Role of Lung Ultrasound in the Detection of Lung Sequelae in Post-COVID-19 Patients: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Material and Methods
2.1. Eligibility Criteria
2.2. Information Sources
2.3. Search Strategy
2.4. Selection Process
2.5. Data Collection Process
2.6. Data Items
2.7. Study Risk of Bias Assessment
2.8. Effect Measures
2.9. Synthesis Methods, Reporting Bias Assessment and Certainty Assessment
3. Results
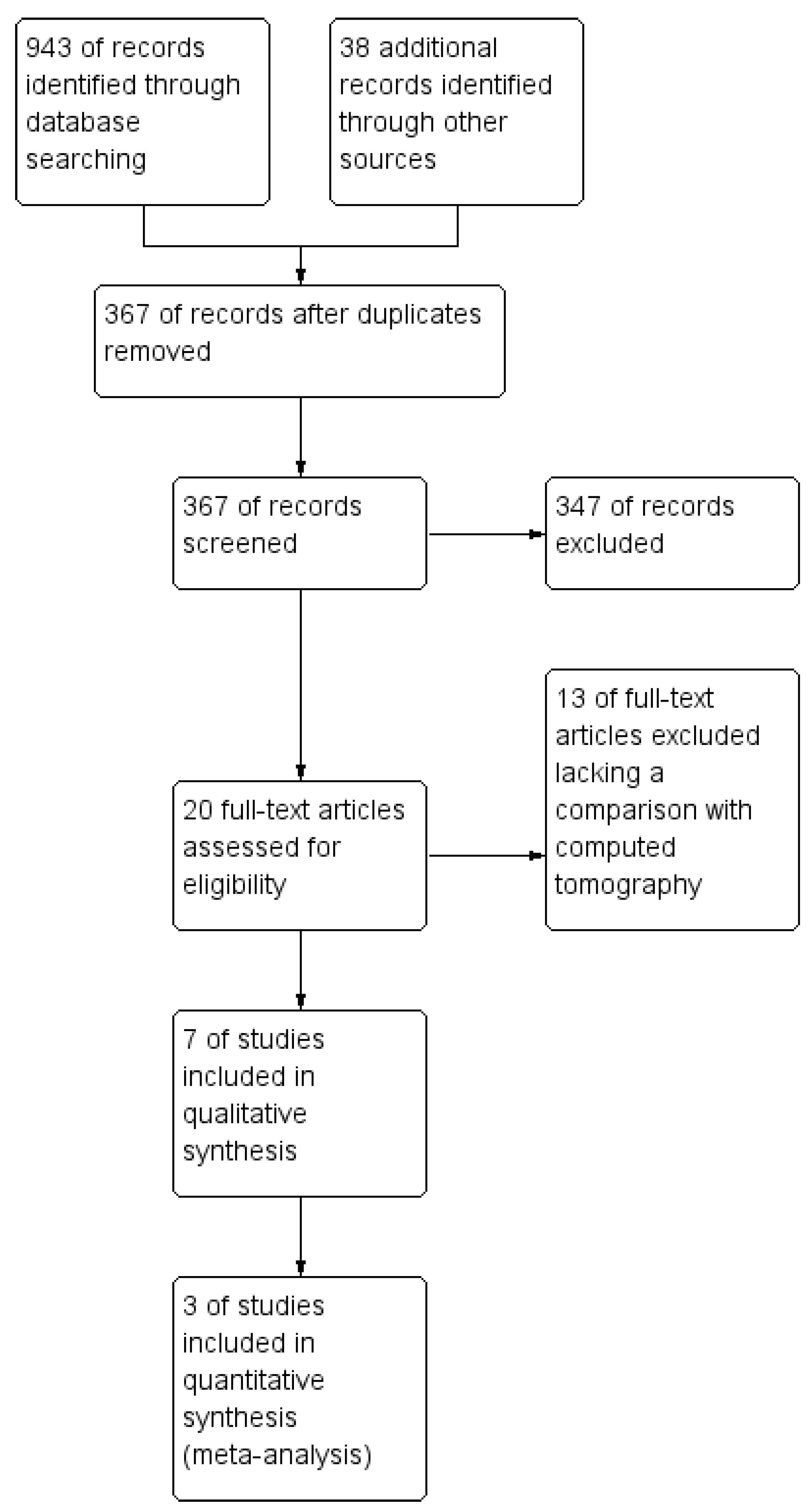
3.1. Study Identification
3.2. Study Characteristics
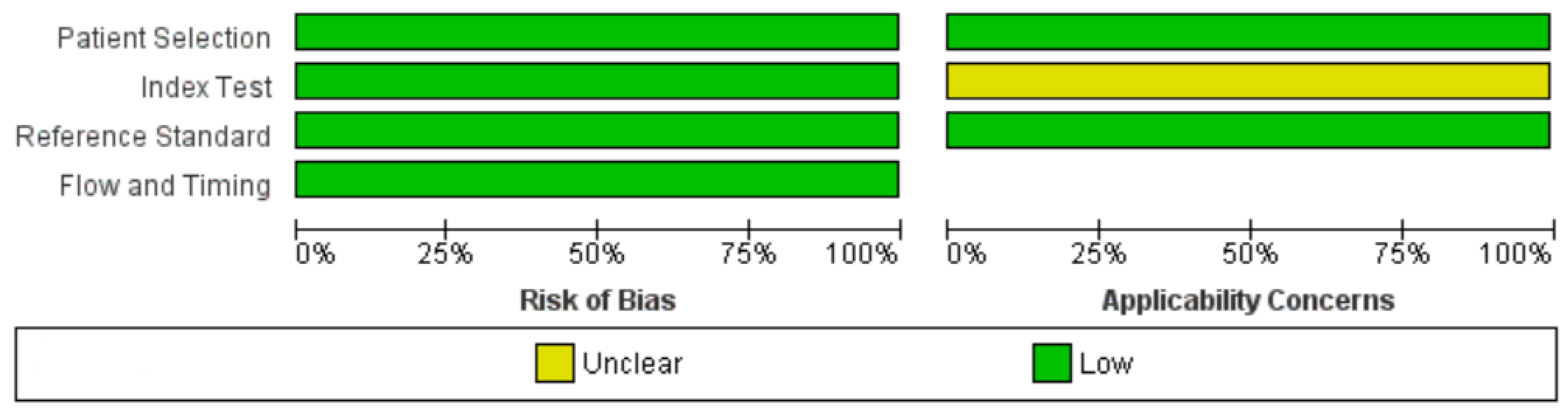
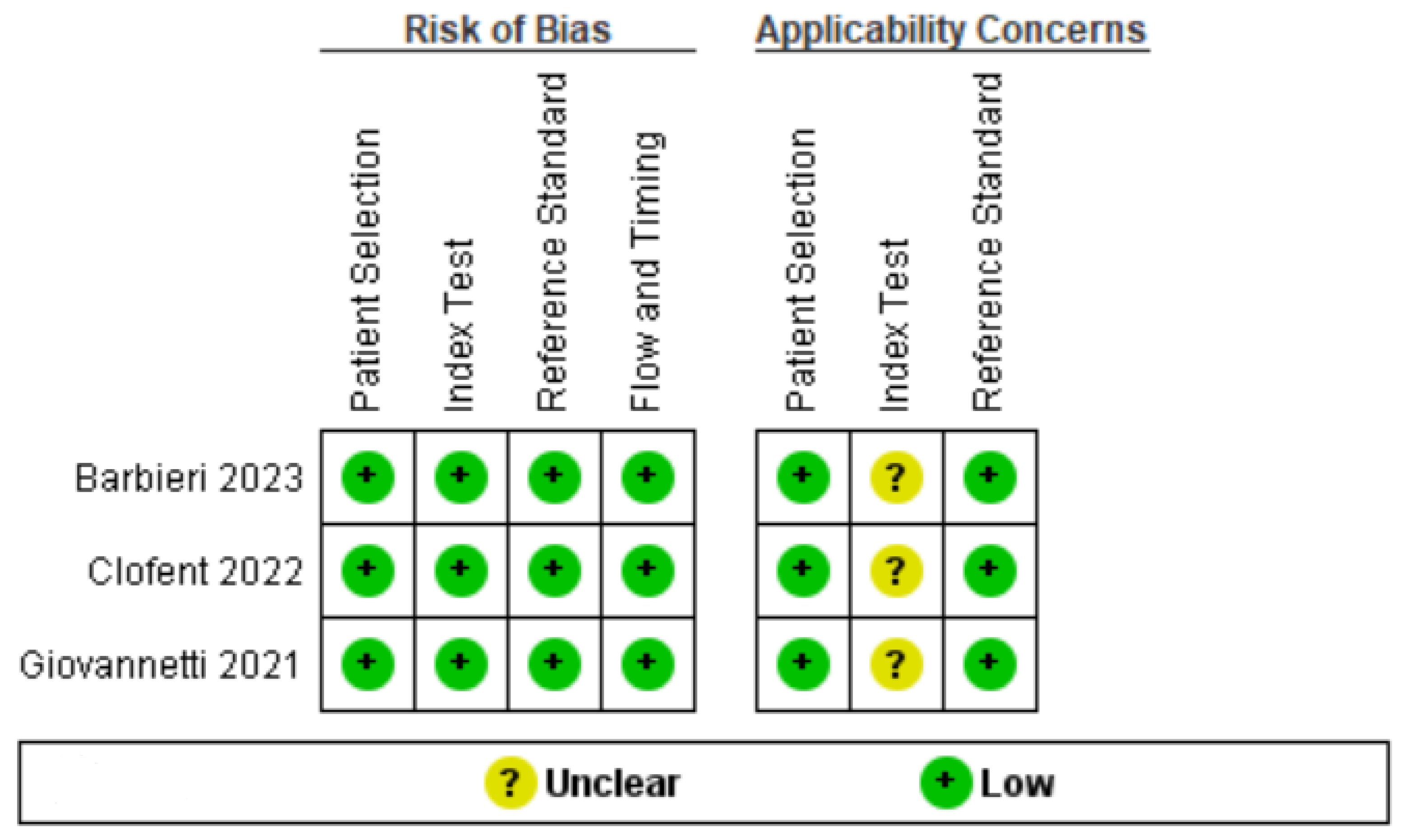
3.3. Quality Assessment
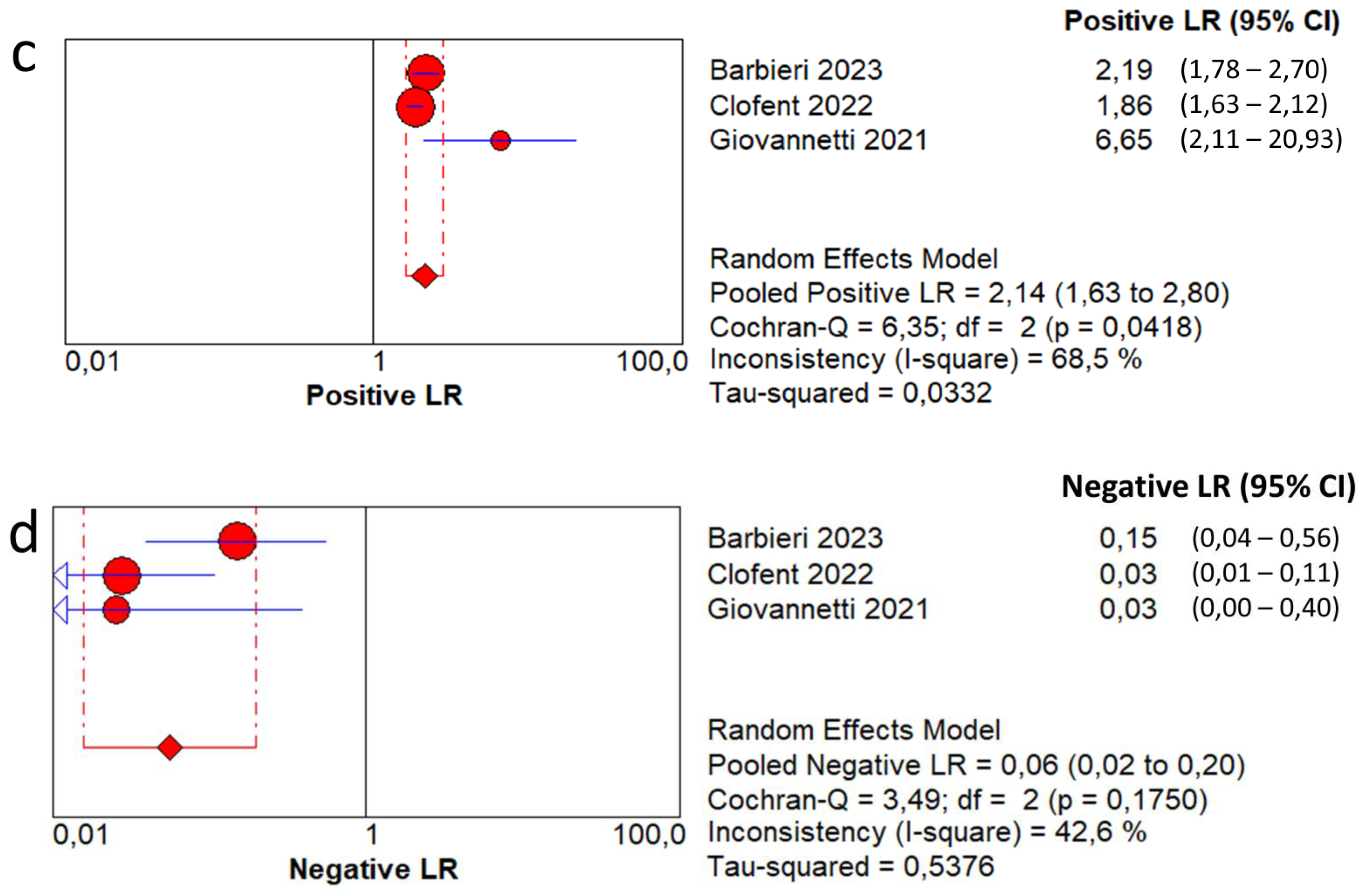
3.4. Overall Diagnostic Accuracy of Lung Ultrasound to Detect Fibrotic-like Changes (MODEL 1)
3.5. Overall Diagnostic Accuracy of Lung Ultrasound to Detect Fibrotic-like Changes (MODEL 2)
3.6. Publication Bias
4. Discussion
Other Pathological Lung Ultrasound Findings
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
- PubMed
- #1 “post COVID-19” [Supplementary Concept]
- #2 “post Severe Acute Respiratory Syndrome Coronavirus 2” [Supplementary Concept]
- #3 “post COVID-19” [Title/Abstract]
- #4 “post SARS-COV-2” [Title/Abstract]
- #5 “post novel coronavirus” [Title/Abstract]
- #6 “post 2019-novel coronavirus” [Title/Abstract]
- #7 “post coronavirus disease-19” [Title/Abstract]
- #8 “post coronavirus disease 2019” [Title/Abstract]
- #9 “post COVID19” [Title/Abstract]
