A Pilot Study on the Effects of Exercise Training on Cardiorespiratory Performance, Quality of Life, and Immunologic Variables in Long COVID
Abstract
1. Introduction
2. Methods and Materials
2.1. Study Design
2.2. Study Enrollment Criteria
2.3. Screening Visit
2.4. Cardiopulmonary Exercise Testing (CPET)
2.5. Overnight Oximetry
2.6. Patient Reported Outcomes
2.7. Physical Activity
2.8. Immunophenotyping and Cytokine Analysis
2.9. Exercise Training
2.10. Post-Training Assessments
2.11. Statistical Analyses
3. Results
3.1. Participants
3.2. Demographics
3.3. Exercise Training Compliance
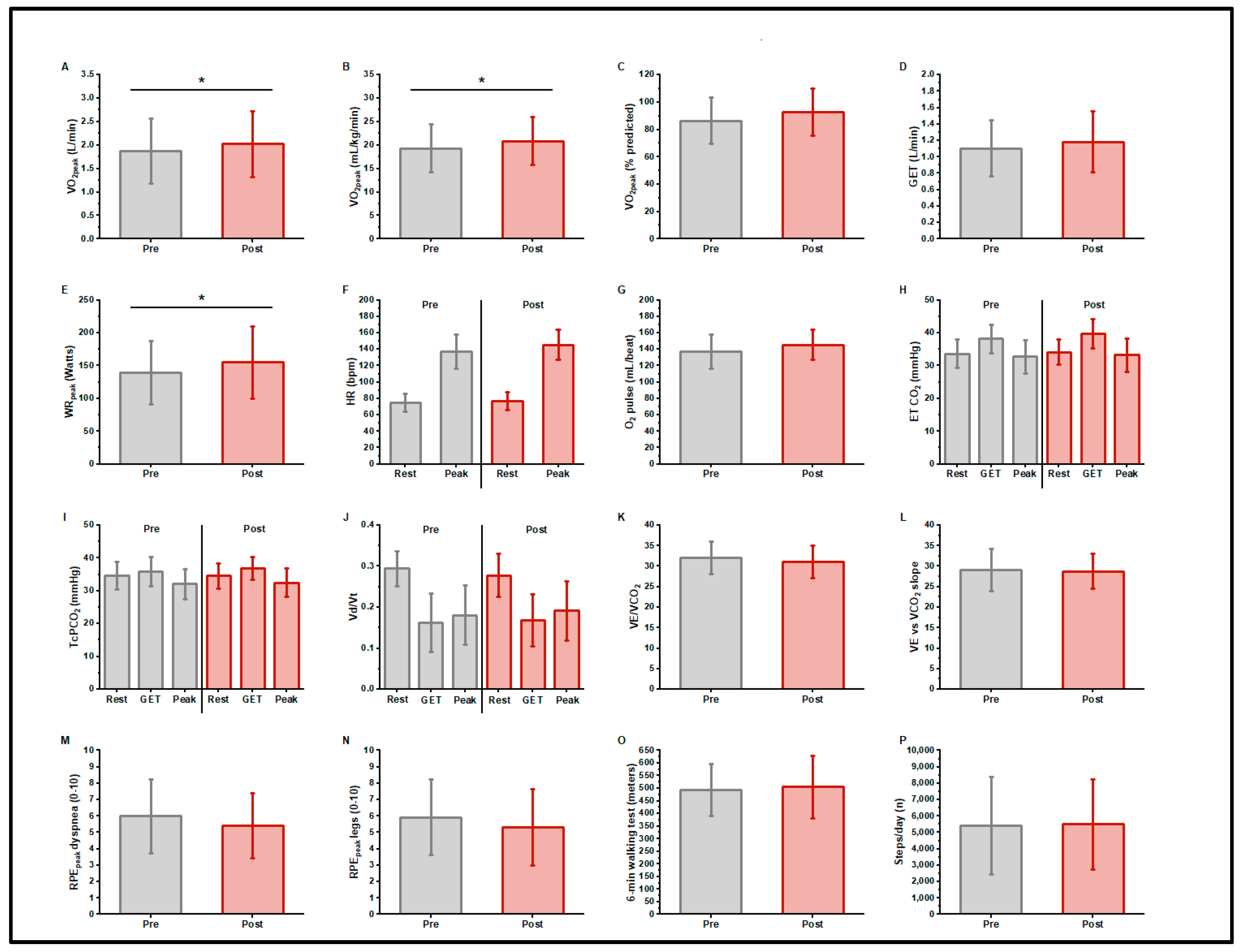
3.4. CPET Pre- and Post-Training
3.5. 6MWD
3.6. 7-Day Activity Monitoring
3.7. Patient-Reported Outcomes/Questionnaires
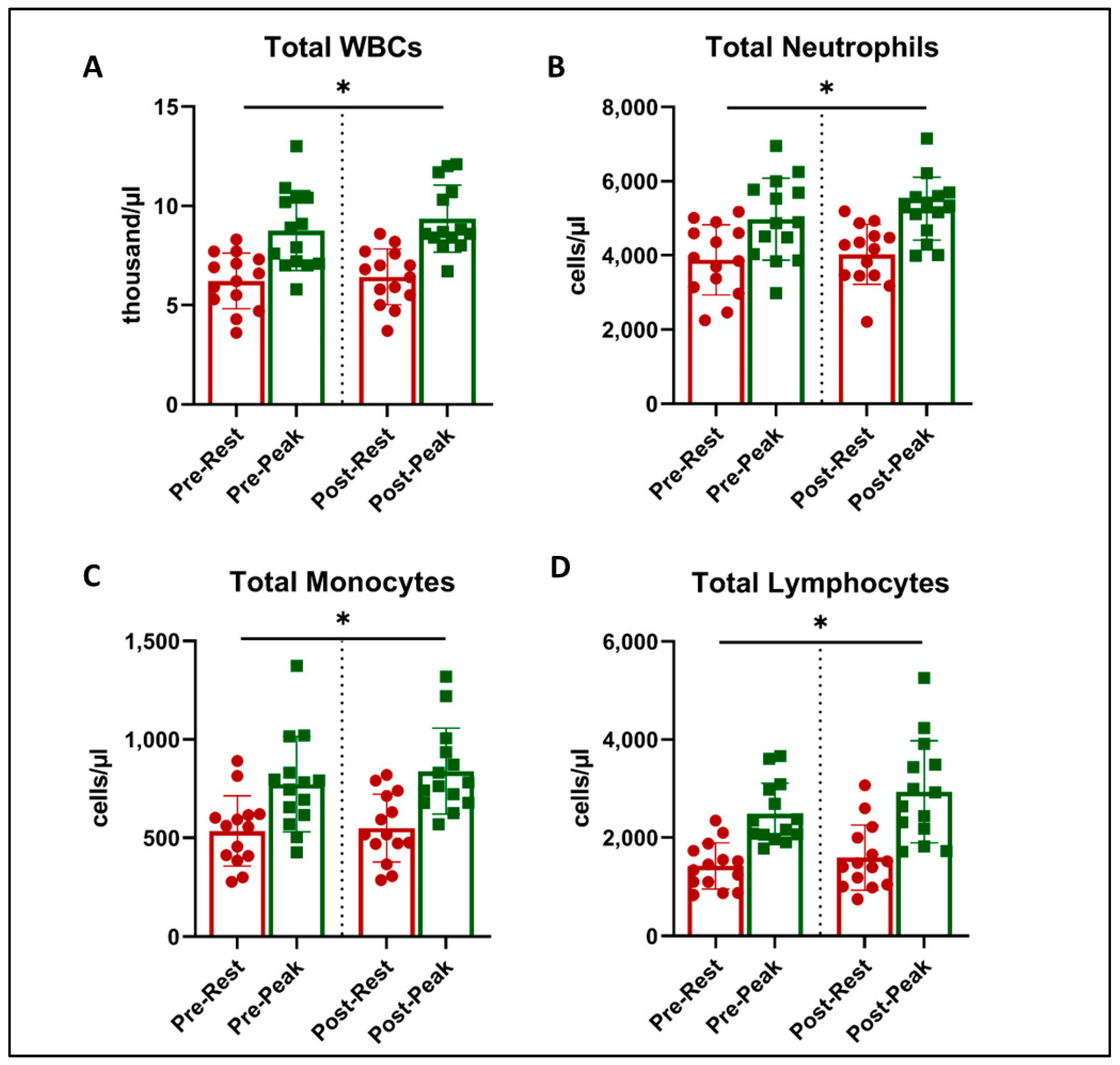
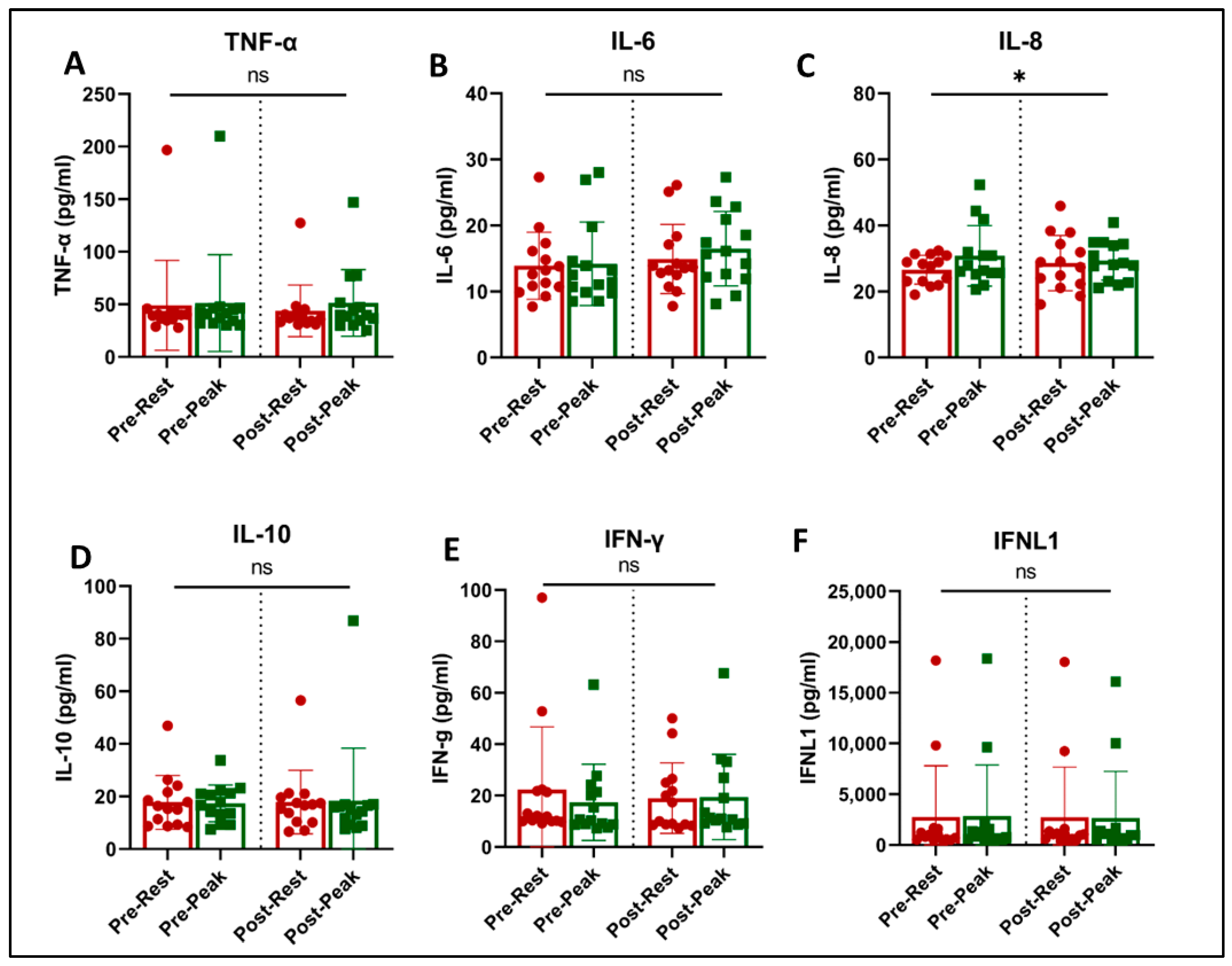
4. Immunologic Measurements
4.1. White Blood Cell (WBC) Numbers
4.2. Immune Cell Subsets
4.3. Plasma Biomarkers and Cytokines
5. Discussion
6. Strengths and Limitations
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borges do Nascimento, I.J.; O’Mathúna, D.P.; von Groote, T.C.; Abdulazeem, H.M.; Weerasekara, I.; Marusic, A.; Puljak, L.; Civile, V.T.; Zakarija-Grkovic, I.; Pericic, T.P.; et al. Coronavirus disease (COVID-19) pandemic: An overview of systematic reviews. BMC Infect. Dis. 2021, 21, 525. [Google Scholar] [CrossRef] [PubMed]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.A.-O.; McCorkell, L.A.-O.; Vogel, J.A.-O.; Topol, E.A.-O. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long covid-mechanisms, risk factors, and management. BMJ 2021, 374, n1648. [Google Scholar] [CrossRef] [PubMed]
- Briggs, A.; Vassall, A. Count the cost of disability caused by COVID-19. Nature 2021, 593, 502–505. [Google Scholar] [CrossRef]
- Astin, R.; Banerjee, A.; Baker, M.R.; Dani, M.; Ford, E.; Hull, J.H.; Lim, P.B.; McNarry, M.; Morten, K.; O’Sullivan, O.; et al. Long COVID: Mechanisms, risk factors and recovery. Exp. Physiol. 2023, 108, 12–27. [Google Scholar] [CrossRef]
- Singh, S.J.; Baldwin, M.M.; Daynes, E.; Evans, R.A.; Greening, N.J.; Jenkins, R.G.; Lone, N.I.; McAuley, H.; Mehta, P.; Newman, J.; et al. Respiratory sequelae of COVID-19: Pulmonary and extrapulmonary origins, and approaches to clinical care and rehabilitation. Lancet Respir. Med. 2023, 11, 709–725. [Google Scholar] [CrossRef]
- Chen, H.; Shi, H.; Liu, X.; Sun, T.; Wu, J.; Liu, Z. Effect of Pulmonary Rehabilitation for Patients With Post-COVID-19: A Systematic Review and Meta-Analysis. Front. Med. 2022, 9, 837420. [Google Scholar] [CrossRef]
