Optimized Treatment of Interleukin (IL-1)-Mediated Autoinflammatory Diseases: Impact of Disease Activity-Based Treatment Adjustments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Related Data
2.2. Treatment Regimen and Definitions
2.3. Definition of Disease Activity
2.4. Outcome
2.5. Analysis
3. Results
3.1. Disease Activity over Time
3.2. Treatment Adjustments
3.2.1. Disease Activity-Based Treatment Adjustments
3.2.2. Specific Dosing Regimen
3.3. Impact on Disease Activity Trajectories
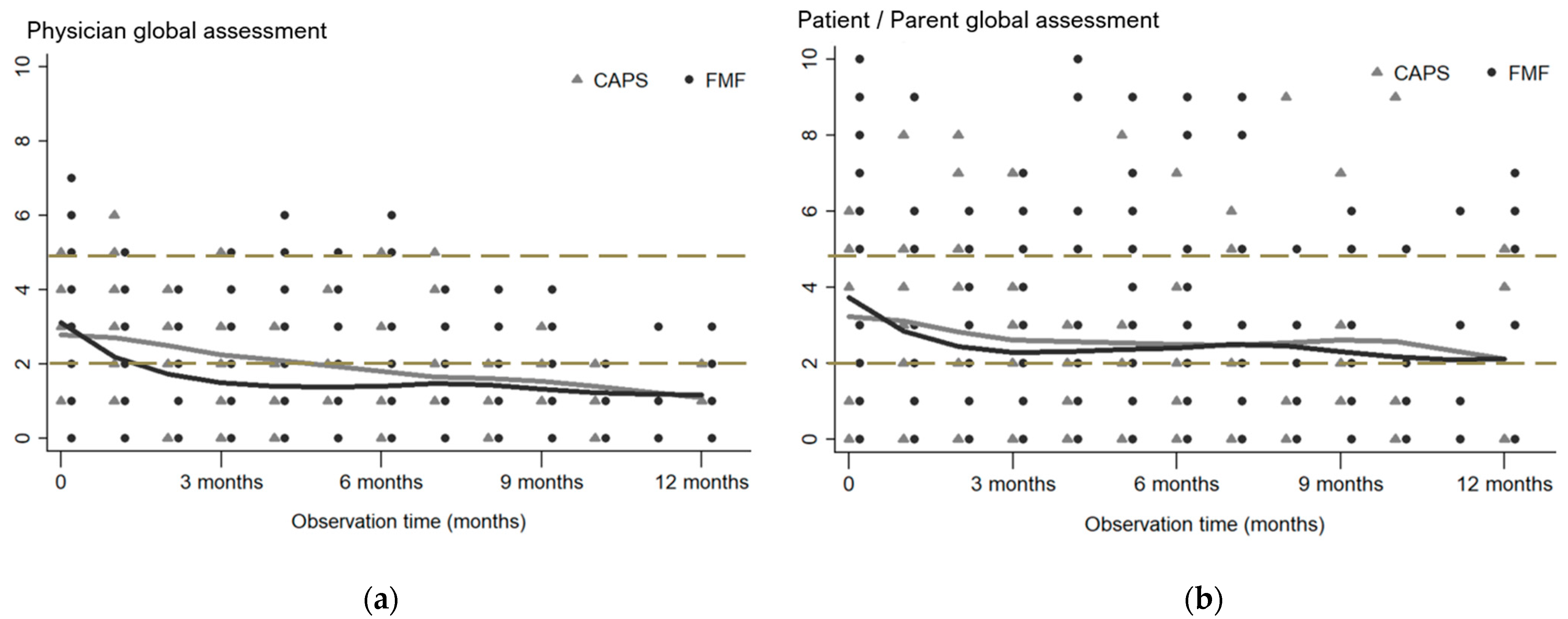
3.3.1. PGA and PPGA
3.3.2. Parameter Trajectories/Changes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Broderick, L.; De Nardo, D.; Franklin, B.S.; Hoffman, H.M.; Latz, E. The inflammasomes and autoinflammatory syndromes. Annu. Rev. Pathol. 2015, 10, 395–424. [Google Scholar] [CrossRef] [PubMed]
- Lachmann, H.J. Periodic fever syndromes. Best. Pract. Res. Clin. Rheumatol. 2017, 31, 596–609. [Google Scholar] [CrossRef] [PubMed]
- Toplak, N.; Frenkel, J.; Ozen, S.; Lachmann, H.J.; Woo, P.; Koné-Paut, I.; De Benedetti, F.; Neven, B.; Hofer, M.; Dolezalova, P.; et al. An International registry on Autoinflammatory diseases: The Eurofever experience. Ann. Rheum. Dis. 2012, 71, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Blank, N.; Kotter, I.; Schmalzing, M.; Rech, J.; Krause, K.; Kohler, B.; Kaudewitz, D.; Nitschke, M.; Haas, C.S.; Lorenz, H.M.; et al. Clinical presentation and genetic variants in patients with autoinflammatory diseases: Results from the German GARROD registry. Rheumatol. Int. 2024, 44, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Cipolletta, S.; Giudici, L.; Punzi, L.; Galozzi, P.; Sfriso, P. Health-related quality of life, illness perception, coping strategies and the distribution of dependency in autoinflammatory diseases. Clin. Exp. Rheumatol. 2019, 37 (Suppl. S121), 156–157. [Google Scholar] [PubMed]
- Erbis, G.; Schmidt, K.; Hansmann, S.; Sergiichuk, T.; Michler, C.; Kuemmerle-Deschner, J.B.; Benseler, S.M. Living with autoinflammatory diseases: Identifying unmet needs of children, adolescents and adults. Pediatr. Rheumatol. Online J. 2018, 16, 81. [Google Scholar] [CrossRef] [PubMed]
- Vasse, M.; Reumaux, H.; Kone-Paut, I.; Quartier, P.; Hachulla, E. Socio-professional impact and quality of life of cryopyrin-associated periodic syndromes in 54 patients in adulthood. Clin. Exp. Rheumatol. 2023, 41, 2039–2043. [Google Scholar] [CrossRef] [PubMed]
