Abstract
Objectives: Fatigue is a prominent feature of long COVID (LC) and may be related to several pathophysiologic mechanisms, including immune hyperstimulation. Aerobic endurance exercise training may be a useful therapy, with appropriate attention to preventing post-exertional malaise. Methods: Fourteen participants completed a pilot study of aerobic exercise training (twenty 1.5 h sessions of over 10 weeks). Cardiorespiratory fitness, 6 min walk distance, quality of life, symptoms, 7-day physical activity, immunophenotype, and inflammatory biomarkers were measured before and after exercise training. Results: The participant characteristics at baseline were as follows: 53.5 ± 11.6 yrs, 53% f, BMI 32.5 ± 8.4, 42% ex-smokers, 15.1 ± 8.8 months since initial COVID-19 infection, low normal pulmonary function testing, O2peak 19.3 ± 5.1 mL/kg/min, 87 ± 17% predicted. After exercise training, participants significantly increased their peak work rate (+16 ± 20 W, p = 0.010) and O2peak (+1.55 ± 2.4 mL/kg/min, p = 0.030). Patients reported improvements in fatigue severity (−11%), depression (−42%), anxiety (−29%), and dyspnea level (−46%). There were no changes in 6MW distance or physical activity. The circulating number of CD3+, CD4+, CD19+, CD14++CD16, and CD16++CD14+ monocytes and CD56+ cells (assessed with flow cytometry) increased with acute exercise (rest to peak) and was not diminished or augmented by exercise training. Plasma concentrations of TNF-α, IL-6, IL-8, IL-10, INF-γ, and INF-λ were normal at study entry and not affected by training. Conclusions: Aerobic endurance exercise training in individuals with LC delivered beneficial effects on cardiorespiratory fitness, quality of life, anxiety, depression, and fatigue without detrimental effects on immunologic function.
1. Introduction
As the initial waves of acute coronavirus disease 2019 (COVID-19) infection subsided [1], a dark ‘second act’ appeared in 5–30% of COVID-19-infected patients, namely the persistence of viral-type symptoms beyond 12 weeks, even in those with mild disease courses. The most common debilitating ‘long COVID’ (LC) symptoms are fatigue (defined as a sensation of extreme and persistent tiredness or lethargy that hinders normal physical activity) and exercise intolerance (defined as an abnormally low capacity for endurance exercise); however, a myriad of other symptoms related to almost every organ system can occur, including shortness of breath, anxiety, cognitive dysfunction (‘brain fog’), headaches, palpitations, muscle aches, transient rashes, joint pains, sleep disturbances, post-exertional malaise (PEM), clotting abnormalities, chest pains, etc. [2,3,4]. Although the LC etiology is currently unknown, it is thought to be related to one or more pathophysiologic mechanisms involving ongoing viral replication, immune hyperstimulation, dysregulation of the RAAS system, changes in the microbiome, etc. [2]. The trajectory for improvement from LC is highly variable, ranging from complete recovery to prolonged disability with a reduced quality of life and potentially premature death [5]. Currently, there are no specific treatments directed at LC, although a number of studies are underway [6].
Pulmonary rehabilitation (PR) is very well established as being beneficial following high-severity acute COVID-19 (including hospitalization, high-flow oxygen therapy, ICU care, intubation, and mechanical ventilation) [7,8,9]. PR improved anxiety, depression, brain fog, and 6min walk distance (6MWD) in these high-severity groups [8,10,11,12,13,14,15]; however, the limitation in these studies was that 6MWD provides a very limited insight into the mechanism of cardiorespiratory performance or the physiologic mechanisms behind exercise training improvements.
We sought to improve the physiologic and immunologic understanding of outpatient (non-severe) LC. Cardiopulmonary exercise testing (CPET) is an important tool to assess dyspnea, prescribe exercise training, and exclude comorbid diseases causing similar symptoms to LC. CPET is also useful to provide insight into the mechanism(s) of exercise limitation. Prior CPET studies in LC [16,17,18] show baseline reductions in peak pulmonary oxygen uptake (O2peak) and pulmonary function (both to roughly 80–90% of predicted), even 6–12 months after acute infection.
Recent exercise training and rehabilitation studies in LC utilizing CPET have reported improvements in peak oxygen uptake, work rate, and quality of life [19,20,21,22] in less severe populations (primarily outpatient COVID-19), but have not investigated the inflammatory state or the effects of aerobic exercise training on immune function, as suggested in early 2021 publications by da Silveira and Nieman [23,24].
The evidence exists that endurance and/or strength exercise training may also improve immune function and surveillance (transient increases in the number of NK cells, cytotoxic T cells, and immature B cells), immunoglobulin levels, and reduce inflammatory CRP levels in patients with chronic diseases [24,25]. However, concerns remain as to whether exercise testing and exercise training in LC are safe, because they have the potential to increase post-exertional malaise (PEM) and worsen immune function [26], as can observed in other post-viral syndromes [27].
Our aim in this pilot study was to determine whether moderate aerobic exercise training mitigates LC symptomatology and improves cardiorespiratory fitness and psychological wellness. We hypothesized that LC patients would benefit from a comprehensive moderate aerobic and exercise training program (individualized to baseline CPET) without detrimental effects on immune function.
2. Methods and Materials
This study was approved by the Institutional Review Board of The Lundquist Institute for Biomedical Innovation at Harbor–UCLA Medical Center (#32558-01; ClinicalTrials.Gov Identifier: NCT05398692). Written informed consent was provided and documented prior to participation.
2.1. Study Design
This was a single-center, pilot clinical trial conducted between February 2022 and December 2023. The study design, interventions, and flow diagram are presented in Figure S1.
2.2. Study Enrollment Criteria
The inclusion criteria included adults who were ≥18 years of age, who suffered a COVID-19 infection > 12 weeks prior to study participation (documented by PCR or by patient report when testing was not available), and who experienced one or more of the following persistent symptoms: fatigue, dyspnea, exercise intolerance, PEM, and/or difficulty breathing. Exclusion criteria included subjects unable to perform a technically acceptable PFT or CPET, a desaturation below 80% during exercise, the recent completion of a pulmonary rehabilitation program, a recent or concurrent interventional clinical trial, pregnant or nursing women, malignancy within the past 2 years, injectable insulin use, HIV, system corticosteroids, or any significant respiratory disease other than LC that would put the subject at risk by participating in the study.
2.3. Screening Visit
After obtaining informed consent, a detailed history and focused physical examination were performed, including vaccine status, a medical record review, and smoking history. Resting blood pressure (WelchAllyn, SureBP, New York, NY, USA), heart rate, oxygen saturation by portable pulse oximeter (Santa Medical, Tustin, CA, USA), and 12-lead ECG (CardioTech, GT300, Nundah, QLD, Australia) were measured. Peripheral blood laboratories were drawn at the screening visit to ensure the potential subject was safe to enter the study. These included a complete blood count with differential (CBCdiff), renal, and liver function tests. In addition, D-dimer, Brain Natriuretic Peptide (ProBNP), and inflammatory markers (Ferritin, CRP, and high-sensitivity Troponin (to exclude myocarditis)) were obtained to assess the inflammatory state of the subject. A 10 min NASA lean test [28] was also performed to exclude postural orthostatic hypotension and heart rate autonomic dysfunction.
A pre-bronchodilator spirometry, body plethysmography, and the single breath diffusing capacity of the lung for carbon monoxide (DLCO) were measured according to ATS/ERS standards [29,30,31,32]. The post-bronchodilator spirometry was measured 20 min after the inhalation of 4 puffs of albuterol (400 mg). Predicted spirometry values were obtained from NHANES III [33]; lung volumes from ERS [34]; and DLCO from Cotes et al. [35]. The 6MW was assessed per ATS/ERS guidelines [36].
2.4. Cardiopulmonary Exercise Testing (CPET)
A ramp incremental CPET at 10–20 watts/min (depending on individual fitness) was performed using an electromagnetically braked cycle ergometer (Excalibur Sport PFM, Lode, Groningen, NL, USA). Intolerance was assessed by the inability to maintain a pedaling cadence > 50 rpm despite encouragement. Gas exchange and ventilatory variables were measured breath by breath (Ultima CPX, MGC Diagnostics, St Paul, MN, USA), while participants breathed through a mouthpiece with a nose clip in place. Heart rate, rhythm disturbances, and ST-T wave changes were monitored using an integrated continuous 12-lead ECG (Mortara, Milwaukee, WI, USA). Exercise blood pressure (Suntech Tango, Morrisville, NC, USA), ratings of perceived dyspnea (RPEdyspnea), and leg fatigue (RPElegs) (modified Borg CR-10 scale [37]) were assessed every 2 min. The gas exchange lactate threshold (GE-LAT) was assessed using the V-slope method and corroborated with ventilatory equivalents (E/O2 and E/CO2) and end-tidal partial pressure responses (PETO2 and PETCO2) [38]. The dead space to tidal volume ratio (VD/VT) was assessed using transcutaneous PCO2 as a valid substitute for arterial PCO2 in the mass balance equation (Tosca 500, Radiometer America, Brea, CA, USA) [39,40]. Predicted values for exercise variables were from Hansen et al. [41].