- Spielmanns, M.; Pekacka-Egli, A.M.; Schoendorf, S.; Windisch, W.; Hermann, M. Effects of a Comprehensive Pulmonary Rehabilitation in Severe Post-COVID-19 Patients. Int. J. Environ. Res. Public. Health 2021, 18, 2695. [Google Scholar] [CrossRef]
- Sánchez-García, J.C.; Reinoso-Cobo, A.; Piqueras-Sola, B.; Cortés-Martín, J.; Menor-Rodríguez, M.J.; Alabau-Dasi, R.; Rodríguez-Blanque, R. Long COVID and Physical Therapy: A Systematic Review. Diseases 2023, 11, 163. [Google Scholar] [CrossRef]
- Burnett, D.M.; Skinner, C.E. Year in Review: Long COVID and Pulmonary Rehabilitation. Respir. Care 2023, 68, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Pouliopoulou, D.V.; Macdermid, J.C.; Saunders, E.; Peters, S.; Brunton, L.; Miller, E.; Quinn, K.L.; Pereira, T.V.; Bobos, P. Rehabilitation Interventions for Physical Capacity and Quality of Life in Adults With Post-COVID-19 Condition: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2023, 6, e2333838. [Google Scholar] [CrossRef] [PubMed]
- Amro, M.; Mohamed, A.; Alawna, M. Effects of increasing aerobic capacity on improving psychological problems seen in patients with COVID-19: A review. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2808–2821. [Google Scholar] [CrossRef]
- Chuang, H.J.; Lin, C.W.; Hsiao, M.Y.; Wang, T.G.; Liang, H.W. Long COVID and rehabilitation. J. Formos. Med. Assoc. 2024, 123 (Suppl. S1), S61–S69. [Google Scholar] [CrossRef]
- Zheng, C.; Chen, X.K.; Sit, C.H.; Liang, X.; Li, M.H.; Ma, A.C.; Wong, S.H. Effect of Physical Exercise-Based Rehabilitation on Long COVID: A Systematic Review and Meta-analysis. Med. Sci. Sports Exerc. 2024, 56, 143–154. [Google Scholar] [CrossRef]
- Alba, G.A.; Ziehr, D.R.; Rouvina, J.N.; Hariri, L.P.; Knipe, R.S.; Medoff, B.D.; Hibbert, K.A.; Kowal, A.; Hoenstine, C.; Ginns, L.C.; et al. Exercise performance in patients with post-acute sequelae of SARS-CoV-2 infection compared to patients with unexplained dyspnea. eClinicalMedicine 2021, 39, 101066. [Google Scholar] [CrossRef]
- Huang, L.; Yao, Q.; Gu, X.; Wang, Q.; Ren, L.; Wang, Y.; Hu, P.; Guo, L.; Liu, M.; Xu, J.; et al. 1-year outcomes in hospital survivors with COVID-19: A longitudinal cohort study. Lancet 2021, 398, 747–758. [Google Scholar] [CrossRef]
- Durstenfeld, M.S.; Peluso, M.J.; Kaveti, P.; Hill, C.; Li, D.; Sander, E.; Swaminathan, S.; Arechiga, V.M.; Lu, S.; Goldberg, S.A.; et al. Reduced Exercise Capacity, Chronotropic Incompetence, and Early Systemic Inflammation in Cardiopulmonary Phenotype Long Coronavirus Disease 2019. J. Infect. Dis. 2023, 228, 542–554. [Google Scholar] [CrossRef]