- Piram, M.; Kone-Paut, I.; Lachmann, H.J.; Frenkel, J.; Ozen, S.; Kuemmerle-Deschner, J.; Stojanov, S.; Simon, A.; Finetti, M.; Sormani, M.P.; et al. Validation of the auto-inflammatory diseases activity index (AIDAI) for hereditary recurrent fever syndromes. Ann. Rheum. Dis. 2014, 73, 2168–2173. [Google Scholar] [CrossRef] [PubMed]
- Ter Haar, N.M.; van Delft, A.L.J.; Annink, K.V.; van Stel, H.; Al-Mayouf, S.M.; Amaryan, G.; Anton, J.; Barron, K.S.; Benseler, S.; Brogan, P.A.; et al. In silico validation of the Autoinflammatory Disease Damage Index. Ann. Rheum. Dis. 2018, 77, 1599–1605. [Google Scholar] [CrossRef]
- Romano, M.; Arici, Z.S.; Piskin, D.; Alehashemi, S.; Aletaha, D.; Barron, K.S.; Benseler, S.; Berard, R.; Broderick, L.; Dedeoglu, F.; et al. The 2021 EULAR/American College of Rheumatology points to consider for diagnosis, management and monitoring of the interleukin-1 mediated autoinflammatory diseases: Cryopyrin-associated periodic syndromes, tumour necrosis factor receptor-associated periodic syndrome, mevalonate kinase deficiency, and deficiency of the interleukin-1 receptor antagonist. Ann. Rheum. Dis. 2022, 81, 907–921. [Google Scholar] [CrossRef]
- Ozen, S.; Demirkaya, E.; Erer, B.; Livneh, A.; Ben-Chetrit, E.; Giancane, G.; Ozdogan, H.; Abu, I.; Gattorno, M.; Hawkins, P.N.; et al. EULAR recommendations for the management of familial Mediterranean fever. Ann. Rheum. Dis. 2016, 75, 644–651. [Google Scholar] [CrossRef] [PubMed]
- De Benedetti, F.; Gattorno, M.; Anton, J.; Ben-Chetrit, E.; Frenkel, J.; Hoffman, H.M.; Kone-Paut, I.; Lachmann, H.J.; Ozen, S.; Simon, A.; et al. Canakinumab for the Treatment of Autoinflammatory Recurrent Fever Syndromes. N. Engl. J. Med. 2018, 378, 1908–1919. [Google Scholar] [CrossRef] [PubMed]
- Hentgen, V.; Vinit, C.; Fayand, A.; Georgin-Lavialle, S. The Use of Interleukine-1 Inhibitors in Familial Mediterranean Fever Patients: A Narrative Review. Front. Immunol. 2020, 11, 971. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S. Treat-to-target: Rationale and strategies. Clin. Exp. Rheumatol. 2012, 30, S2–S6. [Google Scholar] [PubMed]