2.5. Overnight Oximetry
Overnight oximetry was performed on the night following the screening CPET using a WristOx2, (Model 3150, Nonin Medical, Plymouth, MN, USA) to exclude sleep-disordered breathing.
2.6. Patient Reported Outcomes
Participants completed the Short Form Health Survey (SF-36), Fatigue Severity Score (FSS), Patient Health Depression Questionnaire (PHQ-9), General Anxiety Disorder (GAD-7), modified Medical Research Council Dyspnea Scale (mMRC), mini Mental Status Examination (MMSE), Post COVID-19 Functional Status (PCFS), modified DePaul Symptom Questionnaire—Post-Exertional Malaise (mDSQ-PEM), and 7-Day Symptom Diary. See Supplementary Materials for details.
2.7. Physical Activity
Seven-day physical activity was assessed using a waist-worn triaxial accelerometer (Dynaport, McRoberts, The Hague, NL, USA). Participants were instructed to wear the activity monitor for 24 h except when bathing or showering. At least 8 h of active time wear, over at least 4 days, was required for measurement validity.
2.8. Immunophenotyping and Cytokine Analysis
Venous blood samples were obtained at rest and at peak exercise during the pre-training and post-training CPET. The enumeration and phenotyping of major leukocyte populations present in the circulation—neutrophils (CD16+ cells), monocytes (CD14++16−, CD16++CD14+, CD80+, CD86+, CD80+CD86+), T cells (CD3+ T, CD4+ T, CD8+ T, CD4+FOXP3+ Tregs), B cells (CD19+ B, CD19+CD24+CD38+ Bregs), and NK cells (CD56+ NK, CD56hiCD16− NK, CD56lowCD16+ NK)—were performed based on methods previously described [42] with minor modifications (see Supplementary Materials). Plasma TNF-α, IL-6, IL-10, IL-8, IFN-γ, and IFNL1 concentrations were measured using a commercially available ELISA (see Supplementary Materials).
2.9. Exercise Training
Participants performed 10 weeks of twice-weekly, 1.5 h, on-site aerobic training sessions involving cycle and treadmill ergometers. Two types of ergometers were used for subject training (combined equally for each session) to break up the monotony of exercise training and involve different muscle groups. All training sessions were directly supervised. The initial exercise time was 10 min, and participants were verbally assessed for PEM within 48 h of each session, either at their next visit or by phone. If PEM occurred or worsened, the exercise intensity and time were decreased by 50%. If no PEM occurred, the intensity and exercise duration were gradually increased (up to 60 min, treadmill and cycle ergometer combined) by the end of the 10-week training. Cycle ergometer training started at an intensity that was 25% of the initial CPET peak work rate and progressed from a constant work rate to interval training as tolerated. Treadmill constant work rate training was initiated at a 1 MPH speed, 0% incline, and 5 min duration and progressed as individually tolerated. RPE targets were 3–5 during training. A warm up and warm down were performed, and participants were educated with respect to breathing techniques to reduce dyspnea and anxiety, training with Therabands® (Akron, OH, USA), gentle yoga, stretching, balance exercises, and relaxation techniques, as well as given information on nutrition, hydration, and self-care [43].
2.10. Post-Training Assessments
The CPET, patient reported outcomes, physical activity, and immunologic assessments were repeated at the end of the 10-week training program.
2.11. Statistical Analyses
Data are presented as mean ± SD unless otherwise stated. Statistical significance was accepted at p < 0.05. Data were checked for data normality using the Shapiro–Wilk test, histograms, Q-Q plots, and boxplots. As this was a pilot study, no formal a priori sample size calculation was performed. SPSS (version 24.0; IBM, Chicago, IL, USA) was used for statistical analyses.
For the CPET, paired t-tests were used to assess pre- and post-training differences in cardiorespiratory and perceptual responses during the CPET. For questionnaires, paired t-tests assessed changes in depression and anxiety. Wilcoxon signed rank tests assessed changes in fatigue, dyspnea, the magnitude of post-COVID-19 functional status, and cognitive impairment, as the data were not normally distributed. Changes in SF-36 items were analysed using paired t-tests, except for two items that were not normally distributed, where non-parametric sign tests were used. An exact McNemar’s test determined pre- and post-training changes in the proportion of participants reporting PEM. A 2-way repeated-measure ANOVA (day × training) analyzed pre- and post-training differences in 7-day symptom diary items pre- and post-training. When the sphericity assumption was not met, the Greenhouse–Geisser correction was applied.
Two-way repeated-measure ANOVAs (acute exercise × training) were used to assess immune cell number and cytokine concentration pre- and post-training differences immediately before CPET (i.e., rest) and at the peak CPET work rate. When the sphericity assumption was not met, the Greenhouse-Geisser correction was applied. Significant acute exercise and training interaction effects were followed up using paired t-tests and Fisher’s LSD.
3. Results
3.1. Participants
Twenty-one participants consented. The enrollment chart is presented in Figure S2. Five participants were excluded from the study intervention and referred for medical evaluation due to abnormal screening tests, including very high glucose (one); an elevated BNP (one); an ischemic exercise ECG during CPET (one); a high-degree heart block with bradycardia (one); and repeated COVID-19 infection after the first rehabilitation visit (one). Two participants withdrew prior to exercise training: one due to the perceived risk of contracting COVID-19 during exercise training and one due to perceived increased PEM symptoms risk with exercise training. Fourteen participants completed the exercise training study.
3.2. Demographics
Demographics, blood chemistry, CBCDiff, and PFT results at screening are reported in Table 1 for the 14 participants who completed the study. The screening tests were all within normal limits (Table 1). There were no differences in screening test results between those participants who completed the study (n = 14) and those who screen-failed or dropped out (n = 7) (Table 1). No subject had sleep-disordered breathing suggested from the overnight oximetry.

Table 1.
Baseline demographics, long COVID symptoms, screening laboratories, and pulmonary function testing. n = 14 completed study vs. 7 who did not complete study. All values, mean ± SD. FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; TLC, total lung capacity; RV, residual volume; DLCO, diffusing capacity of lung for carbon monoxide. * = p < 0.05 (n = 7) relative to group who completed study (n = 14).
3.3. Exercise Training Compliance
Participants completed 96% of onsite visits during the 10-week, 2 sessions per week, 1.5 h session exercise training program. Twelve sessions (4% of the total visits) were allowed to be completed off-site to accommodate patient travel or weather. Participants started at a low exercise duration and intensity (5–10 min) and advanced, with the goal of reaching 60 min of combined bike and treadmill aerobic exercise by the 10th week. Across all 20 sessions, participants exercised for an average of 43 ± 9 min per session (range 30 to 58 min) and in the final exercise training session, participants averaged 56 ± 7 min of exercise.
