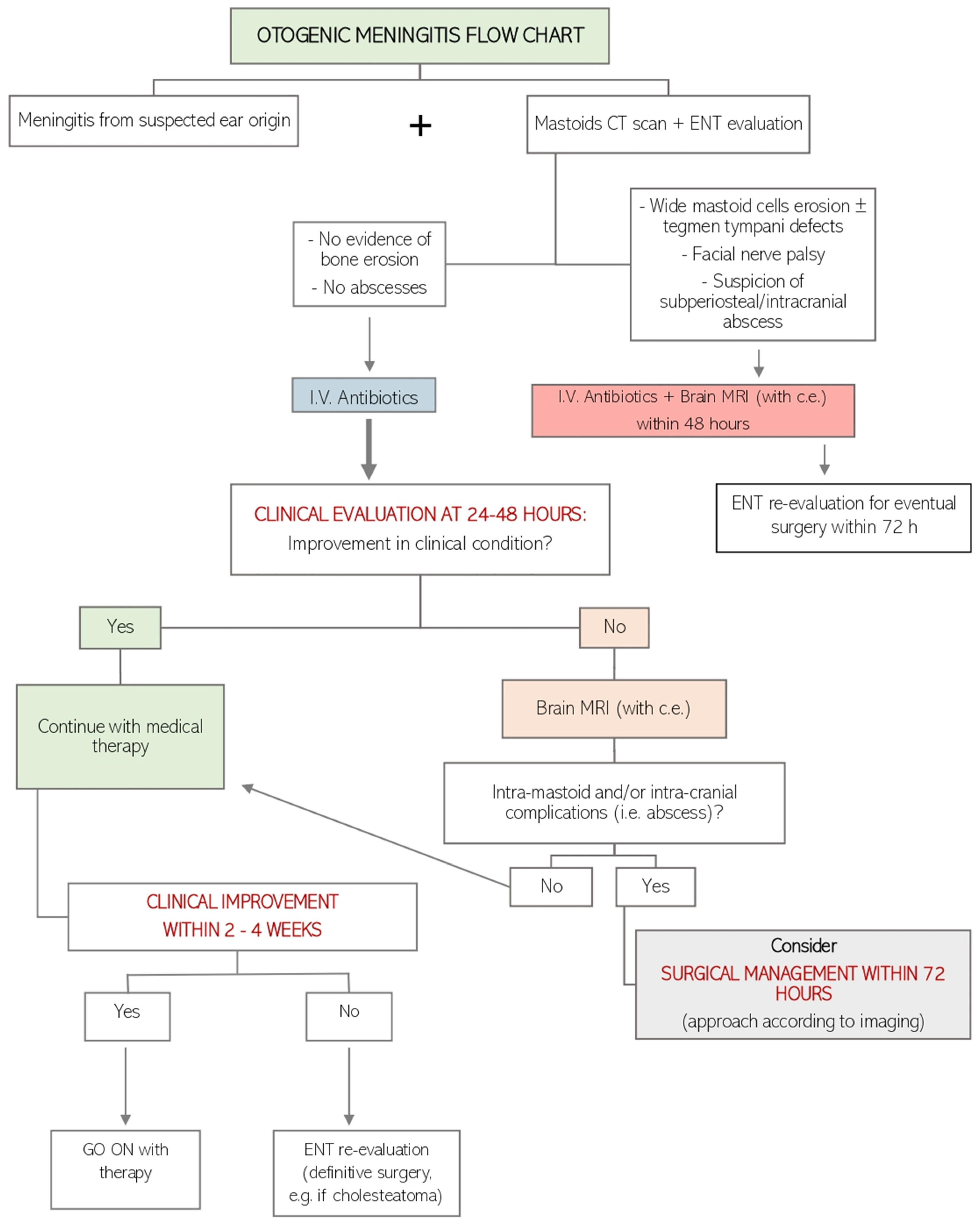
2.2. Our Decision-Making Process
2.3. A Literature Review of Otogenic Meningitis from Acute Otitis Media
3. Results
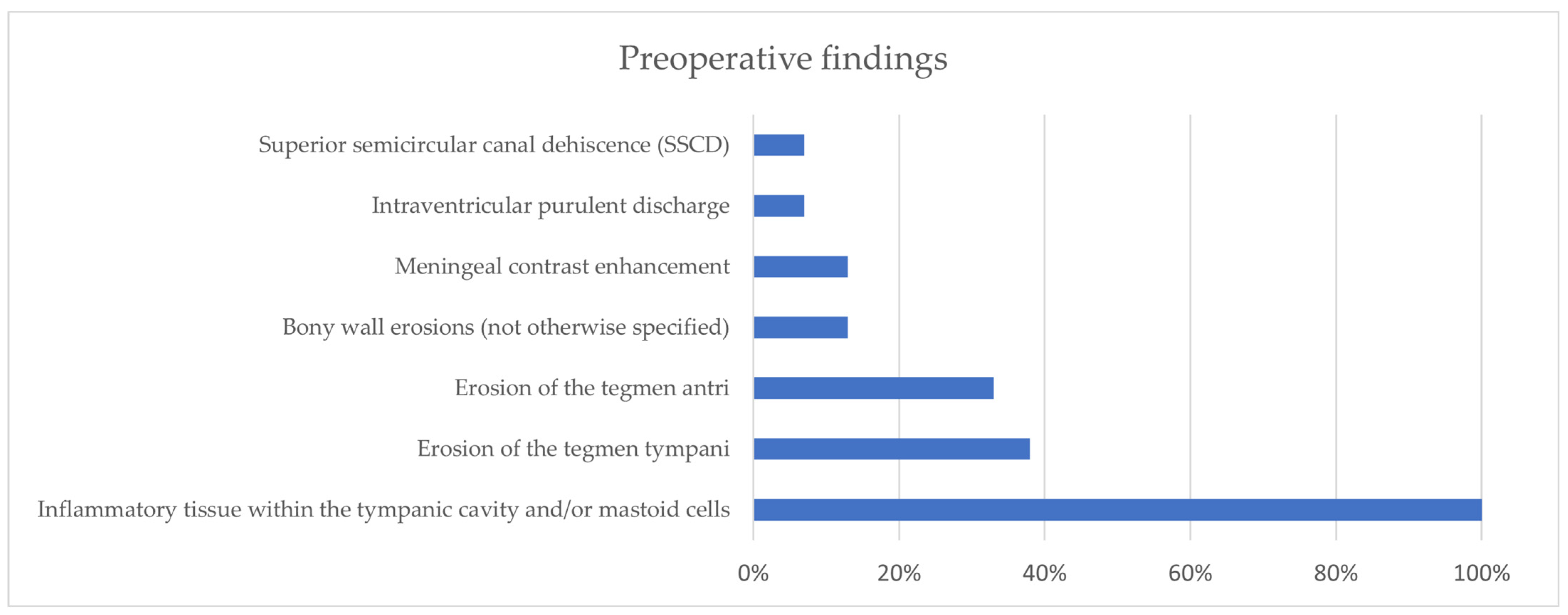
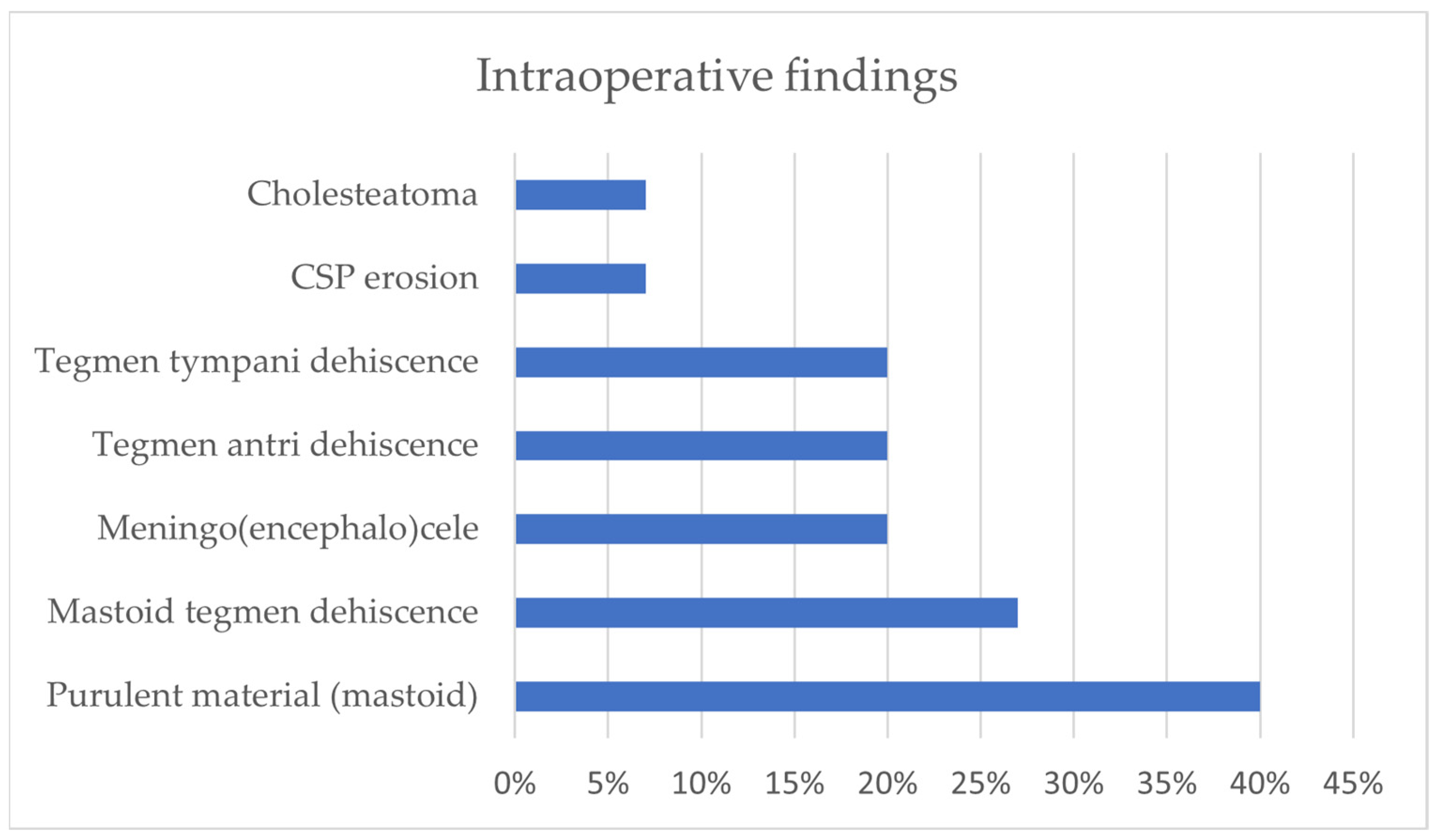
3.1. Our Surgical Experience in Otogenic Meningitis
3.2. Literature Review of Otogenic Meningitis from Acute Otitis Media
4. Discussion
Otogenic Meningitis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bruschini, L.; Fortunato, S.; Tascini, C.; Ciabotti, A.; Leonildi, A.; Bini, B.; Giuliano, S.; Abbruzzese, A.; Berrettini, S.; Menichetti, F.; et al. Otogenic Meningitis: A Comparison of Diagnostic Performance of Surgery and Radiology. Open Forum Infect. Dis. 2017, 4, ofx069. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.I.; Cheang, P.P.; Nunez, D.A. Incidence of meningitis secondary to suppurative otitis media in adults. J. Laryngol. Otol. 2010, 124, 1158–1161. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.P.C.; Shepherd, R.K.; Robins-Browne, R.M.; Clark, G.M.; O’Leary, S.J. Pneumococcal meningitis post-cochlear implantation: Preventative measures. Otolaryngol.-Head Neck Surg. 2010, 143, S9–S14. [Google Scholar] [CrossRef] [PubMed]
- Mittal, Y.; Chappity, P.; Grover, M.; Gupta, G.; Pradhan, P.; Parida, P.K.; Mishra, A. Efficacy of endoscopic repair for CSF otorrhoea in children with recurrent meningitis due to incomplete partition type 1. Int. J. Pediatr. Otorhinolaryngol. 2022, 159, 111215. [Google Scholar] [CrossRef]
- Ng, M.M.Y.; D’Arco, F.; Chorbachi, R.; Nash, R. Oval window perilymph fistula in child with recurrent meningitis and unilateral hearing loss. BMJ Case Rep. 2020, 13, e234744. [Google Scholar] [CrossRef]
- Gregurić, T.; Ries, M.; Hergešić, F.; Košec, A. Bilateral Temporal Bone Meningocele Presenting With Otogenic Meningitis. Ear Nose Throat J. 2021, 100, 581–584. [Google Scholar] [CrossRef]
- Mandalà, M.; Colletti, L. Bacterial meningitis secondary to stapes footplate malformation in a child with an auditory brainstem implant. J. Laryngol. Otol. 2012, 126, 72–75. [Google Scholar] [CrossRef]