- Solomon, D.H.; Bitton, A.; Katz, J.N.; Radner, H.; Brown, E.M.; Fraenkel, L. Review: Treat to target in rheumatoid arthritis: Fact, fiction, or hypothesis? Arthritis Rheumatol. 2014, 66, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Hansmann, S.; Lainka, E.; Horneff, G.; Holzinger, D.; Rieber, N.; Jansson, A.F.; Rosen-Wolff, A.; Erbis, G.; Prelog, M.; Brunner, J.; et al. Consensus protocols for the diagnosis and management of the hereditary autoinflammatory syndromes CAPS, TRAPS and MKD/HIDS: A German PRO-KIND initiative. Pediatr. Rheumatol. Online J. 2020, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, L.; Rolfes, E.; Lieber, M.; Muller, D.; Lainka, E.; Gohar, F.; Klaus, G.; Girschick, H.; Horstermann, J.; Kummerle-Deschner, J.; et al. Treat-to-target strategies for the management of familial Mediterranean Fever in children. Pediatr. Rheumatol. Online J. 2023, 21, 108. [Google Scholar] [CrossRef]
- Georgin-Lavialle, S.; Savey, L.; Cuisset, L.; Boursier, G.; Boffa, J.J.; Delplanque, M.; Bourguiba, R.; Monfort, J.B.; Touitou, I.; Grateau, G.; et al. French protocol for the diagnosis and management of familial Mediterranean fever. Rev. Med. Interne 2023, 44, 602–616. [Google Scholar] [CrossRef] [PubMed]
- Gattorno, M.; Hofer, M.; Federici, S.; Vanoni, F.; Bovis, F.; Aksentijevich, I.; Anton, J.; Arostegui, J.I.; Barron, K.; Ben-Cherit, E.; et al. Classification criteria for autoinflammatory recurrent fevers. Ann. Rheum. Dis. 2019, 78, 1025–1032. [Google Scholar] [CrossRef]
- Kuemmerle-Deschner, J.B.; Ozen, S.; Tyrrell, P.N.; Kone-Paut, I.; Goldbach-Mansky, R.; Lachmann, H.; Blank, N.; Hoffman, H.M.; Weissbarth-Riedel, E.; Hugle, B.; et al. Diagnostic criteria for cryopyrin-associated periodic syndrome (CAPS). Ann. Rheum. Dis. 2017, 76, 942–947. [Google Scholar] [CrossRef]
- Yalcinkaya, F.; Ozen, S.; Ozcakar, Z.B.; Aktay, N.; Cakar, N.; Duzova, A.; Kasapcopur, O.; Elhan, A.H.; Doganay, B.; Ekim, M.; et al. A new set of criteria for the diagnosis of familial Mediterranean fever in childhood. Rheumatology 2009, 48, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Milhavet, F.; Cuisset, L.; Hoffman, H.M.; Slim, R.; El-Shanti, H.; Aksentijevich, I.; Lesage, S.; Waterham, H.; Wise, C.; Sarrauste de Menthiere, C.; et al. The infevers autoinflammatory mutation online registry: Update with new genes and functions. Hum. Mutat. 2008, 29, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Infevers: An Online Database for Autoinflammatory Mutations. Copyright. Available online: https://infevers.umai-montpellier.fr (accessed on 1 November 2023).