- Besnier, F.; Malo, J.; Mohammadi, H.; Clavet, S.; Klai, C.; Martin, N.; Bérubé, B.; Lecchino, C.; Iglesies-Grau, J.; Vincent, T.; et al. Effects of Cardiopulmonary Rehabilitation on Cardiorespiratory Fitness and Clinical Symptom Burden in Long COVID: Results from the COVID-Rehab Randomized Controlled Trial. Am. J. Phys. Med. Rehabil. 2024. [Google Scholar] [CrossRef]
- Mooren, J.M.; Garbsch, R.; Schäfer, H.; Kotewitsch, M.; Waranski, M.; Teschler, M.; Schmitz, B.; Mooren, F.C. Medical Rehabilitation of Patients with Post-COVID-19 Syndrome-A Comparison of Aerobic Interval and Continuous Training. J. Clin. Med. 2023, 12, 6739. [Google Scholar] [CrossRef]
- Araújo, B.T.S.; Barros, A.; Nunes, D.T.X.; Remígio de Aguiar, M.I.; Mastroianni, V.W.; de Souza, J.A.F.; Fernades, J.; Campos, S.L.; Brandão, D.C.; Dornelas de Andrade, A. Effects of continuous aerobic training associated with resistance training on maximal and submaximal exercise tolerance, fatigue, and quality of life of patients post-COVID-19. Physiother. Res. Int. 2023, 28, e1972. [Google Scholar] [CrossRef] [PubMed]
- Jimeno-Almazán, A.; Buendía-Romero, Á.; Martínez-Cava, A.; Franco-López, F.; Sánchez-Alcaraz, B.J.; Courel-Ibáñez, J.; Pallarés, J.G. Effects of a concurrent training, respiratory muscle exercise, and self-management recommendations on recovery from post-COVID-19 conditions: The RECOVE trial. J. Appl. Physiol. 2023, 134, 95–104. [Google Scholar] [CrossRef] [PubMed]
- da Silveira, M.P.; da Silva Fagundes, K.K.; Bizuti, M.R.; Starck, É.; Rossi, R.C.; de Resende, E.S.D.T. Physical exercise as a tool to help the immune system against COVID-19: An integrative review of the current literature. Clin. Exp. Med. 2021, 21, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C. Exercise Is Medicine for Immune Function: Implication for COVID-19. Curr. Sports Med. Rep. 2021, 20, 395–401. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Alawna, M. Role of increasing the aerobic capacity on improving the function of immune and respiratory systems in patients with coronavirus (COVID-19): A review. Diabetes Metab. Syndr. 2020, 14, 489–496. [Google Scholar] [CrossRef]
- Jason, L.A.; Dorri, J.A. ME/CFS and Post-Exertional Malaise among Patients with Long COVID. Neurol. Int. 2022, 15, 1–11. [Google Scholar] [CrossRef]
- Choutka, J.A.-O.; Jansari, V.; Hornig, M.A.-O.; Iwasaki, A.A.-O. Unexplained post-acute infection syndromes. Nat. Med. 2023, 28, 911–923. [Google Scholar] [CrossRef]
- Lee, J.; Vernon, S.D.; Jeys, P.; Ali, W.; Campos, A.; Unutmaz, D.; Yellman, B.; Bateman, L. Hemodynamics during the 10-minute NASA Lean Test: Evidence of circulatory decompensation in a subset of ME/CFS patients. J. Transl. Med. 2020, 18, 314. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef]
