Immune-Molecular Link between Thyroid and Skin Autoimmune Diseases: A Narrative Review
Abstract
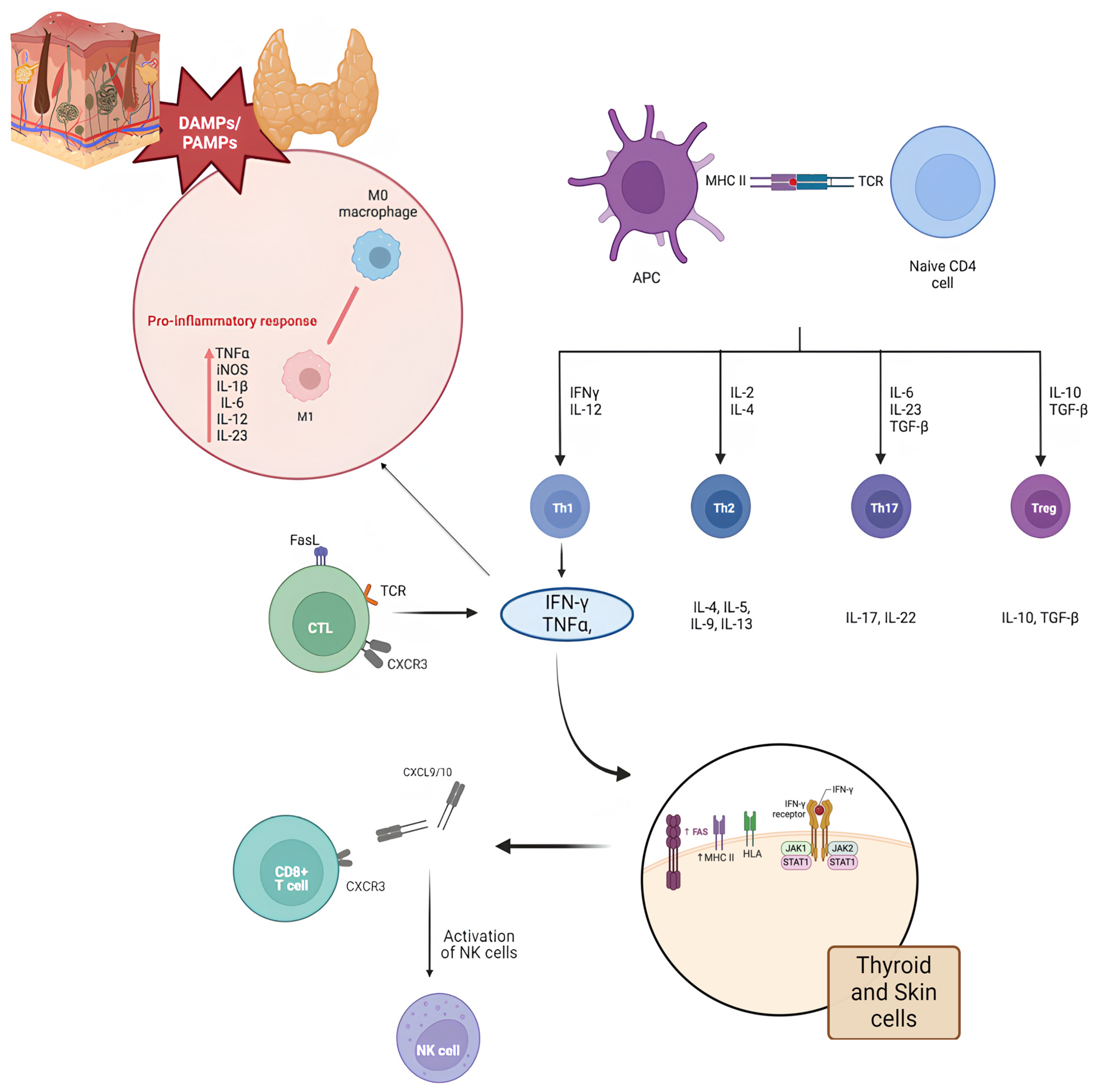
:1. Introduction
2. Discussion
2.1. Psoriasis
2.2. Lichen Planus
2.3. Atopic Dermatitis
2.4. Alopecia Areata
2.5. Vitiligo
- IFNG: encodes IFN-γ, an inflammatory cytokine that activates immune cells.
- STAT1: encodes a signaling protein involved in the IFN-γ response.
- IL1B: encodes interleukin-1 beta, a pro-inflammatory cytokine that recruits immune cells.
- CXCL10: encodes the chemokine CXCL10, which attracts T cells.