- Kuemmerle-Deschner, J.B.; Hofer, F.; Endres, T.; Kortus-Goetze, B.; Blank, N.; Weissbarth-Riedel, E.; Schuetz, C.; Kallinich, T.; Krause, K.; Rietschel, C.; et al. Real-life effectiveness of canakinumab in cryopyrin-associated periodic syndrome. Rheumatology 2016, 55, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Kuemmerle-Deschner, J.B.; Ramos, E.; Blank, N.; Roesler, J.; Felix, S.D.; Jung, T.; Stricker, K.; Chakraborty, A.; Tannenbaum, S.; Wright, A.M.; et al. Canakinumab (ACZ885, a fully human IgG1 anti-IL-1beta mAb) induces sustained remission in pediatric patients with cryopyrin-associated periodic syndrome (CAPS). Arthritis Res. Ther. 2011, 13, R34. [Google Scholar] [CrossRef] [PubMed]
- Welzel, T.; Wildermuth, A.L.; Deschner, N.; Benseler, S.M.; Kuemmerle-Deschner, J.B. Colchicine—An effective treatment for children with a clinical diagnosis of autoinflammatory diseases without pathogenic gene variants. Pediatr. Rheumatol. Online J. 2021, 19, 142. [Google Scholar] [CrossRef] [PubMed]
- Welzel, T.; Oefelein, L.; Twilt, M.; Pfister, M.; Kuemmerle-Deschner, J.B.; Benseler, S.M. Tapering of biological treatment in autoinflammatory diseases: A scoping review. Pediatr. Rheumatol. Online J. 2022, 20, 67. [Google Scholar] [CrossRef]
- Parlar, K.; Ates, M.B.; Onal, M.E.; Bostanci, E.; Azman, F.N.; Ugurlu, S. Factors triggering familial mediterranean fever attacks, do they really exist? Intern. Emerg. Med. 2024. [Google Scholar] [CrossRef] [PubMed]
- Kharouf, F.; Tsemach-Toren, T.; Ben-Chetrit, E. IL-1 inhibition in familial Mediterranean fever: Clinical outcomes and expectations. Clin. Exp. Rheumatol. 2022, 40, 1567–1574. [Google Scholar] [CrossRef]
- Levy, R.; Gerard, L.; Kuemmerle-Deschner, J.; Lachmann, H.J.; Kone-Paut, I.; Cantarini, L.; Woo, P.; Naselli, A.; Bader-Meunier, B.; Insalaco, A.; et al. Phenotypic and genotypic characteristics of cryopyrin-associated periodic syndrome: A series of 136 patients from the Eurofever Registry. Ann. Rheum. Dis. 2015, 74, 2043–2049. [Google Scholar] [CrossRef]
- Ter Haar, N.M.; Jeyaratnam, J.; Lachmann, H.J.; Simon, A.; Brogan, P.A.; Doglio, M.; Cattalini, M.; Anton, J.; Modesto, C.; Quartier, P.; et al. The Phenotype and Genotype of Mevalonate Kinase Deficiency: A Series of 114 Cases From the Eurofever Registry. Arthritis Rheumatol. 2016, 68, 2795–2805. [Google Scholar] [CrossRef] [PubMed]
- Ben-Zvi, I.; Danilesko, I.; Yahalom, G.; Kukuy, O.; Rahamimov, R.; Livneh, A.; Kivity, S. Risk factors for amyloidosis and impact of kidney transplantation on the course of familial Mediterranean fever. Isr. Med. Assoc. J. 2012, 14, 221–224. [Google Scholar] [PubMed]
- Labrousse, M.; Kevorkian-Verguet, C.; Boursier, G.; Rowczenio, D.; Maurier, F.; Lazaro, E.; Aggarwal, M.; Lemelle, I.; Mura, T.; Belot, A.; et al. Mosaicism in autoinflammatory diseases: Cryopyrin-associated periodic syndromes (CAPS) and beyond. A systematic review. Crit. Rev. Clin. Lab. Sci. 2018, 55, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Bas, B.; Sayarlioglu, H.; Yarar, Z.; Dilek, M.; Arik, N.; Sayarlioglu, M. Investigation of the relationship between disease severity and development of amyloidosis and genetic mutation in FMF disease. Ir. J. Med. Sci. 2023, 192, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- Lachmann, H.J.; Papa, R.; Gerhold, K.; Obici, L.; Touitou, I.; Cantarini, L.; Frenkel, J.; Anton, J.; Kone-Paut, I.; Cattalini, M.; et al. The phenotype of TNF receptor-associated autoinflammatory syndrome (TRAPS) at presentation: A series of 158 cases from the Eurofever/EUROTRAPS international registry. Ann. Rheum. Dis. 2014, 73, 2160–2167. [Google Scholar] [CrossRef] [PubMed]