- Belmont, M.J.; Arjmand, E.M. Recurrent acute otitis media associated meningitis in a patient with a contralateral cochlear implant and bilateral cochleovestibular dysplasia. Int. J. Pediatr. Otorhinolaryngol. 2004, 68, 1091–1093. [Google Scholar] [CrossRef] [PubMed]
- Marom, T.; Shemesh, S.; Habashi, N.; Gluck, O.; Tamir, S.O. Adult Otogenic Meningitis in the Pneumococcal Conjugated Vaccines Era. Int. Arch. Otorhinolaryngol. 2020, 24, e175–e181. [Google Scholar] [CrossRef]
- Vâţă, A.; Irimie-Băluţă, E.; Roşu, F.M.; Onofrei, I.M.; Loghin, I.I.; Perţea, M.; Avădanei, A.N.; Miron, M.; Rădulescu, L.; Eşanu, I.; et al. Polymicrobial Bacterial Meningitis in a Patient with Chronic Suppurative Otitis Media: Case Report and Literature Review. Medicina 2023, 59, 1428. [Google Scholar] [CrossRef]
- Thevis, M.; Leow, T.Y.S.; Bekkers, S.; Otten, J.; Waterval, J.J.; Derks, J.; Buil, J.B.; Kunst, H.P.M.; Jansen, T.T.G. Diagnosis, treatment and prognosis of otomastoiditis induced by Fusobacterium necrophorum: A retrospective multicentre cohort study. Anaerobe 2022, 76, 102587. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.; Weir, I.; Silber, T. Otogenic Fusobacterium meningitis, sepsis, and mastoiditis in an adolescent. South. Med. J. 2004, 97, 416–418. [Google Scholar] [CrossRef] [PubMed]
- Slovik, Y.; Kraus, M.; Leiberman, A.; Kaplan, D.M. Role of surgery in the management of otogenic meningitis. J. Laryngol. Otol. 2007, 121, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.P.; Larawin, V.; Molumi, C.P. Intracranial spread of chronic middle ear suppuration. Am. J. Otolaryngol. 2010, 31, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Cucu, A.I.; Patrascu, R.E.; Cosman, M.; Costea, C.F.; Vonica, P.; Blaj, L.A.; Hartie, V.; Istrate, A.C.; Prutianu, I.; Boisteanu, O.; et al. Cerebellar Abscess Secondary to Cholesteatomatous Otomastoiditis-An Old Enemy in New Times. Diagnostics 2023, 13, 3566. [Google Scholar] [CrossRef]