3.4. CPET Pre- and Post-Training
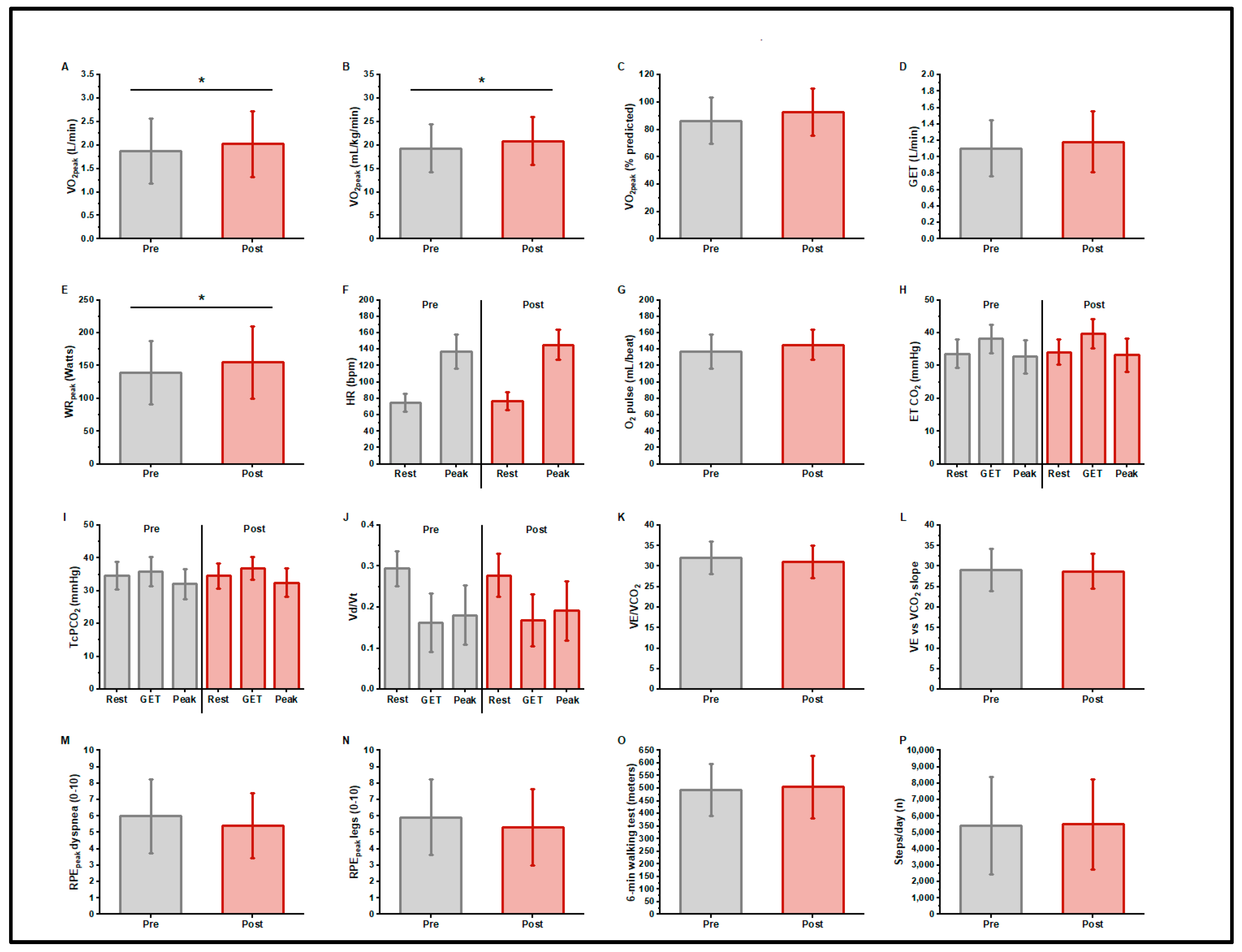
Table 2 and Figure 1 present CPET responses pre- and post-exercise training. Participants began the trial with an impaired cardiorespiratory function, i.e., O2peak (1.88 ± 0.69 L/min, 86.5 ± 16.8% predicted [41], 19.3 ± 5.1 mL/kg/min) and GELAT (1.11 ± 0.34 L/min, 58.7% O2peak); the peak work rate was 139 ± 49 Watts. The mean ventilatory efficiency was normal: the E/CO2 at LAT was 31.9 ± 4.0, the E to CO2 slope was 29.0 ± 5.3. The resting transcutaneous PCO2 was 34.7 ± 4.2 mmHg. The mean VD/VT at GELAT was normal at 0.16 ± 0.07. The peak Borg scores were RPEdyspnea 5.9 ± 2.3 and RPElegs 6.0 ± 2.3. After training, the O2peak was 2.03 ± 0.70 L/min, increasing by a mean of 150 ± 200 mL/min (7.9% increase, p = 0.017) or 1.51 ± 2.3 mL/kg/min (8% increase, p = 0.030). The peak work rate increased 16 ± 20 watts (11.5% increase, p = 0.010). There were no significant changes in indices of ventilatory efficiency (E/CO2 at LAT, or E to CO2 slope) or VD/VT. At peak exercise, RPEdyspnea (5.4 ± 2.0), and RPElegs (5.3 ± 2.3) did not differ in response to training.

Table 2.
Pre- and post-exercise training ramp CPET results (n = 14). O2, oxygen uptake; LAT, estimated lactic acidosis threshold; for O2 at LAT, the % predicted is 40% of peak O2; VE, minute ventilation; CO2, carbon dioxide output; E to CO2 slope, the slope of the relationship between E and CO2 measured between 20 W and the respiratory compensation point; WR, work rate (watts); MVV, maximum voluntary ventilation estimated from the forced expiratory volume in 1 s * 40; peak HR, maximum HR recorded during the CPET. Peak O2 pulse = peak O2/peak HR. All predicted values from Wasserman et al. [41].
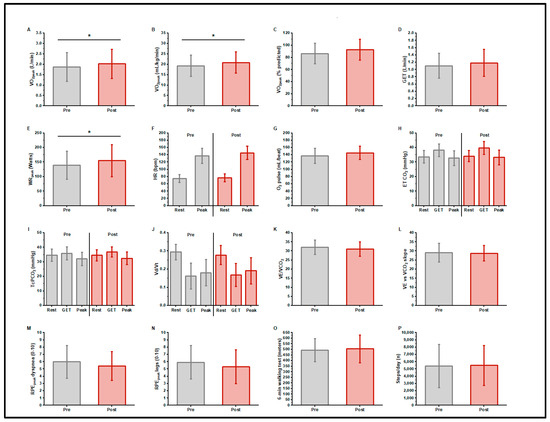
Figure 1.
Cardiopulmonary exercise test responses, 6 min walk distance, and daily step counts, before and after exercise training in long COVID patients. n = 14; O2peak, gas exchange threshold (GET), and peak work rate increased with training (A–E) (* p < 0.05). There was no change in resting or peak heart rate or peak O2 pulse with training (F,G). Indices of ventilatory efficiency did not differ with training (H–L). There were no differences in peak exercise ratings of dyspnea or leg fatigue with training (M,N). There was no change in 6 min walk distance (O) or daily step count (P) with training.
3.5. 6MWD
3.6. 7-Day Activity Monitoring
3.7. Patient-Reported Outcomes/Questionnaires
Most SF-36 subscale scores (Table 3) improved (statistically and with respect to minimally important differences) with exercise training. Improvements were observed in physical functioning, energy/fatigue, emotional well-being, social functioning, and general health. Physical and emotional role limitations and pain changes were not changed with exercise training.

Table 3.
Patent-reported outcomes: pre- and post-exercise training intervention (n = 14). Patient-reported outcomes: 36-item Short Form Survey (SF-36, higher is better), Fatigue Severity Score (lower is better), Patient Health Questionaire-9 (PHQ-9; for depression, lower is better), General Anxiety Disorder-7 (GAD-7; for anxiety, lower is better), mMRC (lower is better), MMSE (mental status, higher is better), Post-COVID-19 Functional Status (PCFS, lower is better). SF-36 change category, (S = Small, M = Moderate, L = Large).
Exercise training was associated with a significant reduction in depression scores (PHQ-9) from moderate to mild, while anxiety (GAD-7) was unchanged (mild, Table 3). There was an important MCID reduction in mean FSS, although this was not statistically significant. There was an MCID reduction in the mMRC dyspnea score after exercise training (Table 3). The statistically significant increase in the MMSE score failed to reach the accepted MCID of 1.5 to 2.0 (Table 3). The PCFS decreased significantly from median; however, an MCID is not well established for this questionnaire.
By mDSQ-PEM criteria, 78.6% of our participants (11/14) qualified as having PEM in the past 6 months based upon a ≥2 score (on a 0–4 range) in both frequency and severity in at least one of the five PEM-related questionnaire items. Nine participants reported a PEM score ≥ 2 after training (64.3%), which was not different from the baseline (p = 0.500).
In some exercise training sessions, participants self-reported lingering fatigue from the prior training session, consistent with the concept of post-exertional symptom exacerbation (PESE) or PEM. Across the study, this occurred an average of 3 sessions out of 30 (range 0–7). PESE rarely required training intensity reduction, and the symptoms responded by the next training visit.
The 7-day symptom diary tracked daily fatigue, shortness of breath, anxiety, cough, brain fog, overall health, and activity levels (Figure S3). Symptoms of fatigue, brain fog, and overall health were significantly reduced following exercise training, but the remainder of the symptom scores, although lower post-training, did not differ statistically.