- Vitale, A.; Lucherini, O.M.; Galeazzi, M.; Frediani, B.; Cantarini, L. Long-term clinical course of patients carrying the Q703K mutation in the NLRP3 gene: A case series. Clin. Exp. Rheumatol. 2012, 30, 943–946. [Google Scholar] [PubMed]
- Paul, S.; Marotte, H.; Kavanaugh, A.; Goupille, P.; Kvien, T.K.; de Longueville, M.; Mulleman, D.; Sandborn, W.J.; Vande Casteele, N. Exposure-Response Relationship of Certolizumab Pegol and Achievement of Low Disease Activity and Remission in Patients with Rheumatoid Arthritis. Clin. Transl. Sci. 2020, 13, 743–751. [Google Scholar] [CrossRef]
- Ternant, D.; Azzopardi, N.; Raoul, W.; Bejan-Angoulvant, T.; Paintaud, G. Influence of Antigen Mass on the Pharmacokinetics of Therapeutic Antibodies in Humans. Clin. Pharmacokinet. 2019, 58, 169–187. [Google Scholar] [CrossRef]
- Caorsi, R.; Lepore, L.; Zulian, F.; Alessio, M.; Stabile, A.; Insalaco, A.; Finetti, M.; Battagliese, A.; Martini, G.; Bibalo, C.; et al. The schedule of administration of canakinumab in cryopyrin associated periodic syndrome is driven by the phenotype severity rather than the age. Arthritis Res. Ther. 2013, 15, R33. [Google Scholar] [CrossRef]
- Knieper, A.M.; Klotsche, J.; Lainka, E.; Berger, T.; Dressler, F.; Jansson, A.F.; Rietschel, C.; Oommen, P.T.; Berendes, R.; Niehues, T.; et al. Familial Mediterranean fever in children and adolescents: Factors for colchicine dosage and predicting parameters for dose increase. Rheumatology 2017, 56, 1597–1606. [Google Scholar] [CrossRef]
- Cohen, K.; Spielman, S.; Semo-Oz, R.; Bitansky, G.; Gerstein, M.; Yacobi, Y.; Vivante, A.; Tirosh, I. Colchicine treatment can be discontinued in a selected group of pediatric FMF patients. Pediatr. Rheumatol. Online J. 2023, 21, 2. [Google Scholar] [CrossRef] [PubMed]
- Shehadeh, K.; Levinsky, Y.; Kagan, S.; Zuabi, T.; Tal, R.; Aviran, N.H.; Butbul Aviel, Y.; Tirosh, I.; Spielman, S.; Miller-Barmak, A.; et al. An “On Demand” canakinumab regimen for treating children with Colchicine-Resistant familial Mediterranean fever—A multicentre study. Int. Immunopharmacol. 2024, 132, 111967. [Google Scholar] [CrossRef] [PubMed]
- Duzova, A.; Bakkaloglu, A.; Besbas, N.; Topaloglu, R.; Ozen, S.; Ozaltin, F.; Bassoy, Y.; Yilmaz, E. Role of A-SAA in monitoring subclinical inflammation and in colchicine dosage in familial Mediterranean fever. Clin. Exp. Rheumatol. 2003, 21, 509–514. [Google Scholar] [PubMed]
- Russell, M.D.; Bukhari, M.; Galloway, J. The price of good health care. Rheumatology 2019, 58, 931–932. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Cameron, C.; Noorbaloochi, S.; Cullis, T.; Tucker, M.; Christensen, R.; Ghogomu, E.T.; Coyle, D.; Clifford, T.; Tugwell, P.; et al. Risk of serious infection in biological treatment of patients with rheumatoid arthritis: A systematic review and meta-analysis. Lancet 2015, 386, 258–265. [Google Scholar] [CrossRef]
- Welzel, T.; Golhen, K.; Atkinson, A.; Gotta, V.; Ternant, D.; Kuemmerle-Deschner, J.B.; Michler, C.; Koch, G.; van den Anker, J.N.; Pfister, M.; et al. Prospective study to characterize adalimumab exposure in pediatric patients with rheumatic diseases. Pediatr. Rheumatol. Online J. 2024, 22, 5. [Google Scholar] [CrossRef] [PubMed]