- Perry, B.P.; Rubinstein, J.T. Meningitis due to acute otitis media and arachnoid granulations. Ann. Otol. Rhinol. Laryngol. 2000, 109, 877–879. [Google Scholar] [CrossRef]
- Penido Nde, O.; Chandrasekhar, S.S.; Borin, A.; Maranhão, A.S.; Gurgel Testa, J.R. Complications of otitis media—A potentially lethal problem still present. Braz. J. Otorhinolaryngol. 2016, 82, 253–262. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aziz M, Omakobia E: Recurrent otogenic intracranial sepsis: A key radiological finding, not to be missed. Case Rep. Otolaryngol. 2019, 2019, 5013932. [CrossRef]
- Abir, M.; Amal, G.; Mouna, B.; Malika, O.; Habiba, B.S.; Jihen, H.; Wassim, K.; Mohamed, A. Clinical features of otogenic cerebral sinovenous thrombosis: Our experience and review of literature. Clin. Case Rep. 2022, 10, e6475. [Google Scholar] [CrossRef]
- Chang, K.H.; Han, M.H.; Roh, J.K.; Kim, I.O.; Han, M.C.; Kim, C.W. Gd-DTPA-enhanced MR imaging of the brain in patients with meningitis: Comparison with CT. AJNR Am. J. Neuroradiol. 1990, 11, 69–76. [Google Scholar] [CrossRef]
- Alonso, R.C.; de la Peña, M.J.; Caicoya, A.G.; Rodriguez, M.R.; Moreno, E.A.; de Vega Fernandez, V.M. Spontaneous skull base meningoen- cephaloceles and cerebrospinal fluid fistulas. Radiographics 2013, 33, 553–570. [Google Scholar] [CrossRef] [PubMed]
- Lim, Z.M.; Friedland, P.L.; Boeddinghaus, R.; Thompson, A.; Rodrigues, S.J.; Atlas, M. Otitic meningitis, superior semicircular canal dehiscence, and encephalocele: A case series. Otol. Neurotol. 2012, 33, 610–612. [Google Scholar] [CrossRef] [PubMed]
- Ciorba, A.; Berto, A.; Borgonzoni, M.; Grasso, D.L.; Martini, A. Pneumocephalus and meningitis as a complication of acute otitis media: Case report. Acta Otorhinolaryngol. Ital. 2007, 27, 87–89. [Google Scholar] [PubMed]
- Migirov, L.; Duvdevani, S.; Kronenberg, J. Otogenic intracranial complications: A review of 28 cases. Acta Otolaryngol. 2005, 125, 819–822. [Google Scholar] [CrossRef]
- Barry, B.; Delattre, J.; Vié, F.; Bedos, J.P.; Géhanno, P. Otogenic intracranial infections in adults. Laryngoscope 1999, 109, 483–487. [Google Scholar] [CrossRef]