- MacIntyre, N.; Crapo, R.O.; Viegi, G.; Johnson, D.C.; van der Grinten, C.P.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Enright, P.; et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur. Respir. J. 2005, 26, 720–735. [Google Scholar] [CrossRef]
- Wanger, J.; Clausen, J.L.; Coates, A.; Pedersen, O.F.; Brusasco, V.; Burgos, F.; Casaburi, R.; Crapo, R.; Enright, P.; van der Grinten, C.P.; et al. Standardisation of the measurement of lung volumes. Eur. Respir. J. 2005, 26, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.L.; Brusasco, V.; Burgos, F.; Cooper, B.G.; Jensen, R.; Kendrick, A.; MacIntyre, N.R.; Thompson, B.R.; Wanger, J. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur. Respir.J. 2017, 49, 1600016. [Google Scholar] [CrossRef] [PubMed]
- Hankinson, J.L.; Odencrantz, J.R.; Fedan, K.B. Spirometric reference values from a sample of the general U.S. population. Am. J. Respir. Crit. Care Med. 1999, 159, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Quanjer, P.H.; Tammeling, G.J.; Cotes, J.E.; Pedersen, O.F.; Peslin, R.; Yernault, J.C. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur. Respir. J.Suppl. 1993, 16, 5–40. [Google Scholar] [CrossRef]
- Cotes, J. Lung Function; Blackwell Scientific Publications: London, UK, 1979; pp. 225–250. [Google Scholar]
- Singh, S.J.; Puhan, M.A.; Andrianopoulos, V.; Hernandes, N.A.; Mitchell, K.E.; Hill, C.J.; Lee, A.L.; Camillo, C.A.; Troosters, T.; Spruit, M.A.; et al. An official systematic review of the European Respiratory Society/American Thoracic Society: Measurement properties of field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1447–1478. [Google Scholar] [CrossRef]
- Borg, S.A. Psychological Basis of Perceived Exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Beaver, W.L.; Wasserman, K.; Whipp, B.J. A new method for detecting the anaerobic threshold by gas exchange. J. Appl. Physiol. 1986, 60, 2020–2027. [Google Scholar] [CrossRef]
- Cao, M.; Stringer, W.W.; Corey, S.; Orogian, A.; Cao, R.; Calmelat, R.; Lin, F.; Casaburi, R.; Rossiter, H.B.; Porszasz, J. Transcutaneous PCO2 for Exercise Gas Exchange Efficiency in Chronic Obstructive Pulmonary Disease. COPD J. Chronic Obstr. Pulm. Dis. 2021, 18, 16–25. [Google Scholar] [CrossRef]
- Stringer, W.A.-O.; Porszasz, J.; Cao, M.; Rossiter, H.B.; Siddiqui, S.; Rennard, S.; Casaburi, R. The effect of long-acting dual bronchodilator therapy on exercise tolerance, dynamic hyperinflation, and dead space during constant work rate exercise in COPD. J. Appl. Physiol. 2021, 130, 2009–2018. [Google Scholar] [CrossRef]