- #10 “post Novel CoV” [Title/Abstract]
- #11 “post 2019-nCoV” [Title/Abstract]
- #12 “post 2019-CoV” [Title/Abstract]
- #13 OR/#1-12
- #14 lung ultrasound* [Title/Abstract]
- #15 lung POCUS [Title/Abstract]
- #16 lung ultrasound [MeSH Terms]
- #17 OR/#14-16
- #18 “lung radiography, thoracic” [MeSH Terms]
- #19 “lung computed tomography” [Title/Abstract]
- #20 “lung radiograph*” [Title/Abstract]
- #21 “lung imagin*” [Title/Abstract]
- #22 OR/#18-#21
- #23 #13 AND #17 AND #22
- Cochrane library
- #1 “post COVID-19”:ti,ab,kw
- #2 “post SARS-COV-2”:ti,ab,kw
- #3 “post Novel coronavirus”:ti,ab,kw
- #4 “post 2019-novel coronavirus”:ti,ab,kw
- #5 “post Novel CoV”:ti,ab,kw
- #6 “post 2019-nCoV”:ti,ab,kw
- #7 “post 2019-CoV”:ti,ab,kw
- #8 “post coronavirus disease-19”:ti,ab,kw
- #9 “post coronavirus disease 2019”:ti,ab,kw
- #10 “post COVID19”:ti,ab,kw
- #11 OR/#1-10
- #12 MeSH descriptor: [Lung Ultrasonography] explode all trees
- #13 (lung ultrasound*):ti,ab,kw
- #14 (lung ultrasonography*):ti,ab,kw
- #15 lung POCUS:ti,ab,kw
- #16 OR/12-15
- #17 #11 AND #16
- Embase
- #1. ‘post COVID-19’:ab,ti
- #2. ‘post SARS-COV-2’:ab,ti
- #3. ‘post novel coronavirus’:ab,ti
- #4. ‘post 2019-novel coronavirus’:ab,ti
- #5. ‘post coronavirus disease-19’:ab,ti
- #6. ‘post coronavirus disease 2019’:ab,ti
- #7. ‘post COVID19’:ab,ti
- #8. ‘post novel cov’:ab,ti
- #9. ‘post 2019-ncov’:ab,ti
- #10. ‘post 2019-cov’:ab,ti
- #11. ‘post coronavirus disease 2019’/exp
- #12. OR/#1-11