2.6. The Role of Immune Checkpoint Inhibitors (ICIs)
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bagnasco, M.; Minciullo, P.L.; Schiavo, M.; Saraceno, G.; Gangemi, S.; Benvenga, S. Urticaria and Thyroid Autoimmunity. Thyroid 2011, 21, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Bracken, S.J.; Abraham, S.; MacLeod, A.S. Autoimmune Theories of Chronic Spontaneous Urticaria. Front. Immunol. 2019, 10, 627. [Google Scholar] [CrossRef]
- Plouhinec, J.-L.; Medina-Ruiz, S.; Borday, C.; Bernard, E.; Vert, J.-P.; Eisen, M.B.; Harland, R.M.; Monsoro-Burq, A.H. A Molecular Atlas of the Developing Ectoderm Defines Neural, Neural Crest, Placode, and Nonneural Progenitor Identity in Vertebrates. PLoS Biol. 2017, 15, e2004045. [Google Scholar] [CrossRef] [PubMed]
- Conrad, C.; Gilliet, M. Psoriasis: From Pathogenesis to Targeted Therapies. Clin. Rev. Allergy Immunol. 2018, 54, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Menter, A.; Krueger, G.G.; Paek, S.Y.; Kivelevitch, D.; Adamopoulos, I.E.; Langley, R.G. Interleukin-17 and Interleukin-23: A Narrative Review of Mechanisms of Action in Psoriasis and Associated Comorbidities. Dermatol. Ther. 2021, 11, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Delle Sedie, A.; Fallahi, P.; Ferrari, S.M.; Maccheroni, M.; Ferrannini, E.; Bombardieri, S.; Riente, L. High Prevalence of Thyroid Autoimmunity and Hypothyroidism in Patients with Psoriatic Arthritis. J. Rheumatol. 2006, 33, 2026–2028. [Google Scholar]
- Fallahi, P.; Ferrari, S.M.; Ruffilli, I.; Elia, G.; Miccoli, M.; Sedie, A.D.; Riente, L.; Antonelli, A. Increased Incidence of Autoimmune Thyroid Disorders in Patients with Psoriatic Arthritis: A Longitudinal Follow-up Study. Immunol. Res. 2017, 65, 681–686. [Google Scholar] [CrossRef]
- Gul, U.; Gonul, M.; Kaya, I.; Aslan, E. Autoimmune Thyroid Disorders in Patients with Psoriasis. Eur. J. Dermatol. EJD 2009, 19, 221–223. [Google Scholar] [CrossRef]
- Vassilatou, E.; Papadavid, E.; Papastamatakis, P.; Alexakos, D.; Koumaki, D.; Katsimbri, P.; Hadjidakis, D.; Dimitriadis, G.; Rigopoulos, D. No Association of Psoriasis with Autoimmune Thyroiditis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 102–106. [Google Scholar] [CrossRef]
- Alidrisi, H.A.; Al Hamdi, K.; Mansour, A.A. Is There Any Association Between Psoriasis and Hashimoto’s Thyroiditis? Cureus 2019, 11, e4269. [Google Scholar] [CrossRef]
- Amato-Cuartas, P.A.; Tabares-Quintero, A.E.; Vélez-Jaramillo, L.F.; Álvarez-Gómez, G.; González-Pérez, L.V.; Martínez-Delgado, C.M.; Robledo-Sierra, J. Coexistence of Thyroid Disease and Oral Lichen Planus in a Colombian Population. Acta Odontol. Latinoam. 2019, 32, 71–74. [Google Scholar] [PubMed]
- Lo Muzio, L.; Santarelli, A.; Campisi, G.; Lacaita, M.; Favia, G. Possible Link between Hashimoto’s Thyroiditis and Oral Lichen Planus: A Novel Association Found. Clin. Oral Investig. 2013, 17, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Shi, L.; Jiang, B.; Zhou, Z.; Shen, X. A Cross-Sectional Study of Oral Lichen Planus Associated With Thyroid Diseases in East China. Front. Endocrinol. 2020, 10, 928. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, J.; Li, C.; Chen, Q.; Hua, H. The Association of Thyroid Disease and Oral Lichen Planus: A Literature Review and Meta-Analysis. Front. Endocrinol. 2017, 8, 310. [Google Scholar] [CrossRef]
- Zhou, T.; Li, D.; Chen, Q.; Hua, H.; Li, C. Correlation Between Oral Lichen Planus and Thyroid Disease in China: A Case–Control Study. Front. Endocrinol. 2018, 9, 330. [Google Scholar] [CrossRef]