- Wasserman, K.; Hansen, J.E.; Sue, D.Y.; Stringer, W.W.; Sietsema, K.; Sun MD, X.-G.; Whipp, B.J. Principles of Exercise Testing and Interpretation: Pathophysiology and Clinical Applications; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2012; Volume 3. [Google Scholar]
- Hasan, M.; Beitz, B.; Rouilly, V.; Libri, V.; Urrutia, A.; Duffy, D.; Cassard, L.; Di Santo, J.P.; Mottez, E.; Quintana-Murci, L.; et al. Semi-automated and standardized cytometric procedures for multi-panel and multi-parametric whole blood immunophenotyping. Clin. Immunol. 2015, 157, 261–276. [Google Scholar] [CrossRef]
- Gloeckl, R.; Zwick, R.H.; Fürlinger, U.; Schneeberger, T.; Leitl, D.; Jarosch, I.; Behrends, U.; Scheibenbogen, C.; Koczulla, A.R. Practical Recommendations for Exercise Training in Patients with Long COVID with or without Post-exertional Malaise: A Best Practice Proposal. Sports Med. Open 2024, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, G.I.; Febbraio, M.A. The immunomodulating role of exercise in metabolic disease. Trends Immunol. 2014, 35, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Koelwyn, G.J.; Wennerberg, E.; Demaria, S.; Jones, L.W. Exercise in Regulation of Inflammation-Immune Axis Function in Cancer Initiation and Progression. Oncology 2015, 29, 908–920, 922. [Google Scholar] [PubMed]
- Pedersen, B.K. Anti-inflammatory effects of exercise: Role in diabetes and cardiovascular disease. Eur. J. Clin. Investig. 2017, 47, 600–611. [Google Scholar] [CrossRef]
- Nieman, D.C.; Wentz, L.M. The compelling link between physical activity and the body’s defense system. J. Sport. Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef]
- Bigley, A.B.; Rezvani, K.; Chew, C.; Sekine, T.; Pistillo, M.; Crucian, B.; Bollard, C.M.; Simpson, R.J. Acute exercise preferentially redeploys NK-cells with a highly-differentiated phenotype and augments cytotoxicity against lymphoma and multiple myeloma target cells. Brain Behav. Immun. 2014, 39, 160–171. [Google Scholar] [CrossRef]
- LaVoy, E.C.; Bollard, C.M.; Hanley, P.J.; Blaney, J.W.; O’Connor, D.P.; Bosch, J.A.; Simpson, R.J. A single bout of dynamic exercise enhances the expansion of MAGE-A4 and PRAME-specific cytotoxic T-cells from healthy adults. Exerc. Immunol. Rev. 2015, 21, 144–153. [Google Scholar]

| Demographics | n = 14 Finished Training | n = 7 (Did Not Continue Study) | ||
|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | |
| Age (years) ± SD | 53.5 | 11.6 | 60.3 | 6.2 |
| % Female (n, %) | 6/43 | 5/71 * | ||
| Race (Asian/Black/Caucasian) % | 7/0/93 | 0/0/100 | ||
| Ethnicity (Non-Hispanic/Hispanic) % | 57/43 | 71/29 | ||
| Height (cm) | 174 | 10.6 | 167 | 15.5 |
| Weight (kg) | 98 | 26.4 | 83 | 28.0 |
| BMI (kg/m2) | 32.5 | 8.4 | 29.5 | 8.4 |