- Geyik, M.F.; Kokoglu, O.F.; Hosoglu, S.; Ayaz, C. Acute bacterial meningitis as a complication of otitis media and related mortality factors. Yonsei Med. J. 2002, 43, 573–578. [Google Scholar] [CrossRef]
- Penido, N.D.O.; Borin, A.; Iha, L.C.; Suguri, V.M.; Onishi, E.; Fukuda, Y.; Cruz, O.L.M. Intracranial complications of otitis media: 15 years of experience in 33 patients. Otolaryngol. Head. Neck Surg. 2005, 132, 37–42. [Google Scholar] [CrossRef]
- Habib, R.G.; Girgis, N.I.; Abu el Ella, A.H.; Farid, Z.; Woody, J. The treatment and outcome of intracranial infections of otogenic origin. J. Trop. Med. Hyg. 1988, 91, 83–86. [Google Scholar]
- Fernandez Rodriguez, R.; Porto Golpe, I.; Santos Perez, S.; Labella Caballero, T. Intracranial complications of otitis: The present-day situation. An. Otorrinolaringol. Ibero Am. 1989, 16, 281–297. (In Spanish) [Google Scholar]
- Gower, D.; McGuirt, W.F. Intracranial complications of acute and chronic infectious ear disease: A problem still with us. Laryngoscope 1983, 93, 1028–1033. [Google Scholar] [CrossRef]
- Isaacson, B.; Mirabal, C.; Kutz JWJr Lee, K.H.; Roland, P.S. Pediatric otogenic intracranial abscesses. Otolaryngol. Head. Neck Surg. 2010, 142, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Mathews, T.J.; Marus, G. Otogenic intradural complications (a review of 37 patients). J. Laryngol. Otol. 1988, 102, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Sennaroglu, L.; Sozeri, B. Otogenic brain abscess: Review of 41 cases. Otolaryngol. Head. Neck Surg. 2000, 123, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Kangsanarak, J.; Navacharoen, N.; Fooanant, S.; Ruckphaopunt, K. Intracranial com- plications of suppurative otitis media: 13 years’ experience. Am. J. Otol. 1995, 16, 104–109. [Google Scholar]