- Zhang, T.; Hou, F.; Liu, D.; Zhou, H.; Sun, Y.; Deng, X.; Xu, Y.; Xiao, Y.; Wang, X.; Wu, C.; et al. Association of Hashimoto’s Thyroiditis and Anti-Thyroid Antibodies with Oral Lichen Planus: A Cross-Sectional Study. Front. Immunol. 2022, 13, 967988. [Google Scholar] [CrossRef]
- Alikhani, M.; Ghalaiani, P.; Askariyan, E.; Khunsaraki, Z.A.; Tavangar, A.; Naderi, A. Association between the Clinical Severity of Oral Lichen Planus and Anti-TPO Level in Thyroid Patients. Braz. Oral Res. 2017, 31, e10. [Google Scholar] [CrossRef]
- Karanikas, G.; Schuetz, M.; Wahl, K.; Paul, M.; Kontur, S.; Pietschmann, P.; Kletter, K.; Dudczak, R.; Willheim, M. Relation of anti-TPO Autoantibody Titre and T-lymphocyte Cytokine Production Patterns in Hashimoto’s Thyroiditis. Clin. Endocrinol. 2005, 63, 191–196. [Google Scholar] [CrossRef]
- Carlé, A.; Bülow Pedersen, I.; Knudsen, N.; Perrild, H.; Ovesen, L.; Banke Rasmussen, L.; Jørgensen, T.; Laurberg, P. Smoking Cessation Is Followed by a Sharp but Transient Rise in the Incidence of Overt Autoimmune Hypothyroidism—A Population-based, Case–Control Study. Clin. Endocrinol. 2012, 77, 764–772. [Google Scholar] [CrossRef]
- Hawkins, B.R.; Lam, K.S.L.; Ma, J.T.C.; Wang, C.; Yeung, R.T.T. Strong Association between HLA DRw9 and Hashimoto’s Thyroiditis in Southern Chinese. Acta Endocrinol. 1987, 114, 543–546. [Google Scholar] [CrossRef]
- Lin, S.; Sun, A. HLA-DR and DQ Antigens in Chinese Patients with Oral Lichen Planus. J. Oral Pathol. Med. 1990, 19, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Tomer, Y.; Davies, T.F. Searching for the Autoimmune Thyroid Disease Susceptibility Genes: From Gene Mapping to Gene Function. Endocr. Rev. 2003, 24, 694–717. [Google Scholar] [CrossRef] [PubMed]
- Alaizari, N.; Al-Maweri, S.; Al-Shamiri, H.; Tarakji, B.; Shugaa-Addin, B. Hepatitis C Virus Infections in Oral Lichen Planus: A Systematic Review and Meta-analysis. Aust. Dent. J. 2016, 61, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Ghodratnama, F.; Wray, D.; Bagg, J. Detection of Serum Antibodies against Cytomegalovirus, Varicella Zoster Virus and Human Herpesvirus 6 in Patients with Recurrent Aphthous Stomatitis. J. Oral Pathol. Med. 1999, 28, 12–15. [Google Scholar] [CrossRef]
- Sultanova, A.; Cistjakovs, M.; Gravelsina, S.; Chapenko, S.; Roga, S.; Cunskis, E.; Nora-Krukle, Z.; Groma, V.; Ventina, I.; Murovska, M. Association of Active Human Herpesvirus-6 (HHV-6) Infection with Autoimmune Thyroid Gland Diseases. Clin. Microbiol. Infect. 2017, 23, 50.e1–50.e5. [Google Scholar] [CrossRef]
- Kazanowska-Dygdała, M.; Duś, I.; Radwan-Oczko, M. The Presence of Helicobacter Pylori in Oral Cavities of Patients with Leukoplakia and Oral Lichen Planus. J. Appl. Oral Sci. 2016, 24, 18–23. [Google Scholar] [CrossRef]
- Tsatsoulis, A. The Role of Stress in the Clinical Expression of Thyroid Autoimmunity. Ann. N. Y. Acad. Sci. 2006, 1088, 382–395. [Google Scholar] [CrossRef]
- Hennessey, J.V. Autoimmune Thyroiditis and Depression. JAMA Psychiatry 2018, 75, 1204. [Google Scholar] [CrossRef]
- Mohan, R.S.; Gupta, A.; Kamarthi, N.; Malik, S.; Goel, S.; Gupta, S. Incidence of Oral Lichen Planus in Perimenopausal Women: A Cross-Sectional Study in Western Uttar Pradesh Population. J.-Life Health 2017, 8, 70. [Google Scholar] [CrossRef]
- Ansar Ahmed, S.; Young, P.R.; Penhale, W.J. The Effects of Female Sex Steroids on the Development of Autoimmune Thyroiditis in Thymectomized and Irradiated Rats. Clin. Exp. Immunol. 1983, 54, 351–358. [Google Scholar]
- Wu, P.; Luo, S.; Zhou, T.; Wang, R.; Qiu, X.; Yuan, P.; Yang, Y.; Han, Q.; Jiang, L. Possible Mechanisms Involved in the Cooccurrence of Oral Lichen Planus and Hashimoto’s Thyroiditis. Mediat. Inflamm. 2020, 2020, 6309238. [Google Scholar] [CrossRef] [PubMed]