- #13. ‘lung ultrasound’/exp
- #14. Lung pocus:ti,ab
- #15. Lung ultrasound:ti,ab
- #16. OR/#13-15
- #17. #12 AND #16
References
- Cheung, O.Y.; Chan, J.W.; Ng, C.K.; Koo, C.K. The spectrum of pathological changes in severe acute respiratory syndrome (SARS). Histopathology 2004, 45, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.K.; Chan, J.W.; Kwan, T.L.; To, T.S.; Chan, Y.H.; Ng, F.Y.; Mok, T.Y. Six month radiological and physiological outcomes in severe acute respiratory syndrome (SARS) survivors. Thorax 2004, 59, 889–891. [Google Scholar] [CrossRef] [PubMed]
- Hui, D.S.; Wong, K.T.; Ko, F.W.; Tam, L.S.; Chan, D.P.; Woo, J.; Sung, J.J. The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest 2005, 128, 2247–2261. [Google Scholar] [CrossRef] [PubMed]
- Carfì, A.; Bernabei, R.; Landi, F. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, C.; Hu, Y.; Li, C.; Ren, Q.; Zhang, X.; Shi, H.; Zhou, M. Temporal Changes of CT Findings in 90 Patients with COVID-19 Pneumonia: A Longitudinal Study. Radiology 2020, 296, E55–E64. [Google Scholar] [CrossRef]
- Guler, S.A.; Ebner, L.; Aubry-Beigelman, C.; Bridevaux, P.O.; Brutsche, M.; Clarenbach, C.; Garzoni, C.; Geiser, T.K.; Lenoir, A.; Mancinetti, M.; et al. Pulmonary function and radiological features 4 months after COVID-19: First results from the national prospective observational Swiss COVID-19 lung study. Eur. Respir. J. 2021, 57, 2003690. [Google Scholar] [CrossRef]