4. Immunologic Measurements
4.1. White Blood Cell (WBC) Numbers
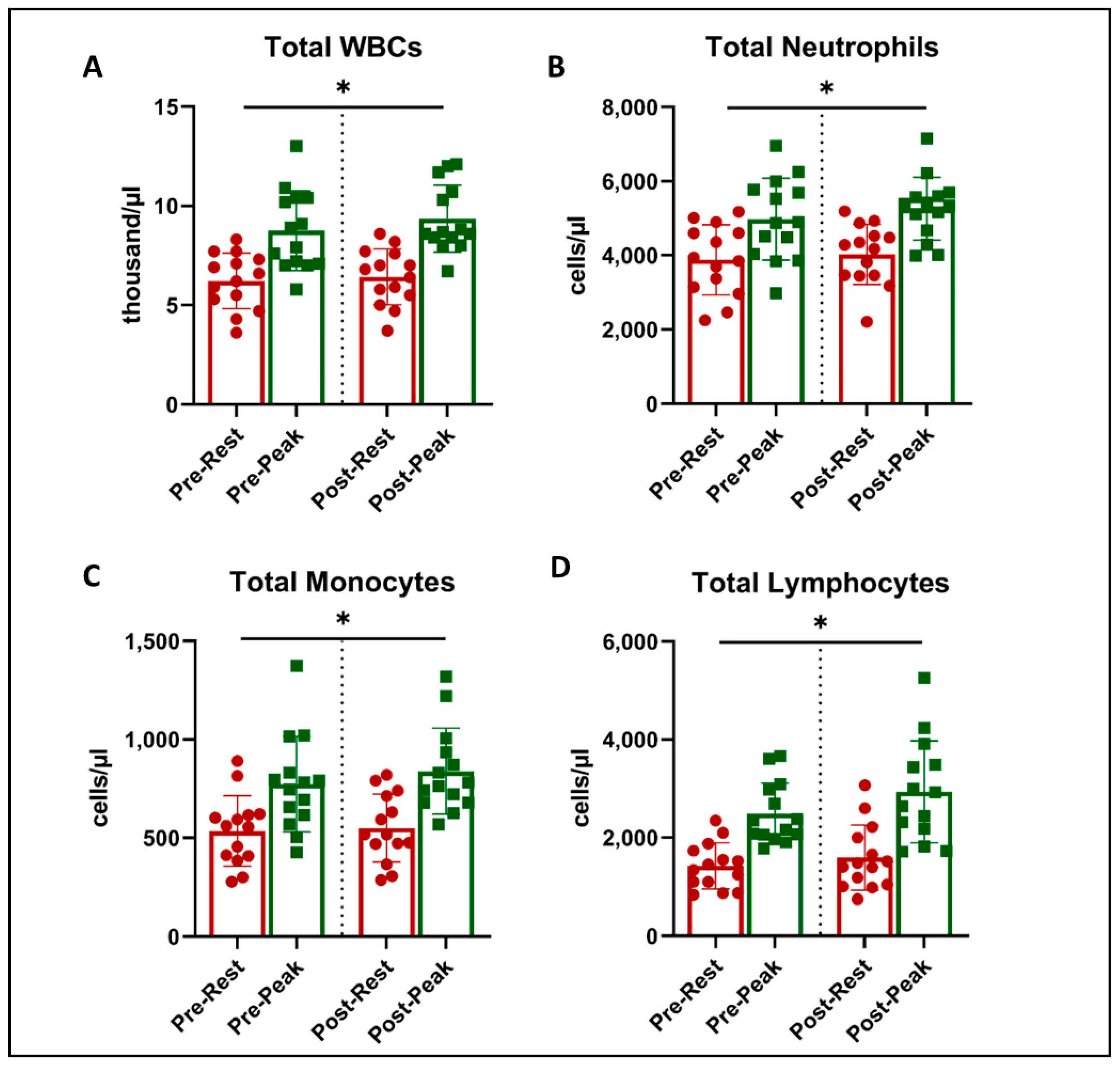
There was a main effect of acute exercise on WBC counts (ANOVA p < 0.05), with expected and significant increases from rest to peak exercise during CPET (Figure 2). However, there was no effect of exercise training on WBC counts or an interaction.
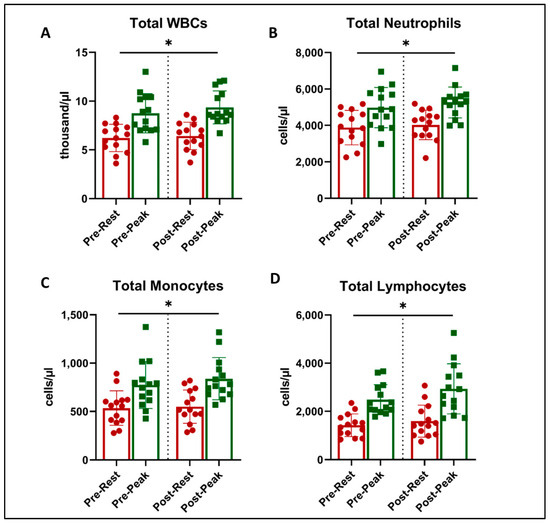
Figure 2.
Total leukocyte counts at rest (red) and peak exercise during CPET (green) before and after exercise training in long COVID patients. There was an increase in cell numbers related to acute exercise in total WBC counts (A), total Neutrophils (B), Total Lymphocytes (C), and Total Monocytes (D) (* = all p < 0.001, 2-way ANOVA). There was no effect on this response with exercise training.
4.2. Immune Cell Subsets
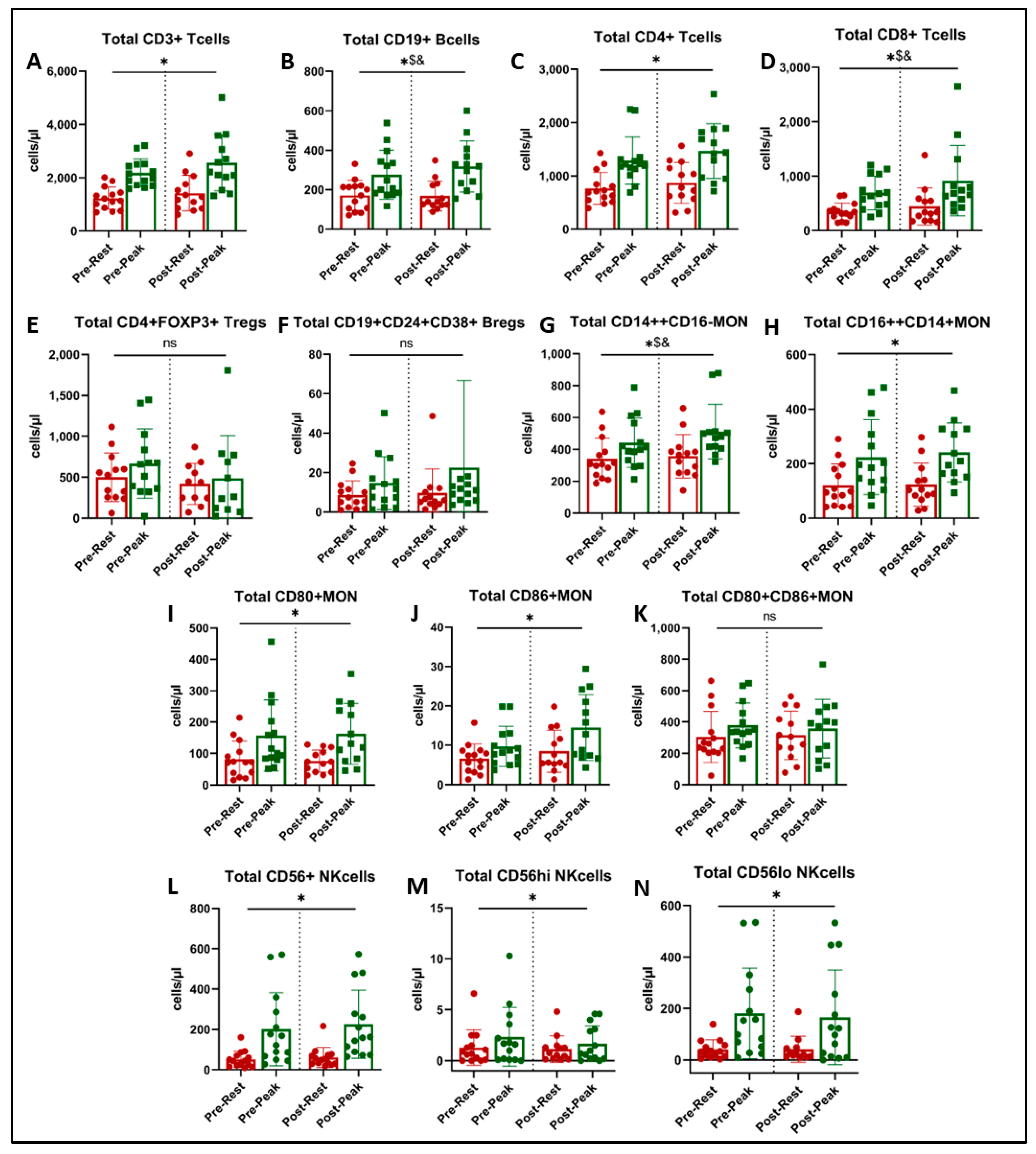
There was a significant main effect of acute exercise on CD3+ T cells (p < 0.001, Figure 3A), CD4+ helper T cells (p < 0.001, Figure 3C), CD16++CD14+ non-classical monocytes (p < 0.001, Figure 3H), CD80+ and CD86+ monocytes (p < 0.001, Figure 3I,J), and CD56+ NK cells and their two subsets (p < 0.001, Figure 3L–N). There was no effect of acute exercise or exercise training on FOXP3+ regulatory T cells (Figure 3E), CD24+CD38+ regulatory B cells (Figure 3F), or CD80+CD86+ activated monocytes (Figure 3K).