| Smoking Status (Never/Ex) % | 58/42 | 86/14 * | ||
| Time Since Initial COVID Infection (Months) | 15.1 | 8.8 | 21.7 | 12.0 |
| Severity of COVID Infection (Non Hospitalized, n, %) | 11/79 | 7/100 * | ||
| Systolic BP (mmHg) | 129 | 14 | 128 | 18 |
| Diastolic MB (mmHg) | 73 | 10 | 79 | 9 |
| Activity Level at Study Entry (Sedentary/Walking/Regular Workouts) % | 29/57/14 | 43/43/14 | ||
| Primary Long COVID Symptoms (Inclusion Criteria) | n | % | n | % |
| Fatigue | 12 | 86 | 7 * | 100 |
| Dyspnea | 5 | 36 | 1 | 14 |
| Exercise Intolerance | 6 | 46 | 4 | 57 |
| Post-Exertional Malaise | 8 | 57 | 4 | 57 |
| Difficulty Breathing | 5 | 36 | 1 | 14 |
| Brain Fog | 14 | 100 | 7 | 100 |
| Screening Laboratories (Normal Range) | Male/Female Mean | Male/Female S.D. | Male/Female Mean | Male/Female S.D. |
| Hemoglobin (Range: male 13.2–17.1, female 117–15.5 gm/dL) | 14.9/13.4 | 1.1/0.9 | 16.3/14.0 | 0.6/1.0 |
| Hematocrit (male 38.5–50%, female 35–45%) | 44.1/39.3 | 3.0/2.6 | 47.7/41.8 | 1.8/2.6 |
| AST (10–30 U/L) | 20 | 6.4 | 30 | 29.3 |
| ALT (6–29 U/L) | 24 | 15.2 | 47 | 59.9 |
| Bilirubin (0.2–1.2 mg/dL) | 0.6 | 0.3 | 0.7 | 0.4 |
| Creatinine (Range: F 0.5–1.1 mg/dL. M 0.7–1.3 mg/dL) | 0.9 | 0.2 | 0.8 | 0.2 |
| D-Dimer (normal < 0.5 mcg/mL) | 0.44 | 0.18 | 1.15 * | 0.96 |
| Ferritin (38–280 ng/mL) | 105 | 95 | 74 | 48 |
| CRP (normal < 8.0 ng/mL) | 4.3 | 5.8 | 8.1 | 9.5 |
| Pro-BNP (normal < 253 pg/mL) | 43 | 49 | 132 | 181 |
| High-Sensitivty Troponin (normal < 15 ng/L) | 6.8 | 2.2 | 6.6 | 1.8 |
| Pulmonary Function Testing | Mean | S.D. | Mean | S.D. |
| FEV1 (L) | 3.11 | 0.69 | 2.61 | 0.99 |
| FEV1 (% predicted) | 94 | 19 | 91 | 14 |
| FEV1 (% change with BD) | 5 | 5 | 7 | 6 |
| FVC (L) | 3.89 | 0.85 | 3.41 | 1.36 |
| FVC (% predicted) | 92 | 16 | 92 | 10 |
| FEV1/FVC | 80 | 8 | 77 | 9 |
| TLC (L) | 5.42 | 1.11 | 5.43 | 1.76 |
| TLC (% predicted) | 86 | 12 | 94 | 9 |
| DLCO (mL∙min−1∙mmHg−1) | 23.7 | 5.9 | 22.0 | 7.2 |
| DLCO (% predicted) | 93 | 18 | 96 | 10 |
| 6-Minute Walk Distance (m) | 498 | 105 | 436 | 140 |
| Exercise and Activity Responses to Training (N = 14) | |||||||
|---|---|---|---|---|---|---|---|
| Value | Pre | S.D. | Post | S.D. | Absolute Change | % Change | p Value |
| CPET | |||||||
| Peak Oxygen Uptake | |||||||
| Absolute (L/min) | 1.88 | 0.69 | 2.03 | 0.70 | 0.15 ± 0.20 | 7.4% | 0.017 |
| mL/Kg/min | 19.3 | 5.1 | 20.9 | 5.1 | 1.55 ± 2.4 | 7.4% | 0.030 |
| % Predicted | 87 | 17 | 93 | 17 | 6 ± 1.0 | 6.7 | 0.815 |
| Gas Exchange LAT (GELAT, L/min) | 1.11 | 0.34 | 1.18 | 0.37 | 0.06 ± 0.19 | 5.1% | 0.250 |
| AT as a % of Peak O2 | 59 | 58 | |||||
| Peak Work Rate (W) | 139 | 49 | 155 | 55 | 16 ± 20 | 10.3% | 0.010 |