- Bieber, T. Atopic Dermatitis. N. Engl. J. Med. 2008, 358, 1483–1494. [Google Scholar] [CrossRef] [PubMed]
- Steinman, L. A Brief History of T(H)17, the First Major Revision in the T(H)1/T(H)2 Hypothesis of T Cell-Mediated Tissue Damage. Nat. Med. 2007, 13, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Wing, J.B.; Sakaguchi, S. Foxp3+ T(Reg) Cells in Humoral Immunity. Int. Immunol. 2014, 26, 61–69. [Google Scholar] [CrossRef]
- Josefowicz, S.Z.; Niec, R.E.; Kim, H.Y.; Treuting, P.; Chinen, T.; Zheng, Y.; Umetsu, D.T.; Rudensky, A.Y. Extrathymically Generated Regulatory T Cells Control Mucosal TH2 Inflammation. Nature 2012, 482, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Ivert, L.U.; Wahlgren, C.-F.; Lindelöf, B.; Dal, H.; Bradley, M.; Johansson, E.K. Association between Atopic Dermatitis and Autoimmune Diseases: A Population-Based Case-Control Study. Br. J. Dermatol. 2021, 185, 335–342. [Google Scholar] [CrossRef]
- Brunner, P.M.; Silverberg, J.I.; Guttman-Yassky, E.; Paller, A.S.; Kabashima, K.; Amagai, M.; Luger, T.A.; Deleuran, M.; Werfel, T.; Eyerich, K.; et al. Increasing Comorbidities Suggest That Atopic Dermatitis Is a Systemic Disorder. J. Investig. Dermatol. 2017, 137, 18–25. [Google Scholar] [CrossRef]
- Lo, W.-C.; Arsenescu, R.I.; Friedman, A. Mathematical Model of the Roles of T Cells in Inflammatory Bowel Disease. Bull. Math. Biol. 2013, 75, 1417–1433. [Google Scholar] [CrossRef]
- Wu, L.; Hwang, C.; Chung, P.; Hua, T.; Chen, Y.; Chu, S.; Lee, D.; Chang, Y.; Wang, W.; Liu, H.; et al. Autoimmune Disease Comorbidities in Patients with Atopic Dermatitis: A Nationwide Case–Control Study in Taiwan. Pediatr. Allergy Immunol. 2014, 25, 586–592. [Google Scholar] [CrossRef]
- Pedullá, M.; Fierro, V.; Papacciuolo, V.; Alfano, R.; Ruocco, E. Atopy as a Risk Factor for Thyroid Autoimmunity in Children Affected with Atopic Dermatitis. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1057–1060. [Google Scholar] [CrossRef]
- Pedullà, M.; Vincenzo Fierro, P.M. Skin Disease and Thyroid Autoimmunity in Atopic South Italian Children. World J. Clin. Pediatr. 2016, 5, 288–292. [Google Scholar] [CrossRef]
- Marwaha, R.K.; Tandon, N.; Karak, A.K.; Gupta, N.; Verma, K.; Kochupillai, N. Hashimoto’s Thyroiditis: Countrywide Screening of Goitrous Healthy Young Girls in Postiodization Phase in India. J. Clin. Endocrinol. Metab. 2000, 85, 3798–3802. [Google Scholar] [CrossRef] [PubMed]
- Jaksić, J.; Dumić, M.; Filipović, B.; Ille, J.; Cvijetić, M.; Gjurić, G. Thyroid Diseases in a School Population with Thyromegaly. Arch. Dis. Child. 1994, 70, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Mendiratta, V.; Himadri, H.; Verma, D.; Aggarwal, M.; Yadav, J. Is Atopic Dermatitis a Risk Factor for Thyroid Autoimmunity?—A Cross-Sectional Study from a Tertiary Care Center in India. Indian Dermatol. Online J. 2024, 15, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hong, C.; Lian, X.; Wen, L.; Xu, K.; Tian, Z.; Si, W.; Li, Y. Correlations of Thyroid Autoantibodies with Allergic Diseases: A Case-Control Study of 434 Chinese Patients. Medicine 2022, 101, e29871. [Google Scholar] [CrossRef]
- Altrichter, S.; Peter, H.-J.; Pisarevskaja, D.; Metz, M.; Martus, P.; Maurer, M. IgE Mediated Autoallergy against Thyroid Peroxidase--a Novel Pathomechanism of Chronic Spontaneous Urticaria? PLoS ONE 2011, 6, e14794. [Google Scholar] [CrossRef]
- Saylam Kurtipek, G.; Cihan, F.G.; Erayman Demirbaş, Ş.; Ataseven, A. The Frequency of Autoimmune Thyroid Disease in Alopecia Areata and Vitiligo Patients. BioMed Res. Int. 2015, 2015, 435947. [Google Scholar] [CrossRef]