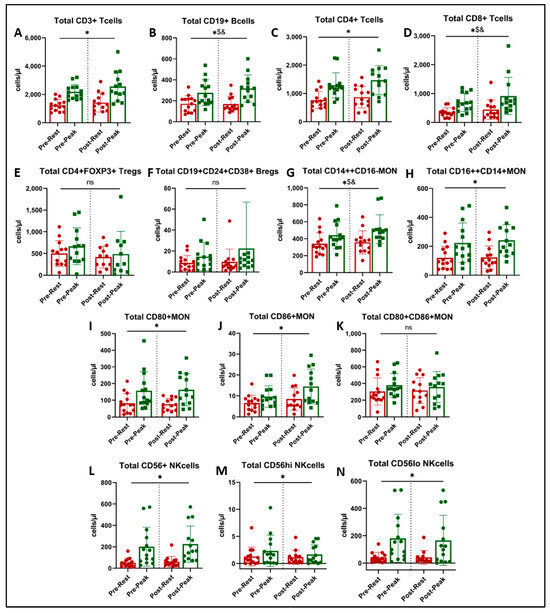
Figure 3.
Immune cell subtype counts at rest (red) and at peak exercise during CPET (green) before and after exercise training in long COVID patients. * = main effect of acute exercise (A,C,H,I,J,L,M,N). $ = acute exercise x training interaction (B,D,G). & = p < 0.05 in paired t-test (post-hoc, (B,D,G)). There was no effect of exercise training on immune cell subset counts (ns = not significant, panels E,F,K).
We found a significant interaction (acute exercise x training) on the number of CD19+ B cells (Figure 3B, p = 0.030) and CD14++CD16− classical monocytes (Figure 3G, p = 0.043). Post-hoc analysis showed that the acute exercise response in CD19+ B cells at peak was greater post-training (Figure 3B, p = 0.033), and peak CD14++CD16− classical monocytes approached significance (Figure 3G, p = 0.051).
4.3. Plasma Biomarkers and Cytokines
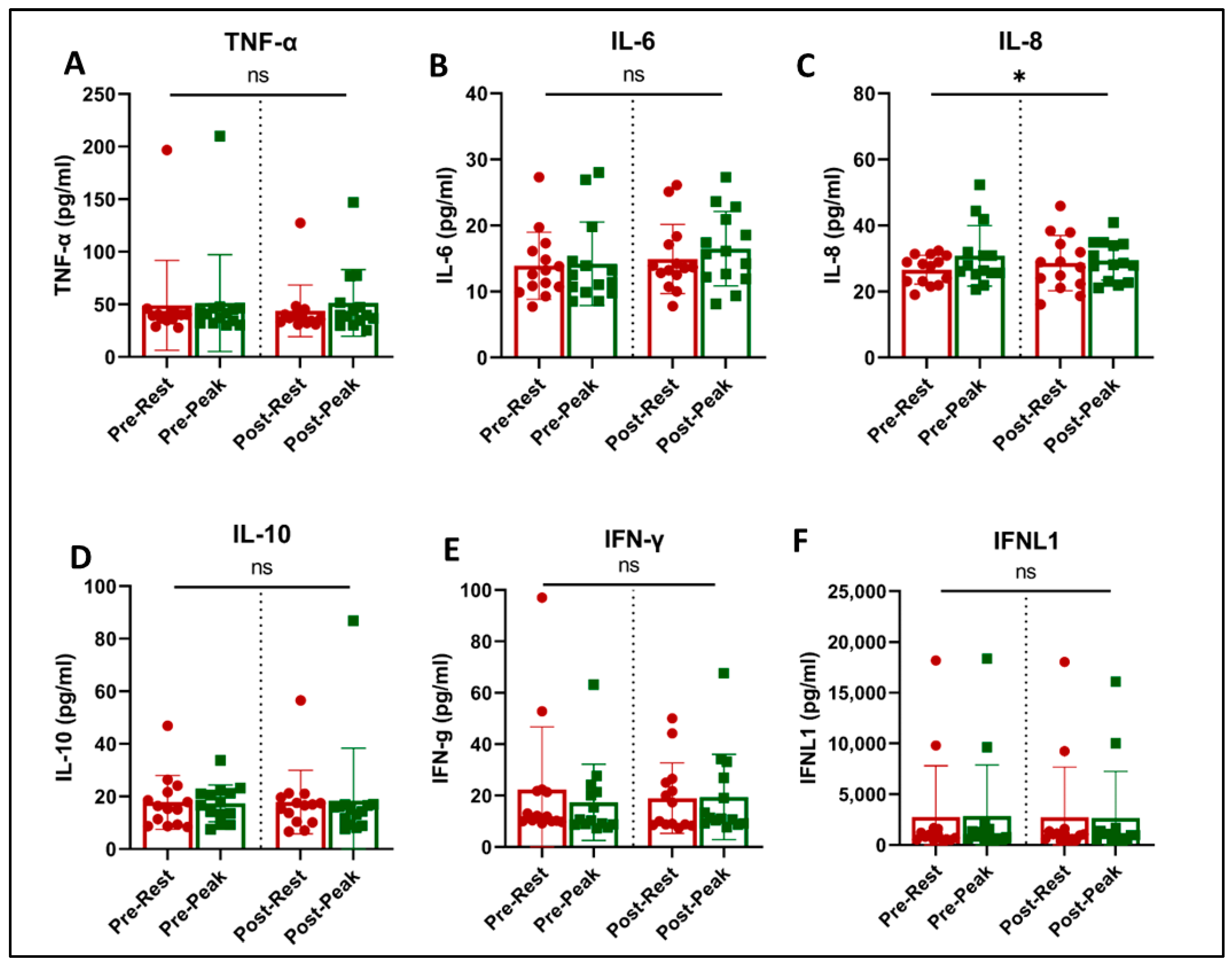
Acute exercise and training effects on plasma inflammatory biomarkers and cytokines are shown in Figure 4. Acute exercise increased the level of IL-8 (p = 0.042, Figure 4C) and showed a trend towards an increase in IL-6 concentrations (p = 0.061, Figure 4B). There was no effect of acute exercise or exercise training on the levels of TNF-α, IL-10, INF-γ, and INFλ (1FNLl).
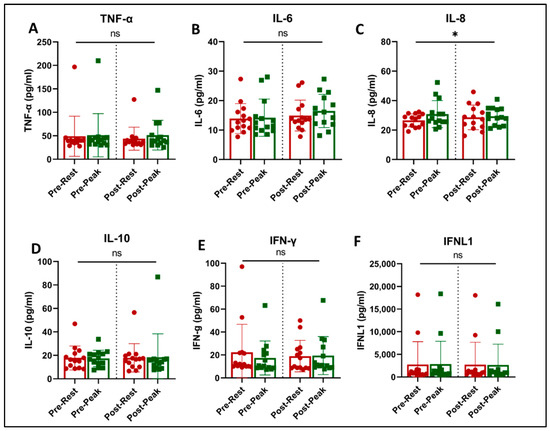
Figure 4.
Plasma immune biomarkers at rest (red) and at peak exercise during CPET (green) before and after exercise training in long COVID patients. * = slight increase in IL-8 with acute exercise (p = 0.042, (C)). There was no effect of training on any of the evaluated immune biomarkers. ns = not significant.