| Resting Heart Rate (b/min) | 75 | 11 | 77 | 11 | 2 ± 8 | 2.6% | 0.421 |
| Peak Heart Rate (b/min) | 137 | 21 | 146 | 18 | 9 ± 15 | 6.5% | 0.052 |
| % Predicted Peak Heart Rate | 83% | 14% | 88% | 10% | |||
| Peak O2 Pulse (mL/beat) | 13.9 | 5.2 | 14.1 | 5.1 | 0.11 ± 2.1 | 0.7% | 0.844 |
| Rest ET CO2 (mmHg) | 33.6 | 4.3 | 34.2 | 3.9 | 0.6 ± 2.6 | 1.8% | 0.445 |
| GE-LAT ET CO2 (mmHg) | 38.2 | 4.4 | 39.7 | 4.5 | 1.5 ± 2.0 | 3.9% | 0.017 |
| Peak ETCO2 (mmHg) | 32.7 | 5.0 | 33.2 | 5.0 | 0.5 ± 2.3 | 1.5% | 0.457 |
| Rest TcPCO2 (mmHg) | 34.7 | 4.2 | 34.6 | 3.8 | 0.08 ± 2.28 | 0.2% | 0.899 |
| GE-LAT TcPCO2 | 35.8 | 4.4 | 36.8 | 3.4 | 1.0 ± 2.21 | 2.7% | 0.120 |
| Peak TcPCO2 (mmHg) | 32.1 | 4.6 | 32.6 | 4.4 | 0.4 ± 2.05 | 1.2% | 0.464 |
| Vd/Vt at Rest | 0.29 | 0.04 | 0.28 | 0.05 | 0.016 ± 0.05 | 5.5% | 0.249 |
| Vd/Vt at GE-LAT | 0.16 | 0.07 | 0.17 | 0.06 | 0.006 ± 0.043 | 3.7% | 0.628 |
| Vd/Vt at Peak | 0.18 | 0.07 | 0.19 | 0.07 | 0.01 ± 0.05 | 5.5% | 0.437 |
| E/CO2 @ LAT | 32.0 | 3.9 | 31.1 | 3.9 | 0.9 ± 2.4 | 2.8% | 0.176 |
| E to CO2 slope | 29.1 | 5.1 | 28.7 | 4.2 | 0.37 ± 3.35 | 1.2% | 0.685 |
| Peak RPE1dyspnea | 6.0 | 2.3 | 5.4 | 2.0 | 0.6 ± 1.9 | 10.1% | 0.293 |
| Peak RPE2leg | 5.9 | 2.3 | 5.3 | 2.3 | 0.6 ± 2.3 | 10.0% | 0.342 |
| 6MW Distance | 498 | 105 | 505 | 123 | 8 ± 57 | 1% | 0.619 |
| Activity Monitoring (7 days) | |||||||
| Steps/Day (n) | 5425 | 2960 | 5505 | 2756 | 79 ± 1829 | 1.5% | 0.437 |
| Total Estimated Energy Expenditure (Kcal/24 h) | 2758 | 684 | 2772 | 601 | 14 ± 243 | −1.2% | 0.416 |
| Sedentary Time (hours/minutes) | 18:16 | 3:06 | 18:29 | 3:01 | −0:04 | −0.2% | 0.357 |
| Light Activity (hours/minutes) | 1:15 | 0:30 | 1:15 | 0:33 | 0:00 | 0.6% | 0.173 |
| Moderate Activity (hours/minutes) | 1:09 | 0:35 | 1:03 | 0:22 | −0:03 | −0.2% | 0.241 |
| Vigorous Activity (hours/minutes) | 0:10 | 0:12 | 0:25 | 0:44 | 0:18 | 164.4% | 0.113 |
| Patient-Reported Outcomes | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Absolute Change | Percent Change | Change Category | MCID | p Value | |||
| Mean | S.D. | Mean | S.D. | ||||||
| SF-36 (Higher is Better) | |||||||||
| Physical Functioning | 40 | 21 | 64 | 22 | 24 ± 6.5 | 60% | M | S 10, M 20, L 30 | 0.003 |
| Energy/Fatigue | 24 | 17 | 39 | 23 | 15 ± 15 | 63% | S | S 12.5, M 25, L 37.5 | 0.002 |
| Emotional Well-Being | 53 | 25 | 71 | 18 | 18 ± 13 | 34% | M | S 8.3, M 16.7, L 25 | <0.001 |
| Social Functioning | 38 | 30 | 58 | 28 | 20 ± 12 | 52% | S | S 12.5, M 25, L 37.5 | <0.001 |
| Pain | 53 | 27 | 63 | 22 | 10 ± 18 | 18% | S | S 10, M 20, L 27.5 | 0.072 |
| General Health | 38 | 18 | 51 | 23 | 14 ± 18 | 37% | S | S 10, M 20, L 30 | 0.010 |