- Valenta, R.; Maurer, D.; Steiner, R.; Seiberler, S.; Sperr, W.R.; Valent, P.; Spitzauer, S.; Kapiotis, S.; Smolen, J.; Stingl, G. Immunoglobulin E Response to Human Proteins in Atopic Patients. J. Investig. Dermatol. 1996, 107, 203–208. [Google Scholar] [CrossRef]
- Maurer, M.; Altrichter, S.; Schmetzer, O.; Scheffel, J.; Church, M.K.; Metz, M. Immunoglobulin E-Mediated Autoimmunity. Front. Immunol. 2018, 9, 689. [Google Scholar] [CrossRef]
- Bertino, L.; Guarneri, F.; Cannavò, S.P.; Casciaro, M.; Pioggia, G.; Gangemi, S. Oxidative Stress and Atopic Dermatitis. Antioxidants 2020, 9, 196. [Google Scholar] [CrossRef]
- Wasserman, D.; Guzman-Sanchez, D.A.; Scott, K.; McMichael, A. Alopecia Areata. Int. J. Dermatol. 2007, 46, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Pratt, C.H.; King, L.E.; Messenger, A.G.; Christiano, A.M.; Sundberg, J.P. Alopecia Areata. Nat. Rev. Dis. Primer 2017, 3, 17011. [Google Scholar] [CrossRef] [PubMed]
- Toussi, A.; Barton, V.R.; Le, S.T.; Agbai, O.N.; Kiuru, M. Psychosocial and Psychiatric Comorbidities and Health-Related Quality of Life in Alopecia Areata: A Systematic Review. J. Am. Acad. Dermatol. 2021, 85, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Gilhar, A.; Etzioni, A.; Paus, R. Alopecia Areata. N. Engl. J. Med. 2012, 366, 1515–1525. [Google Scholar] [CrossRef]
- Shellow, W.V.R.; Edwards, J.E.; Koo, J.Y.M. Profile of Alopecia Areata: A Questionnaire Analysis of Patient and Family. Int. J. Dermatol. 1992, 31, 186–189. [Google Scholar] [CrossRef]
- Blaumeiser, B.; Van Der Goot, I.; Fimmers, R.; Hanneken, S.; Ritzmann, S.; Seymons, K.; Betz, R.C.; Ruzicka, T.; Wienker, T.F.; De Weert, J.; et al. Familial Aggregation of Alopecia Areata. J. Am. Acad. Dermatol. 2006, 54, 627–632. [Google Scholar] [CrossRef]
- Petukhova, L.; Patel, A.V.; Rigo, R.K.; Bian, L.; Verbitsky, M.; Sanna-Cherchi, S.; Erjavec, S.O.; Abdelaziz, A.R.; Cerise, J.E.; Jabbari, A.; et al. Integrative Analysis of Rare Copy Number Variants and Gene Expression Data in Alopecia Areata Implicates an Aetiological Role for Autophagy. Exp. Dermatol. 2020, 29, 243–253. [Google Scholar] [CrossRef]
- Strazzulla, L.C.; Wang, E.H.C.; Avila, L.; Lo Sicco, K.; Brinster, N.; Christiano, A.M.; Shapiro, J. Alopecia Areata: Disease Characteristics, Clinical Evaluation, and New Perspectives on Pathogenesis. J. Am. Acad. Dermatol. 2018, 78, 1–12. [Google Scholar] [CrossRef]
- Puavilai, S.; Puavilai, G.; Charuwichitratana, S.; Sakuntabhai, A.; Sriprachya-Anunt, S. Prevalence of Thyroid Diseases in Patients with Alopecia Areata. Int. J. Dermatol. 1994, 33, 632–633. [Google Scholar] [CrossRef]
- Forouzan, P.; Cohen, P.R. Systemic Lupus Erythematosus Presenting as Alopecia Areata. Cureus 2020, 12, e8724. [Google Scholar] [CrossRef]
- Milgraum, S.S.; Mitchell, A.J.; Bacon, G.E.; Rasmussen, J.E. Alopecia Areata, Endocrine Function, and Autoantibodies in Patients 16 Years of Age or Younger. J. Am. Acad. Dermatol. 1987, 17, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Lewiński, A.; Broniarczyk-Dyła, G.; Sewerynek, E.; Zerek-Mełeń, G.; Szkudliński, M. Abnormalities in Structure and Function of the Thyroid Gland in Patients with Alopecia Areata. J. Am. Acad. Dermatol. 1990, 23, 768–769. [Google Scholar] [CrossRef] [PubMed]
- Seyrafi, H.; Akhiani, M.; Abbasi, H.; Mirpour, S.; Gholamrezanezhad, A. Evaluation of the Profile of Alopecia Areata and the Prevalence of Thyroid Function Test Abnormalities and Serum Autoantibodies in Iranian Patients. BMC Dermatol. 2005, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Bakry, O.A.; Basha, M.A.; El Shafiee, M.K.; Shehata, W.A. Thyroid Disorders Associated with Alopecia Areata in Egyptian Patients. Indian J. Dermatol. 2014, 59, 49–55. [Google Scholar] [CrossRef]