5. Discussion
Our pilot study investigated the effects of a CPET-based exercise training program on physiologic, immunologic, and patient-reported outcomes in LC. We found that exercise training was well tolerated and resulted in increased cardiorespiratory fitness and marked improvements in symptoms and quality of life without adverse immunologic function effects. The pathophysiologic mechanism(s) of the observed limitations and subsequent improvements with exercise training observed in this study are not well understood. The proposed mechanisms for long COVID include persistent viral replication, ongoing immune overstimulation, dysregulation of the RAAS system, changes in the microbiome, and changes in organ function from COVID-19 infection [44]. Clearly, several of these mechanisms could be affected by exercise training, and future studies will hopefully further elucidate the contributions of these various mechanisms.
Our LC participants had an impaired O2peak at baseline, as is observed in the LC CPET literature; however, 20 sessions of CPET-based exercise training over 10 weeks significantly increased the O2peak and peak work rate. Training was individualized to the initial CPET results and was begun at low intensity to avoid PESE/PEM. We encountered PESE/PEM on average in 3 of the 20 training sessions per participant, and in these cases, we scaled back the training prior to slowly advancing again. Despite a low initial training duration (10 min) and intensity (25% of the initial CPET peak work rate), with occasional intensity decreases due to symptom exacerbation, participants reached an average training session duration of 43 ± 9 min of aerobic exercise per session over the 20 sessions and 56 ± 7 min at the end of the training program. Overall, a CPET-guided exercise prescription of aerobic exercise training with symptom education was effective in increasing cardiorespiratory fitness in LC patients.
We did not find any substantial ventilatory or gas exchange abnormalities in our subjects. Specifically, we found that the gas exchange efficiency assessed by E/CO2, the E-CO2 slope, and VD/VT were normal prior to training and did not change with training. The PTCCO2 and PETCO2 values were slightly low (~34–35 mmHg) at rest but behaved normally with exercise.
An important point of this pilot study is that CPET prior to an exercise training intervention in those with LC can uncover unexpected comorbidities attributed to LC symptoms (e.g., fatigue, dyspnea, exercise intolerance, PEM, etc.) We excluded participants with potential contraindications to exercise training, including myocarditis (troponin and BNP), autonomic insufficiency (NASA lean test), and serious comorbidities that might masquerade as LC symptoms (e.g., coronary artery disease, cardiac rate disturbances, metabolic disorders) by performing a CPET prior to exercise training. These screening procedures identified 5 of 21 patients (25%) requiring a medical referral and evaluation, with two situations being life threatening. In addition, the similarity between LC symptoms of fatigue, exercise limitation, and chest pains and the symptoms of significant cardiovascular or metabolic disease warrants detailed investigation prior to exercise training and may lead to an alternative diagnosis from LC. We therefore strongly believe that all LC patients should undergo detailed screening procedures including CPET before embarking on new exercise training programs.
Arguments against performing CPET testing or exercise training in LC relate to the findings with other post-viral syndromes (e.g., Myalgic Encephalomyelitis/Chronic Fatigue Syndrome/) where structured exercise may worsen PEM. In this study, we were careful to assess for PEM at entry with a standardized questionnaire (DePaul, retrograde to 6 months) and review any new or worsened symptoms within 48 h of each exercise training session. The recall range of 6 months for the DePaul questionnaire may be judged too wide; however, we wanted to ensure that we had the largest range of understanding of our participant’s symptomatology. Although we found that 11 out of 14 participants identified PEM > 2 using the DePaul questionnaire criteria, being sensitive to symptom fluctuations in our approach, including flexible adjustments of training intensity and duration, allowed participants to increase their cardiorespiratory fitness in response to an aerobic exercise training program without an increase in PEM. A recent review of exercise training (with and without PEM) in LC by Gloeckl, et al. provides evidence-based practical advice regarding the optimal exercise program, including aerobic training, resistance exercise, and inspiratory muscle training programs for long COVID, with and without PEM, similar to the current study [43].
Patient-reported outcomes showed marked quality of life improvements, including improvements in physical functioning, energy/fatigue, emotional well-being, social functioning, and general health (SF-36) and reductions in depression (PHQ-9). In addition, functional disability due to dyspnea (mMRC), and cognitive impairment (MMSE) improved (Table 3).
We hypothesized that the immunophenotype and balance of inflammation/anti-inflammatory forces in LC would improve with aerobic exercise training. This was based on prior studies showing that exercise training enhances the innate immune function and reduces systemic inflammation in other chronic metabolic and inflammatory diseases [45,46,47]. However, we did not observe significant changes in circulating cell counts or immune biomarkers with exercise training. Nevertheless, there was a transient increase in immune cell subtypes involved in exercise-induced immunosurveillance [45,48]. This aligns with previous research indicating that exercise boosts the recirculation of cytotoxic T cells, immature B cells, and NK cells, which can augment immune surveillance and the suppression of tumorigenesis [46], and similar immune improvements have been demonstrated in other conditions [49,50]. Thus, each bout of moderate exercise appears to temporarily enhance immunosurveillance, whereby regular exercise may provide multiple health benefits, including reduced morbidity and lower systemic inflammation [24].
Our results suggest that the transient change in immune cell subpopulations and biomarkers due to acute exercise in our LC participants was not adversely affected by exercise training and resulted in significant cardiorespiratory fitness and quality of life improvements. In fact, fatigue at entry and fatigue improvements with training did not appear to be associated with immune hyperstimulation, suggesting other etiologies of exercise limitation.
6. Strengths and Limitations
The strengths of this study include in-depth physiologic and questionnaire screening, CPET testing before and after exercise training, activity monitoring, multiple patient-reported outcome assessments, detailed acute exercise and training immunologic data, and screening for comorbid diseases mimicking LC symptoms. The potential limitations are those related to a pilot study: a small sample size, (exacerbated by screen failures due to previously unidentified medical issues and concerns about reinfection or PEM), no control group (subjects were their own control pre/post training), and potential variability in the participant phenotype, since no gold standard biomarker currently exists for LC syndrome.
7. Conclusions
Our hypothesis that LC patients would benefit from a comprehensive moderate aerobic endurance exercise training program prescribed using a baseline CPET was affirmed. Exercise training appears safe to perform in LC patients, when there is great attention to screening for unrecognized medical conditions that may mimic or exacerbate LC symptoms and when the training intensity and duration are flexibly adjusted during the training sessions to limit flare ups of PESE/PEM symptoms. Exercise training substantially improved patient-reported outcomes of mental health, well-being, and symptoms of depression. The resting immune system profile, and the immune response to acute exercise, was unaffected by exercise training despite reductions in fatigue-related symptoms, suggesting that immune hyperstimulation may not be the primary mechanism of LC fatigue symptoms in our study. LC patients benefit from a comprehensive moderate aerobic exercise training program structured to their individual cardiorespiratory fitness, as guided by CPET.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm13185590/s1, Figure S1: Study Interventions and Participant Flow by Visit.; Figure S2: Enrollment Flow Chart. Figure S3: Seven Day Symptom Diary; Figure S4: Gating strategy for the lineage panel; Figure S5: Gating strategy for T cells subsets; Figure S6: Gating strategy for regulatory T cells; Figure S7: Gating strategy for regulatory B cells; Figure S8: Gating strategy for the activated monocytes; Table S1: Antibody characteristics used for immune cell phenotyping.
Author Contributions
Conceptualization, A.A., W.W.S., R.C. and H.B.R.; methodology, A.A., J.P., M.I., H.B.R. and W.W.S.; software, A.A., J.P., W.W.S., C.G., A.S. and K.K.S.; validation, A.A. and W.W.S.; formal analysis, A.A., K.K.S. and W.W.S.; investigation, A.A., J.T., J.P., C.F., H.B.R. and W.W.S.; resources, A.A., J.T., J.P., C.L., C.F., H.B.R. and W.W.S.; data curation, A.A., C.G., K.K.S. and W.W.S.; writing—original draft preparation, A.A., C.G. and W.W.S.; writing—review and editing, A.A., C.G., M.I., C.F., J.T., A.S., KW, J.P., C.L., H.B.R., R.C. and W.W.S.; visualization, C.G., A.A. and W.W.S.; supervision, A.A., J.T., J.P., C.F. and W.W.S.; project administration, A.A., J.T., J.P. and W.W.S.; funding acquisition, A.A. and W.W.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Pulmonary Education and Research Foundation and the UCLA David Geffen School of Medicine (DGSoM)—Ventura County Community Foundation (VCCF) Long COVID 19 Research Award.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of the Lundquist Institute (protocol code (#32558-01 on 10 January 2022).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available with appropriate requests to the corresponding author.