- Kavrul Kayaalp, G.; Caglayan, S.; Demirkan, F.G.; Guliyeva, V.; Otar Yener, G.; Ozturk, K.; Demir, F.; Ozdel, S.; Cakan, M.; Sonmez, H.E.; et al. Is it possible to extend the dose interval of canakinumab treatment in children with familial Mediterranean fever? PeRA group experience. Pediatr. Rheumatol. Online J. 2023, 21, 140. [Google Scholar] [CrossRef] [PubMed]
- Ruperto, N.; Martini, A. International research networks in pediatric rheumatology: The PRINTO perspective. Curr. Opin. Rheumatol. 2004, 16, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Wittkowski, H.; Kuemmerle-Deschner, J.B.; Austermann, J.; Holzinger, D.; Goldbach-Mansky, R.; Gramlich, K.; Lohse, P.; Jung, T.; Roth, J.; Benseler, S.M.; et al. MRP8 and MRP14, phagocyte-specific danger signals, are sensitive biomarkers of disease activity in cryopyrin-associated periodic syndromes. Ann. Rheum. Dis. 2011, 70, 2075–2081. [Google Scholar] [CrossRef]
- Cakan, M.; Karadag, S.G.; Tanatar, A.; Sonmez, H.E.; Ayaz, N.A. The Value of Serum Amyloid A Levels in Familial Mediterranean Fever to Identify Occult Inflammation during Asymptomatic Periods. J. Clin. Rheumatol. 2021, 27, 1–4. [Google Scholar] [CrossRef]
- Lieber, M.; Kallinich, T.; Lohse, P.; Klotsche, J.; Holzinger, D.; Foell, D.; Wittkowski, H. Increased serum concentrations of neutrophil-derived protein S100A12 in heterozygous carriers of MEFV mutations. Clin. Exp. Rheumatol. 2015, 33, S113–S116. [Google Scholar] [PubMed]
- Desthieux, C.; Hermet, A.; Granger, B.; Fautrel, B.; Gossec, L. Patient-Physician Discordance in Global Assessment in Rheumatoid Arthritis: A Systematic Literature Review with Meta-Analysis. Arthritis Care Res. 2016, 68, 1767–1773. [Google Scholar] [CrossRef] [PubMed]
- Floris, A.; Espinosa, G.; Serpa Pinto, L.; Kougkas, N.; Lo Monaco, A.; Lopalco, G.; Orlando, I.; Bertsias, G.; Cantarini, L.; Cervera, R.; et al. Discordance between patient and physician global assessment of disease activity in Behcet’s syndrome: A multicenter study cohort. Arthritis Res. Ther. 2020, 22, 278. [Google Scholar] [CrossRef] [PubMed]
- Challa, D.N.V.; Crowson, C.S.; Davis, J.M., 3rd. The Patient Global Assessment of Disease Activity in Rheumatoid Arthritis: Identification of Underlying Latent Factors. Rheumatol. Ther. 2017, 4, 201–208. [Google Scholar] [CrossRef]
- Monti, S.; Delvino, P.; Klersy, C.; Coppa, G.; Milanesi, A.; Montecucco, C. Factors influencing patient-reported outcomes in ANCA-associated vasculitis: Correlates of the Patient Global Assessment. Semin. Arthritis Rheum. 2022, 56, 152048. [Google Scholar] [CrossRef]
- Incesu, C.; Kayaalp, G.K.; Demirkan, F.G.; Koker, O.; Cakmak, F.; Akgun, O.; Ayaz, N.A.; Omeroglu, R.N. The assessment of fatigue and sleep quality among children and adolescents with familial Mediterranean fever: A case-control and correlation study. Eur. J. Pediatr. 2024. [Google Scholar] [CrossRef]


| Total n = 56 (100%) | FMF n = 46 (82%) | CAPS n = 9 (16%) | TRAPS n = 1 (2%) | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Female 1 | 19 (34) | 17 (37) | 2 (22) | 0 |
| Symptom onset, age in years 2 | 2.5 (0.5; 4.1) | 2.9 (1.6; 4.7) | 0.3 (0.2; 0.5) | 2.7 |
| Diagnosis, age in years 2 | 4.9 (3.0; 7.7) | 5.1 (3.6; 7.5) | 2.8 (1.9; 4.5) | 12.9 |