- Park, S.-M.; Oh, Y.-J.; Lew, B.-L.; Sim, W.-Y. The Association among Thyroid Dysfunction, Thyroid Autoimmunity, and Clinical Features of Alopecia Areata: A Retrospective Study. J. Am. Acad. Dermatol. 2019, 81, 602–605. [Google Scholar] [CrossRef]
- Lee, S.; Lee, Y.B.; Kim, B.J.; Lee, W.-S. Screening of Thyroid Function and Autoantibodies in Patients with Alopecia Areata: A Systematic Review and Meta-Analysis. J. Am. Acad. Dermatol. 2019, 80, 1410–1413.e4. [Google Scholar] [CrossRef]
- Kaur, G.; Kuldeep, C.M.; Bhargava, P.; Mathur, D.K.; Sharda, S.; Chaturvedi, P. Insignificant Correlation between Thyroid Hormone and Antithyroid Peroxidase Antibodies in Alopecia Areata Patients in Northern Rajasthan. Int. J. Trichology 2017, 9, 149–153. [Google Scholar] [CrossRef]
- Rahnama, Z.; Farajzadeh, S.; Mohamamdi, S.; Masoudi, M. Prevalence of Thyroid Disorders in Patients with Alopecia Areata. J. Pak. Assoc. Dermatol. 2014, 24, 246–250. [Google Scholar]
- Gönül, M.; Külcü Çakmak, S.; Ünal, E.; Bıyıklı, Z. Alopesi Areatalı Hastalar Hastalıkları Hakkında Ne Düşünüyorlar? Turk. J. Dermatol. Türk Dermatoloji Derg. 2013, 7, 192–195. [Google Scholar] [CrossRef]
- Naik, P.P.; Farrukh, S.N. Association between Alopecia Areata and Thyroid Dysfunction. Postgrad. Med. 2021, 133, 895–898. [Google Scholar] [CrossRef]
- Bertolini, M.; McElwee, K.; Gilhar, A.; Bulfone-Paus, S.; Paus, R. Hair Follicle Immune Privilege and Its Collapse in Alopecia Areata. Exp. Dermatol. 2020, 29, 703–725. [Google Scholar] [CrossRef] [PubMed]
- Gilhar, A.; Laufer-Britva, R.; Keren, A.; Paus, R. Frontiers in Alopecia Areata Pathobiology Research. J. Allergy Clin. Immunol. 2019, 144, 1478–1489. [Google Scholar] [CrossRef] [PubMed]
- Freyschmidt-Paul, P.; McElwee, K.J.; Hoffmann, R.; Sundberg, J.P.; Vitacolonna, M.; Kissling, S.; Zöller, M. Interferon-Gamma-Deficient Mice Are Resistant to the Development of Alopecia Areata. Br. J. Dermatol. 2006, 155, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Yenin, J.Z.; Serarslan, G.; Yönden, Z.; Ulutaş, K.T. Investigation of Oxidative Stress in Patients with Alopecia Areata and Its Relationship with Disease Severity, Duration, Recurrence and Pattern. Clin. Exp. Dermatol. 2015, 40, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, P.; Arican, O.; Belge Kurutas, E.; Mulayim, K. Oxidative Stress Biomarkers and Adenosine Deaminase over the Alopecic Area of the Patients with Alopecia Areata. Balk. Med. J. 2016, 33, 188–192. [Google Scholar] [CrossRef]
- Acharya, P.; Mathur, M.C. Oxidative Stress in Alopecia Areata: A Systematic Review and Meta-Analysis. Int. J. Dermatol. 2020, 59, 434–440. [Google Scholar] [CrossRef]
- Rajabi, F.; Drake, L.A.; Senna, M.M.; Rezaei, N. Alopecia Areata: A Review of Disease Pathogenesis. Br. J. Dermatol. 2018, 179, 1033–1048. [Google Scholar] [CrossRef]
- Lousada, M.B.; Lachnit, T.; Edelkamp, J.; Rouillé, T.; Ajdic, D.; Uchida, Y.; Di Nardo, A.; Bosch, T.C.G.; Paus, R. Exploring the Human Hair Follicle Microbiome. Br. J. Dermatol. 2021, 184, 802–815. [Google Scholar] [CrossRef]
- Pinto, D.; Sorbellini, E.; Marzani, B.; Rucco, M.; Giuliani, G.; Rinaldi, F. Scalp Bacterial Shift in Alopecia Areata. PLoS ONE 2019, 14, e0215206. [Google Scholar] [CrossRef]
- Moreno-Arrones, O.M.; Serrano-Villar, S.; Perez-Brocal, V.; Saceda-Corralo, D.; Morales-Raya, C.; Rodrigues-Barata, R.; Moya, A.; Jaen-Olasolo, P.; Vano-Galvan, S. Analysis of the Gut Microbiota in Alopecia Areata: Identification of Bacterial Biomarkers. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 400–405. [Google Scholar] [CrossRef]