Acknowledgments
The authors would like to express their gratitude to Carly Heffernan; Olivia Heutlinger; Elizabeth Dineen; and Long-Co Nguyen for their support in recruiting efforts for this study.
Conflicts of Interest
Asghar Abbasi is supported by TRDRP (28FT-0017), NIH (R43HL167289, 2R44HL167289-02), and the Johnny Carson Foundation. A.A. has also received research foundation support from the Pulmonary Education and Research Foundation and the UCLA David Geffen School of Medicine (DGSoM)—Ventura County Community Foundation (VCCF) Long COVID 19 Research Award. Chiara Gattoni—No Disclosure. Michelina Iacovino—No Disclosure. Carrie Ferguson is supported by a grant from NIH (R01HL166850). She is involved in contracted clinical research with United Therapeutics, Genentech, Regeneron, Respira Therapeutics, and Mezzion. She reports consulting fees from Respira Therapeutics. She is a visiting Associate Professor at the University of Leeds, UK. Jacqueline Tosolini—No Disclosure. Ashrita Singh—No Disclosure. Kyaw Soe—No Disclosure. Janos Porszasz—No Disclosure. Charles Lanks—No Disclosure. Harry Rossiter is supported by grants from NIH (R01HL151452, R01HL166850, R01HL153460, P50HD098593, R01DK122767), the Tobacco-Related Disease Research Program (T31IP1666), and the Department of Defense/USAMRAA (HT9425-24-1-0249). He reports consulting fees from the NIH RECOVER-ENERGIZE working group (1OT2HL156812), and is involved in contracted clinical research with Astellas, GlaxoSmithKline, Genentech, Intervene Immune, Mezzion, Novartis, Regeneron, Respira, and United Therapeutics. He is a visiting Professor at the University of Leeds, UK. Richard Casaburi serves on the advisory board and is a stockholder for Inogen, Inc. William W. Stringer is supported by research grants from the NIH (UG3HL155798), and contracted site research with Genentech, AstraZeneca, Allerdia, and PureTech. Site Long COVID Research involves two RECOVER studies (Vital and Neuro, 0T2HL156812-01). Dr Stringer receives research foundation support from the Pulmonary Education and Research Foundation and the UCLA David Geffen School of Medicine (DGSoM)—Ventura County Community Foundation (VCCF) Long COVID 19 Research Award. He reports consulting fees from Verona, Genetech, and Vyaire. He is the co-author of a clinical exercise testing physiology textbook for Lippincott.
References
- Borges do Nascimento, I.J.; O’Mathúna, D.P.; von Groote, T.C.; Abdulazeem, H.M.; Weerasekara, I.; Marusic, A.; Puljak, L.; Civile, V.T.; Zakarija-Grkovic, I.; Pericic, T.P.; et al. Coronavirus disease (COVID-19) pandemic: An overview of systematic reviews. BMC Infect. Dis. 2021, 21, 525. [Google Scholar] [CrossRef] [PubMed]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.A.-O.; McCorkell, L.A.-O.; Vogel, J.A.-O.; Topol, E.A.-O. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long covid-mechanisms, risk factors, and management. BMJ 2021, 374, n1648. [Google Scholar] [CrossRef] [PubMed]
- Briggs, A.; Vassall, A. Count the cost of disability caused by COVID-19. Nature 2021, 593, 502–505. [Google Scholar] [CrossRef]
- Astin, R.; Banerjee, A.; Baker, M.R.; Dani, M.; Ford, E.; Hull, J.H.; Lim, P.B.; McNarry, M.; Morten, K.; O’Sullivan, O.; et al. Long COVID: Mechanisms, risk factors and recovery. Exp. Physiol. 2023, 108, 12–27. [Google Scholar] [CrossRef]
- Singh, S.J.; Baldwin, M.M.; Daynes, E.; Evans, R.A.; Greening, N.J.; Jenkins, R.G.; Lone, N.I.; McAuley, H.; Mehta, P.; Newman, J.; et al. Respiratory sequelae of COVID-19: Pulmonary and extrapulmonary origins, and approaches to clinical care and rehabilitation. Lancet Respir. Med. 2023, 11, 709–725. [Google Scholar] [CrossRef]
- Chen, H.; Shi, H.; Liu, X.; Sun, T.; Wu, J.; Liu, Z. Effect of Pulmonary Rehabilitation for Patients With Post-COVID-19: A Systematic Review and Meta-Analysis. Front. Med. 2022, 9, 837420. [Google Scholar] [CrossRef]
- Spielmanns, M.; Pekacka-Egli, A.M.; Schoendorf, S.; Windisch, W.; Hermann, M. Effects of a Comprehensive Pulmonary Rehabilitation in Severe Post-COVID-19 Patients. Int. J. Environ. Res. Public. Health 2021, 18, 2695. [Google Scholar] [CrossRef]
- Sánchez-García, J.C.; Reinoso-Cobo, A.; Piqueras-Sola, B.; Cortés-Martín, J.; Menor-Rodríguez, M.J.; Alabau-Dasi, R.; Rodríguez-Blanque, R. Long COVID and Physical Therapy: A Systematic Review. Diseases 2023, 11, 163. [Google Scholar] [CrossRef]
- Burnett, D.M.; Skinner, C.E. Year in Review: Long COVID and Pulmonary Rehabilitation. Respir. Care 2023, 68, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Pouliopoulou, D.V.; Macdermid, J.C.; Saunders, E.; Peters, S.; Brunton, L.; Miller, E.; Quinn, K.L.; Pereira, T.V.; Bobos, P. Rehabilitation Interventions for Physical Capacity and Quality of Life in Adults With Post-COVID-19 Condition: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2023, 6, e2333838. [Google Scholar] [CrossRef] [PubMed]
- Amro, M.; Mohamed, A.; Alawna, M. Effects of increasing aerobic capacity on improving psychological problems seen in patients with COVID-19: A review. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2808–2821. [Google Scholar] [CrossRef]
- Chuang, H.J.; Lin, C.W.; Hsiao, M.Y.; Wang, T.G.; Liang, H.W. Long COVID and rehabilitation. J. Formos. Med. Assoc. 2024, 123 (Suppl. S1), S61–S69. [Google Scholar] [CrossRef]
- Zheng, C.; Chen, X.K.; Sit, C.H.; Liang, X.; Li, M.H.; Ma, A.C.; Wong, S.H. Effect of Physical Exercise-Based Rehabilitation on Long COVID: A Systematic Review and Meta-analysis. Med. Sci. Sports Exerc. 2024, 56, 143–154. [Google Scholar] [CrossRef]
- Alba, G.A.; Ziehr, D.R.; Rouvina, J.N.; Hariri, L.P.; Knipe, R.S.; Medoff, B.D.; Hibbert, K.A.; Kowal, A.; Hoenstine, C.; Ginns, L.C.; et al. Exercise performance in patients with post-acute sequelae of SARS-CoV-2 infection compared to patients with unexplained dyspnea. eClinicalMedicine 2021, 39, 101066. [Google Scholar] [CrossRef]
- Huang, L.; Yao, Q.; Gu, X.; Wang, Q.; Ren, L.; Wang, Y.; Hu, P.; Guo, L.; Liu, M.; Xu, J.; et al. 1-year outcomes in hospital survivors with COVID-19: A longitudinal cohort study. Lancet 2021, 398, 747–758. [Google Scholar] [CrossRef]
- Durstenfeld, M.S.; Peluso, M.J.; Kaveti, P.; Hill, C.; Li, D.; Sander, E.; Swaminathan, S.; Arechiga, V.M.; Lu, S.; Goldberg, S.A.; et al. Reduced Exercise Capacity, Chronotropic Incompetence, and Early Systemic Inflammation in Cardiopulmonary Phenotype Long Coronavirus Disease 2019. J. Infect. Dis. 2023, 228, 542–554. [Google Scholar] [CrossRef]
- Besnier, F.; Malo, J.; Mohammadi, H.; Clavet, S.; Klai, C.; Martin, N.; Bérubé, B.; Lecchino, C.; Iglesies-Grau, J.; Vincent, T.; et al. Effects of Cardiopulmonary Rehabilitation on Cardiorespiratory Fitness and Clinical Symptom Burden in Long COVID: Results from the COVID-Rehab Randomized Controlled Trial. Am. J. Phys. Med. Rehabil. 2024. [Google Scholar] [CrossRef]
- Mooren, J.M.; Garbsch, R.; Schäfer, H.; Kotewitsch, M.; Waranski, M.; Teschler, M.; Schmitz, B.; Mooren, F.C. Medical Rehabilitation of Patients with Post-COVID-19 Syndrome-A Comparison of Aerobic Interval and Continuous Training. J. Clin. Med. 2023, 12, 6739. [Google Scholar] [CrossRef]