- González, J.; Benítez, I.D.; Carmona, P.; Santisteve, S.; Monge, A.; Moncusí-Moix, A.; Gort-Paniello, C.; Pinilla, L.; Carratalá, A.; Zuil, M.; et al. Pulmonary Function and Radiologic Features in Survivors of Critical COVID-19: A 3-Month Prospective Cohort. Chest 2021, 160, 187–198. [Google Scholar] [CrossRef]
- Culebras, M.; Loor, K.; Sansano, I.; Persiva, Ó.; Clofent, D.; Polverino, E.; Felipe, A.; Osorio, J.; Muñoz, X.; Álvarez, A. Histological Findings in Transbronchial Cryobiopsies Obtained From Patients After COVID-19. Chest 2022, 161, 647–650. [Google Scholar] [CrossRef]
- Zheng, Z.; Yao, Z.; Wu, K.; Zheng, J. Patient follow-up after discharge after COVID-19 pneumonia: Considerations for infectious control. J. Med. Virol. 2020, 92, 2412–2419. [Google Scholar] [CrossRef]
- Raghu, G.; Wilson, K.C. COVID-19 interstitial pneumonia: Monitoring the clinical course in survivors. Lancet Respir. Med. 2020, 8, 839–842. [Google Scholar] [CrossRef]
- Rubin, G.D.; Ryerson, C.J.; Haramati, L.B.; Sverzellati, N.; Kanne, J.P.; Raoof, S.; Schluger, N.W.; Volpi, A.; Yim, J.J.; Martin, I.B.K.; et al. The Role of Chest Imaging in Patient Management During the COVID-19 Pandemic: A Multinational Consensus Statement From the Fleischner Society. Chest 2020, 158, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Chotirmall, S.H.; Rello, J.; Alba, G.A.; Ginns, L.C.; Krishnan, J.A.; Rogers, R.; Bendstrup, E.; Burgel, P.R.; Chalmers, J.D.; et al. Updated guidance on the management of COVID-19: From an American Thoracic Society/European Respiratory Society coordinated International Task Force (29 July 2020). Eur. Respir. Rev. 2020, 29, 200287. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, G.; Gargani, L.; Lepri, V.; Spinelli, S.; Romei, C.; De Liperi, A.; Chimera, D.; Pistelli, F.; Carrozzi, L.; Corradi, F.; et al. Long-term lung ultrasound follow-up in patients after COVID-19 pneumonia hospitalization: A prospective comparative study with chest computed tomography. Eur. J. Intern. Med. 2023, 110, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Clofent, D.; Polverino, E.; Felipe, A.; Granados, G.; Arjona-Peris, M.; Andreu, J.; Sánchez-Martínez, A.L.; Varona, D.; Cabanzo, L.; Escudero, J.M.; et al. Lung Ultrasound as a First-Line Test in the Evaluation of Post-COVID-19 Pulmonary Sequelae. Front. Med. 2022, 8, 815732. [Google Scholar] [CrossRef] [PubMed]
- Giovannetti, G.; De Michele, L.; De Ceglie, M.; Pierucci, P.; Mirabile, A.; Vita, M.; Palmieri, V.O.; Carpagnano, G.E.; Scardapane, A.; D’Agostino, C. Lung ultrasonography for long-term follow-up of COVID-19 survivors compared to chest CT scan. Respir. Med. 2021, 181, 106384. [Google Scholar] [CrossRef]
- Boccatonda, A.; Grignaschi, A.; Lanotte, A.M.G.; Cocco, G.; Vidili, G.; Giostra, F.; Schiavone, C. Role of Lung Ultrasound in the Management of Patients with Suspected SARS-CoV-2 Infection in the Emergency Department. J. Clin. Med. 2022, 11, 2067. [Google Scholar] [CrossRef]