- Taïeb, A.; Picardo, M.; on behalf of the other VETF members. The Definition and Assessment of Vitiligo: A Consensus Report of the Vitiligo European Task Force. Pigment Cell Res. 2007, 20, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Steve, B.F. Further Investigations in the Treatment of Vitiligo. Va. Med. Mon. 1945, 71, 6–17. [Google Scholar]
- Yuan, J.; Sun, C.; Jiang, S.; Lu, Y.; Zhang, Y.; Gao, X.-H.; Wu, Y.; Chen, H.-D. The Prevalence of Thyroid Disorders in Patients With Vitiligo: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2019, 9, 803. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.-C.; Yang, T.-H.; Huang, Y.-C. Vitiligo and Thyroid Disease: A Systematic Review and Meta-Analysis. Eur. J. Dermatol. 2018, 28, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Alkhateeb, A.; Fain, P.R.; Thody, A.; Bennett, D.C.; Spritz, R.A. Epidemiology of Vitiligo and Associated Autoimmune Diseases in Caucasian Probands and Their Families. Pigment Cell Res. 2003, 16, 208–214. [Google Scholar] [CrossRef]
- Laberge, G.; Mailloux, C.M.; Gowan, K.; Holland, P.; Bennett, D.C.; Fain, P.R.; Spritz, R.A. Early Disease Onset and Increased Risk of Other Autoimmune Diseases in Familial Generalized Vitiligo. Pigment Cell Res. 2005, 18, 300–305. [Google Scholar] [CrossRef]
- Foley, L.M.; Lowe, N.J.; Misheloff, E.; Tiwari, J.L. Association of HLA-DR4 with Vitiligo. J. Am. Acad. Dermatol. 1983, 8, 39–40. [Google Scholar] [CrossRef]
- Liu, J.; Li, M.; Chen, H.; Zhong, S.; Yang, S.; Du, W.; Hao, J.; Zhang, T.; Zhang, X.; Zeegers, M. Association of Vitiligo with HLA-A2: A Meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 205–213. [Google Scholar] [CrossRef]
- Bouayad, A. Thyroid Autoimmunity in Relation to HLA-DRB1 and HLA-DQB1 Polymorphism in Nonsegmental Vitiligo: A Cross-Sectional-Study. Am. J. Transl. Res. 2024, 16, 524–530. [Google Scholar] [CrossRef]
- Yang, S.; Wang, J.; Gao, M.; Liu, H.; Sun, L.; He, P.; Liu, J.; Zhang, A.; Cui, Y.; Liang, Y.; et al. Association of HLA-DQA1 and DQB1 Genes with Vitiligo in Chinese Hans. Int. J. Dermatol. 2005, 44, 1022–1027. [Google Scholar] [CrossRef]
- Buc, M.; Fazekasová, H.; Cechová, E.; Hegyi, E.; Kolibásová, K.; Ferencík, S. Occurrence Rates of HLA-DRB1, HLA-DQB1, and HLA-DPB1 Alleles in Patients Suffering from Vitiligo. Eur. J. Dermatol. 1998, 8, 13–15. [Google Scholar] [PubMed]
- Romo-Tena, J.; Gómez-Martín, D.; Alcocer-Varela, J. CTLA-4 and Autoimmunity: New Insights into the Dual Regulator of Tolerance. Autoimmun. Rev. 2013, 12, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Birlea, S.A.; LaBerge, G.S.; Procopciuc, L.M.; Fain, P.R.; Spritz, R.A. CTLA4 and Generalized Vitiligo: Two Genetic Association Studies and a Meta-analysis of Published Data. Pigment Cell Melanoma Res. 2009, 22, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wan, S.; Qu, M.; Ren, B.; Liu, L.; Shen, H. The Relationship between PTPN22 R620W Polymorphisms and the Susceptibility to Autoimmune Thyroid Diseases: An Updated Meta-Analysis. Immunol. Investig. 2022, 51, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Changotra, H. Association of Protein Tyrosine Phosphatase, Non-Receptor Type 22 +1858C→T Polymorphism and Susceptibility to Vitiligo: Systematic Review and Meta-Analysis. Indian J. Dermatol. Venereol. Leprol. 2017, 83, 183. [Google Scholar] [CrossRef] [PubMed]
- Spritz, R.A. Shared Genetic Relationships Underlying Generalized Vitiligo and Autoimmune Thyroid Disease. Thyroid 2010, 20, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Alkhateeb, A. Mapping of an Autoimmunity Susceptibility Locus (AIS1) to Chromosome 1p31.3-P32.2. Hum. Mol. Genet. 2002, 11, 661–667. [Google Scholar] [CrossRef]