| First study visit, age in years 2 | 4.9 (3.3; 8.1) | 5.1 (3.7; 8.0) | 3.2 (1.9; 4.5) | 13.0 |
| Follow-up, years 2 | 2.1 (1.4; 2.7) | 1.9 (1.4; 2.5) | 2.5 (2.3; 2.9) | 3.0 |
| Genetic variants | ||||
| Pathogenic/likely pathogenic 1 | 28 (50) | 25 (54) | 2 (22) | 1 (100) |
| VUS 1 | 11 (20) | 4 (9) | 7 (78) | 0 |
| Total n = 56 | FMF n = 46 | CAPS n = 9 | TRAPS n = 1 | |
|---|---|---|---|---|
| First episode of increased disease activity, n (%) | 36 (64) | 30 (65) | 5 (56) | 1 (100) |
| Treatment adjustment in 28 out of 36 children * | ||||
| New treatment start, n (%) | 19 (34) | 15 (33) | 3 (33) | 1(100) |
| Treatment switch n, (%) | 4 (7) | 3 (7) | 1 (11) | 0 (0) |
| Dose increase n, (%) | 8 (14) | 7 (15) | 1 (11) | 0 (0) |
| Administration frequency increase, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Subsequent episode of increased disease activity, n (%) | 17 (30) | 13 (28) | 4 (44) | 0 (0) |
| Treatment adjustments in 7 out of 17 children * | ||||
| New treatment start, n (%) | 2 (4) | 2 (4) | 0 (0) | 0 (0) |
| Treatment switch, n (%) | 1 (2) | 1 (2) | 0 (0) | 0 (0) |
| Dose increase, n (%) | 3 (5) | 1 (2) | 2 (22) | 0 (0) |
| Administration frequency increase, n (%) | 1 (2) | 0 (0) | 1 (11) | 0 (0) |
| IL-1 AID Subgroups | |||||||||
| CAPS | FMF | TRAPS | |||||||
| beta 1 | 95%CI | p Value | beta 1 | 95%CI | p Value | beta 1 | 95%CI | p Value | |
| PGA | −0.12 | −0.16; −0.08 | <0.001 | −0.15 | −0.24; −0.06 | 0.001 | −0.11 | −0.17; −0.06 | <0.001 |
| PPGA | −0.09 | −0.19; 0.02 | 0.100 | −0.11 | −0.32; 0.10 | 0.304 | −0.08 | −0.21; 0.05 | 0.247 |
| CRP | −0.05 | −0.10; −0.01 | 0.026 | −0.10 | −0.23; 0.03 | 0.130 | −0.04 | −0.09; 0.01 | 0.093 |
| SAA | −2.49 | −5.68; 0.71 | 0.127 | −4.21 | −10.62; 2.21 | 0.199 | −2.26 | −5.99; 1.47 | 0.236 |
| Genotypes | |||||||||
| Pathogenic/likely pathogenic variants | VUS | No genetic testing/no VUS or (likely) pathogenic variants detected | |||||||
| beta 1 | 95%CI | p value | beta 1 | 95%CI | p value | beta 1 | 95%CI | p value | |
| PGA | −0.13 | −0.19; −0.08 | <0.001 | −0.09 | −0.17; −0.01 | 0.020 | −0.15 | −0.26; −0.05 | 0.004 |
| PPGA | −0.09 | −0.23; 0.06 | 0.225 | −0.08 | −0.22; 0.06 | 0.280 | −0.07 | −0.28; 0.14 | 0.526 |
| CRP | −0.07 | −0.16; 0.01 | 0.073 | −0.05 | −0.12; 0.01 | 0.111 | −0.04 | −0.13; 0.05 | 0.444 |
| SAA | −3.18 | −8.97; 2.62 | 0.283 | −2.67 | −6.15; 0.80 | 0.132 | −1.73 | −10.25; 6.79 | 0.691 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Welzel, T.; Zapf, B.; Klotsche, J.; Satirer, Ö.; Benseler, S.M.; Kuemmerle-Deschner, J.B. Optimized Treatment of Interleukin (IL-1)-Mediated Autoinflammatory Diseases: Impact of Disease Activity-Based Treatment Adjustments. J. Clin. Med. 2024, 13, 2319. https://doi.org/10.3390/jcm13082319
Welzel T, Zapf B, Klotsche J, Satirer Ö, Benseler SM, Kuemmerle-Deschner JB. Optimized Treatment of Interleukin (IL-1)-Mediated Autoinflammatory Diseases: Impact of Disease Activity-Based Treatment Adjustments. Journal of Clinical Medicine. 2024; 13(8):2319. https://doi.org/10.3390/jcm13082319
Chicago/Turabian StyleWelzel, Tatjana, Beate Zapf, Jens Klotsche, Özlem Satirer, Susanne M. Benseler, and Jasmin B. Kuemmerle-Deschner. 2024. "Optimized Treatment of Interleukin (IL-1)-Mediated Autoinflammatory Diseases: Impact of Disease Activity-Based Treatment Adjustments" Journal of Clinical Medicine 13, no. 8: 2319. https://doi.org/10.3390/jcm13082319