- Araújo, B.T.S.; Barros, A.; Nunes, D.T.X.; Remígio de Aguiar, M.I.; Mastroianni, V.W.; de Souza, J.A.F.; Fernades, J.; Campos, S.L.; Brandão, D.C.; Dornelas de Andrade, A. Effects of continuous aerobic training associated with resistance training on maximal and submaximal exercise tolerance, fatigue, and quality of life of patients post-COVID-19. Physiother. Res. Int. 2023, 28, e1972. [Google Scholar] [CrossRef] [PubMed]
- Jimeno-Almazán, A.; Buendía-Romero, Á.; Martínez-Cava, A.; Franco-López, F.; Sánchez-Alcaraz, B.J.; Courel-Ibáñez, J.; Pallarés, J.G. Effects of a concurrent training, respiratory muscle exercise, and self-management recommendations on recovery from post-COVID-19 conditions: The RECOVE trial. J. Appl. Physiol. 2023, 134, 95–104. [Google Scholar] [CrossRef] [PubMed]
- da Silveira, M.P.; da Silva Fagundes, K.K.; Bizuti, M.R.; Starck, É.; Rossi, R.C.; de Resende, E.S.D.T. Physical exercise as a tool to help the immune system against COVID-19: An integrative review of the current literature. Clin. Exp. Med. 2021, 21, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C. Exercise Is Medicine for Immune Function: Implication for COVID-19. Curr. Sports Med. Rep. 2021, 20, 395–401. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Alawna, M. Role of increasing the aerobic capacity on improving the function of immune and respiratory systems in patients with coronavirus (COVID-19): A review. Diabetes Metab. Syndr. 2020, 14, 489–496. [Google Scholar] [CrossRef]
- Jason, L.A.; Dorri, J.A. ME/CFS and Post-Exertional Malaise among Patients with Long COVID. Neurol. Int. 2022, 15, 1–11. [Google Scholar] [CrossRef]
- Choutka, J.A.-O.; Jansari, V.; Hornig, M.A.-O.; Iwasaki, A.A.-O. Unexplained post-acute infection syndromes. Nat. Med. 2023, 28, 911–923. [Google Scholar] [CrossRef]
- Lee, J.; Vernon, S.D.; Jeys, P.; Ali, W.; Campos, A.; Unutmaz, D.; Yellman, B.; Bateman, L. Hemodynamics during the 10-minute NASA Lean Test: Evidence of circulatory decompensation in a subset of ME/CFS patients. J. Transl. Med. 2020, 18, 314. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef]
- MacIntyre, N.; Crapo, R.O.; Viegi, G.; Johnson, D.C.; van der Grinten, C.P.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Enright, P.; et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur. Respir. J. 2005, 26, 720–735. [Google Scholar] [CrossRef]
- Wanger, J.; Clausen, J.L.; Coates, A.; Pedersen, O.F.; Brusasco, V.; Burgos, F.; Casaburi, R.; Crapo, R.; Enright, P.; van der Grinten, C.P.; et al. Standardisation of the measurement of lung volumes. Eur. Respir. J. 2005, 26, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.L.; Brusasco, V.; Burgos, F.; Cooper, B.G.; Jensen, R.; Kendrick, A.; MacIntyre, N.R.; Thompson, B.R.; Wanger, J. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur. Respir.J. 2017, 49, 1600016. [Google Scholar] [CrossRef] [PubMed]
- Hankinson, J.L.; Odencrantz, J.R.; Fedan, K.B. Spirometric reference values from a sample of the general U.S. population. Am. J. Respir. Crit. Care Med. 1999, 159, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Quanjer, P.H.; Tammeling, G.J.; Cotes, J.E.; Pedersen, O.F.; Peslin, R.; Yernault, J.C. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur. Respir. J.Suppl. 1993, 16, 5–40. [Google Scholar] [CrossRef]
- Cotes, J. Lung Function; Blackwell Scientific Publications: London, UK, 1979; pp. 225–250. [Google Scholar]
- Singh, S.J.; Puhan, M.A.; Andrianopoulos, V.; Hernandes, N.A.; Mitchell, K.E.; Hill, C.J.; Lee, A.L.; Camillo, C.A.; Troosters, T.; Spruit, M.A.; et al. An official systematic review of the European Respiratory Society/American Thoracic Society: Measurement properties of field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1447–1478. [Google Scholar] [CrossRef]
- Borg, S.A. Psychological Basis of Perceived Exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Beaver, W.L.; Wasserman, K.; Whipp, B.J. A new method for detecting the anaerobic threshold by gas exchange. J. Appl. Physiol. 1986, 60, 2020–2027. [Google Scholar] [CrossRef]
- Cao, M.; Stringer, W.W.; Corey, S.; Orogian, A.; Cao, R.; Calmelat, R.; Lin, F.; Casaburi, R.; Rossiter, H.B.; Porszasz, J. Transcutaneous PCO2 for Exercise Gas Exchange Efficiency in Chronic Obstructive Pulmonary Disease. COPD J. Chronic Obstr. Pulm. Dis. 2021, 18, 16–25. [Google Scholar] [CrossRef]
- Stringer, W.A.-O.; Porszasz, J.; Cao, M.; Rossiter, H.B.; Siddiqui, S.; Rennard, S.; Casaburi, R. The effect of long-acting dual bronchodilator therapy on exercise tolerance, dynamic hyperinflation, and dead space during constant work rate exercise in COPD. J. Appl. Physiol. 2021, 130, 2009–2018. [Google Scholar] [CrossRef]
- Wasserman, K.; Hansen, J.E.; Sue, D.Y.; Stringer, W.W.; Sietsema, K.; Sun MD, X.-G.; Whipp, B.J. Principles of Exercise Testing and Interpretation: Pathophysiology and Clinical Applications; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2012; Volume 3. [Google Scholar]
- Hasan, M.; Beitz, B.; Rouilly, V.; Libri, V.; Urrutia, A.; Duffy, D.; Cassard, L.; Di Santo, J.P.; Mottez, E.; Quintana-Murci, L.; et al. Semi-automated and standardized cytometric procedures for multi-panel and multi-parametric whole blood immunophenotyping. Clin. Immunol. 2015, 157, 261–276. [Google Scholar] [CrossRef]
- Gloeckl, R.; Zwick, R.H.; Fürlinger, U.; Schneeberger, T.; Leitl, D.; Jarosch, I.; Behrends, U.; Scheibenbogen, C.; Koczulla, A.R. Practical Recommendations for Exercise Training in Patients with Long COVID with or without Post-exertional Malaise: A Best Practice Proposal. Sports Med. Open 2024, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, G.I.; Febbraio, M.A. The immunomodulating role of exercise in metabolic disease. Trends Immunol. 2014, 35, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Koelwyn, G.J.; Wennerberg, E.; Demaria, S.; Jones, L.W. Exercise in Regulation of Inflammation-Immune Axis Function in Cancer Initiation and Progression. Oncology 2015, 29, 908–920, 922. [Google Scholar] [PubMed]
- Pedersen, B.K. Anti-inflammatory effects of exercise: Role in diabetes and cardiovascular disease. Eur. J. Clin. Investig. 2017, 47, 600–611. [Google Scholar] [CrossRef]
- Nieman, D.C.; Wentz, L.M. The compelling link between physical activity and the body’s defense system. J. Sport. Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef]
- Bigley, A.B.; Rezvani, K.; Chew, C.; Sekine, T.; Pistillo, M.; Crucian, B.; Bollard, C.M.; Simpson, R.J. Acute exercise preferentially redeploys NK-cells with a highly-differentiated phenotype and augments cytotoxicity against lymphoma and multiple myeloma target cells. Brain Behav. Immun. 2014, 39, 160–171. [Google Scholar] [CrossRef]
- LaVoy, E.C.; Bollard, C.M.; Hanley, P.J.; Blaney, J.W.; O’Connor, D.P.; Bosch, J.A.; Simpson, R.J. A single bout of dynamic exercise enhances the expansion of MAGE-A4 and PRAME-specific cytotoxic T-cells from healthy adults. Exerc. Immunol. Rev. 2015, 21, 144–153. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).