- Gönüllü, E.; Özkan, N.; Soysal, A.; Acıoğlu, E.; Tavil, E.B.; Ötgün, S.N.; Karaböcüoğlu, M. Nontypeable Haemophilus influenzae Otitis Media: Mastoiditis and Meningitis Complicated with Central Venous Thrombosis in an Immunocompetent Child. Case Rep. Infect. Dis. 2021, 2021, 8845200. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duygu, E.; Şevik Eliçora, S. Our experience on the management of acute mastoiditis in pediatric acute otitis media patients. Int. J. Pediatr. Otorhinolaryngol. 2020, 138, 110372. [Google Scholar] [CrossRef]
- Sakamoto, M.; Ito, K.; Sugasawa, M.; Taniguchi, M. Otogenic meningitis caused by the pneumococci that had acquired resistance to cephalosporins. Otolaryngol. Head Neck Surg. 2001, 124, 350–351. [Google Scholar] [CrossRef]
- Odani, N.; Kitazono, H.; Deshpande, G.A.; Hiraoka, E. Severe Sepsis due to Otogenic Pneumococcal Meningitis with Pneumocephalus without Meningeal Symptoms. Intern. Med. 2015, 54, 1661–1664. [Google Scholar] [CrossRef][Green Version]
- Liourdi, D.; Litsardopoulos, P.; Dimitropoulou, D.; Fatourou, A.; Argyriou, A.A. Pneumococcal Otogenic Meningitis Complicated With Pneumocephalus and Coma State. Cureus 2020, 12, e10917. [Google Scholar] [CrossRef]
- Østergaard, C.; Høiby, N.; Konradsen, H.; Samuelsson, S. Prehospital diagnostic and therapeutic management of otogenic Streptococcus pneumoniae meningitis. Scand. J. Infect. Dis. 2006, 38, 172–180. [Google Scholar] [CrossRef]
- Damergis, J.A.; Chee, K.; Amitai, A. Otogenic pneumococcal meningitis with pneumocephalus. J. Emerg. Med. 2010, 39, e109-12. [Google Scholar] [CrossRef] [PubMed]
- Job, A.; Kurien, M.; Jacob, A.; Mathew, J. Bilateral simultaneous hearing preservation mastoidectomy in otogenic meningitis. Ann. Otol. Rhinol. Laryngol. 1998, 107, 872–875. [Google Scholar] [CrossRef] [PubMed]
- Gaudin, J.; Thayalakulasingam, T. Invasive Pneumococcal Disease and COVID-19 With Acute Otitis Media and a Tegmen Tympani Defect. Cureus 2023, 15, e44869. [Google Scholar] [CrossRef] [PubMed]
- Samuel, J.; Fernandes, C.M.C.; Steinberg, J.L. Intracranial otogenic complications: A persisting problem. Laryngoscope 1986, 96, 272–278. [Google Scholar] [CrossRef]
- Samuel, J.; Fernandes, C.M.C. Otogenic complications with an intact tympanic membrane. Laryngoscope 1985, 95, 1387–1390. [Google Scholar] [CrossRef]