| Median | IQR | Median | IQR | Change Category | MCID (S = Small, M = Moderate, L-Large) | p Value | |||
| Role Limitations—Physical | 0 | 0 | 25 | 75 | M | S 12.5, M 25, L 37.5 | 0.070 | ||
| Role Limitations—Emotional | 0 | 100 | 33 | 100 | S | S 8.3, M 16.7, L 25 | 0.125 | ||
| PHQ-9 (Depression, Lower is Better) | 12 | 7 | 7 | 6 | −5 ± 4 | −42% | Moderate- > Mild | 5 = Mild, 10 = Moderate, 15 = Mod. Severe, and 20 = Severe) | 0.001 |
| GAD-7 (Anxiety, Lower is Better) | 7 | 6 | 5 | 5 | −2 ± 4.7 | −29% | Mild- > Mild | 0–4, Minimal, 5–10 Mild, 10–14 Moderate, and 15–21 Severe Anxiety | 0.125 |
| Median | IQR | Median | IQR | Change Category | MCID | p Value | |||
| Fatigue Severity Score (Lower is Better) | 53.5 | 14.5 | 44.5 | 32.2 | At MCID | 5–11 | 0.177 | ||
| mMRC (Lower is Better) | 2 | 1 | 0.5 | 2 | Below MCID | 1 unit | 0.015 | ||
| MMSE (Higher is Better) | 28 | 2 | 29 | 2 | Below MCID | 1.5 to 2.0 | 0.010 | ||
| Post-COVID-19 Functional Status (Lower is Better) | 2.5 | 1 | 2 | 2.25 | - | Not established | 0.020 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbasi, A.; Gattoni, C.; Iacovino, M.; Ferguson, C.; Tosolini, J.; Singh, A.; Soe, K.K.; Porszasz, J.; Lanks, C.; Rossiter, H.B.; et al. A Pilot Study on the Effects of Exercise Training on Cardiorespiratory Performance, Quality of Life, and Immunologic Variables in Long COVID. J. Clin. Med. 2024, 13, 5590. https://doi.org/10.3390/jcm13185590
Abbasi A, Gattoni C, Iacovino M, Ferguson C, Tosolini J, Singh A, Soe KK, Porszasz J, Lanks C, Rossiter HB, et al. A Pilot Study on the Effects of Exercise Training on Cardiorespiratory Performance, Quality of Life, and Immunologic Variables in Long COVID. Journal of Clinical Medicine. 2024; 13(18):5590. https://doi.org/10.3390/jcm13185590
Chicago/Turabian StyleAbbasi, Asghar, Chiara Gattoni, Michelina Iacovino, Carrie Ferguson, Jacqueline Tosolini, Ashrita Singh, Kyaw Khaing Soe, Janos Porszasz, Charles Lanks, Harry B. Rossiter, and et al. 2024. "A Pilot Study on the Effects of Exercise Training on Cardiorespiratory Performance, Quality of Life, and Immunologic Variables in Long COVID" Journal of Clinical Medicine 13, no. 18: 5590. https://doi.org/10.3390/jcm13185590
APA StyleAbbasi, A., Gattoni, C., Iacovino, M., Ferguson, C., Tosolini, J., Singh, A., Soe, K. K., Porszasz, J., Lanks, C., Rossiter, H. B., Casaburi, R., & Stringer, W. W. (2024). A Pilot Study on the Effects of Exercise Training on Cardiorespiratory Performance, Quality of Life, and Immunologic Variables in Long COVID. Journal of Clinical Medicine, 13(18), 5590. https://doi.org/10.3390/jcm13185590