- Lu, J.; Song, L.; Luan, J.; Feng, Y.; Wang, Y.; Cao, X.; Lu, Y. Identification of Shared Biomarkers and Immune Infiltration Signatures between Vitiligo and Hashimoto’s Thyroiditis. Clin. Cosmet. Investig. Dermatol. 2024, 17, 311–327. [Google Scholar] [CrossRef]
- Steitz, J.; Wenzel, J.; Gaffal, E.; Tüting, T. Initiation and Regulation of CD8+T Cells Recognizing Melanocytic Antigens in the Epidermis: Implications for the Pathophysiology of Vitiligo. Eur. J. Cell Biol. 2004, 83, 797–803. [Google Scholar] [CrossRef]
- Deng, Q.; Luo, Y.; Chang, C.; Wu, H.; Ding, Y.; Xiao, R. The Emerging Epigenetic Role of CD8+T Cells in Autoimmune Diseases: A Systematic Review. Front. Immunol. 2019, 10, 856. [Google Scholar] [CrossRef]
- Ralli, M.; Angeletti, D.; Fiore, M.; D’Aguanno, V.; Lambiase, A.; Artico, M.; De Vincentiis, M.; Greco, A. Hashimoto’s Thyroiditis: An Update on Pathogenic Mechanisms, Diagnostic Protocols, Therapeutic Strategies, and Potential Malignant Transformation. Autoimmun. Rev. 2020, 19, 102649. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-L.; Ko, C.-H. The Role of Oxidative Stress in Vitiligo: An Update on Its Pathogenesis and Therapeutic Implications. Cells 2023, 12, 936. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, M.; Bagnato, G.; Cristani, M.; Borgia, F.; Spatari, G.; Tigano, V.; Saja, A.; Guarneri, F.; Cannavò, S.P.; Gangemi, S. Oxidation Products Are Increased in Patients Affected by Non-Segmental Generalized Vitiligo. Arch. Dermatol. Res. 2017, 309, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, R.M.; CampennÌ, A.; Giuffrida, G.; Casciaro, M.; Barbalace, M.C.; Hrelia, S.; Trimarchi, F.; CannavÒ, S.; Gangemi, S. Oxidative Stress as a Key Feature of Autoimmune Thyroiditis: An Update. Minerva Endocrinol. 2020, 45, 326–344. [Google Scholar] [CrossRef] [PubMed]
- Kochman, J.; Jakubczyk, K.; Bargiel, P.; Janda-Milczarek, K. The Influence of Oxidative Stress on Thyroid Diseases. Antioxidants 2021, 10, 1442. [Google Scholar] [CrossRef]
- Li, D.; Liang, G.; Calderone, R.; Bellanti, J.A. Vitiligo and Hashimoto’s Thyroiditis: Autoimmune Diseases Linked by Clinical Presentation, Biochemical Commonality, and Autoimmune/Oxidative Stress-Mediated Toxicity Pathogenesis. Med. Hypotheses 2019, 128, 69–75. [Google Scholar] [CrossRef]
- Sutanto, H.; Safira, A.; Fetarayani, D. From tumor to tolerance: A comprehensive review of immune checkpoint inhibitors and immune-related adverse events. Asia Pac. Allergy 2024, 14, 124–138. [Google Scholar] [CrossRef]
- Dougan, M.; Pietropaolo, M. Time to dissect the autoimmune etiology of cancer antibody immunotherapy. J. Clin. Investig. 2020, 130, 51–61. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carlucci, P.; Spataro, F.; Cristallo, M.; Di Gioacchino, M.; Nettis, E.; Gangemi, S. Immune-Molecular Link between Thyroid and Skin Autoimmune Diseases: A Narrative Review. J. Clin. Med. 2024, 13, 5594. https://doi.org/10.3390/jcm13185594
Carlucci P, Spataro F, Cristallo M, Di Gioacchino M, Nettis E, Gangemi S. Immune-Molecular Link between Thyroid and Skin Autoimmune Diseases: A Narrative Review. Journal of Clinical Medicine. 2024; 13(18):5594. https://doi.org/10.3390/jcm13185594
Chicago/Turabian StyleCarlucci, Palma, Federico Spataro, Mattia Cristallo, Mario Di Gioacchino, Eustachio Nettis, and Sebastiano Gangemi. 2024. "Immune-Molecular Link between Thyroid and Skin Autoimmune Diseases: A Narrative Review" Journal of Clinical Medicine 13, no. 18: 5594. https://doi.org/10.3390/jcm13185594