- Migirov, L. Computed tomographic versus surgical findings in complicated acute otomastoiditis. Ann. Otol. Rhinol. Laryngol. 2003, 112, 675–677. [Google Scholar] [CrossRef]
- Holt, G.R.; Gates, G.A. Masked mastoiditis. Laryngoscope 1983, 93, 1034–1037. [Google Scholar] [CrossRef]
- Migirov, L.; Weissburd, S.; Wolf, M. Mastoidectomy in the elderly. ORL J. Otorhinolaryngol. Relat. Spec. 2010, 72, 80–83. [Google Scholar] [CrossRef]
- Okada, M.; Hato, N.; Okada, Y.; Sato, E.; Yamada, H.; Hakuba, N.; Gyo, K. A case of hypertrophic cranial pachymeningitis associated with invasive Aspergillus mastoiditis. Auris Nasus Larynx 2015, 42, 488–491. [Google Scholar] [CrossRef]
- Felisati, G.; Di Berardino, F.; Maccari, A.; Sambataro, G. Rapid evolution of acute mastoiditis: Three case reports of otogenic meningitis in adults. Am. J. Otolaryngol. 2004, 25, 442–446. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubini, A.; Ronzani, G.; D’Alessandro, E.; Marchioni, D. Management of Otogenic Meningitis: A Proposal for Practical Guidelines from a Multicenter Experience with a Systematic Review. J. Clin. Med. 2024, 13, 5509. https://doi.org/10.3390/jcm13185509
Rubini A, Ronzani G, D’Alessandro E, Marchioni D. Management of Otogenic Meningitis: A Proposal for Practical Guidelines from a Multicenter Experience with a Systematic Review. Journal of Clinical Medicine. 2024; 13(18):5509. https://doi.org/10.3390/jcm13185509
Chicago/Turabian StyleRubini, Alessia, Guglielmo Ronzani, Edoardo D’Alessandro, and Daniele Marchioni. 2024. "Management of Otogenic Meningitis: A Proposal for Practical Guidelines from a Multicenter Experience with a Systematic Review" Journal of Clinical Medicine 13, no. 18: 5509. https://doi.org/10.3390/jcm13185509
APA StyleRubini, A., Ronzani, G., D’Alessandro, E., & Marchioni, D. (2024). Management of Otogenic Meningitis: A Proposal for Practical Guidelines from a Multicenter Experience with a Systematic Review. Journal of Clinical Medicine, 13(18), 5509. https://doi.org/10.3390/jcm13185509