- Barskova, T.; Gargani, L.; Guiducci, S.; Randone, S.B.; Bruni, C.; Carnesecchi, G.; Conforti, M.L.; Porta, F.; Pignone, A.; Caramella, D.; et al. Lung ultrasound for the screening of interstitial lung disease in very early systemic sclerosis. Ann. Rheum. Dis. 2013, 72, 390–395. [Google Scholar] [CrossRef]
- Boccatonda, A.; Andreetto, L.; Vicari, S.; Campello, E.; Simioni, P.; Ageno, W. The Diagnostic Role of Lung Ultrasound and Contrast-Enhanced Ultrasound in Pulmonary Embolism. Semin. Thromb. Hemost. 2024, 50, 842–850. [Google Scholar] [CrossRef]
- Boccatonda, A.; Cocco, G.; D’Ardes, D.; Delli Pizzi, A.; Vidili, G.; De Molo, C.; Vicari, S.; Serra, C.; Cipollone, F.; Schiavone, C.; et al. Infectious Pneumonia and Lung Ultrasound: A Review. J. Clin. Med. 2023, 12, 1402. [Google Scholar] [CrossRef]
- Brogi, E.; Gargani, L.; Bignami, E.; Barbariol, F.; Marra, A.; Forfori, F.; Vetrugno, L. Thoracic ultrasound for pleural effusion in the intensive care unit: A narrative review from diagnosis to treatment. Crit. Care 2017, 21, 325. [Google Scholar] [CrossRef]
- Inchingolo, R.; Copetti, R.; Smargiassi, A.; Gerardi, R.E.; Conte, E.G.; Corbo, G.M.; Gatto, A.; Pierandrei, C.; Capossela, L.; Lazzareschi, I.; et al. Air bronchogram integrated lung ultrasound score to monitor community-acquired pneumonia in a pilot pediatric population. J. Ultrasound 2021, 24, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D. Lung ultrasound in the critically ill. Curr. Opin. Crit. Care 2014, 20, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.; Goldstein, I.; Mourgeon, E.; Cluzel, P.; Grenier, P.; Rouby, J.J. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology 2004, 100, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.A. BLUE-protocol and FALLS-protocol: Two applications of lung ultrasound in the critically ill. Chest 2015, 147, 1659–1670. [Google Scholar] [CrossRef]
- Volpicelli, G.; Gargani, L.; Perlini, S.; Spinelli, S.; Barbieri, G.; Lanotte, A.; Casasola, G.G.; Nogué-Bou, R.; Lamorte, A.; Agricola, E.; et al. Lung ultrasound for the early diagnosis of COVID-19 pneumonia: An international multicenter study. Intensive Care Med. 2021, 47, 444–454. [Google Scholar] [CrossRef]
- Altersberger, M.; Goliasch, G.; Khafaga, M.; Schneider, M.; Cho, Y.; Winkler, R.; Funk, G.C.; Binder, T.; Huber, G.; Zwick, R.H.; et al. Echocardiography and Lung Ultrasound in Long COVID and Post-COVID Syndrome, a Review Document of the Austrian Society of Pneumology and the Austrian Society of Ultrasound in Medicine. J. Ultrasound Med. 2023, 42, 269–277. [Google Scholar] [CrossRef]
- Alilou, S.; Zangiabadian, M.; Pouramini, A.; Jaberinezhad, M.; Shobeiri, P.; Ghozy, S.; Haseli, S.; Beizavi, Z. Radiological Findings as Predictors of COVID-19 Lung Sequelae: A Systematic Review and Meta-analysis. Acad. Radiol. 2023, 30, 3076–3085. [Google Scholar] [CrossRef]
- González-Suárez, S.; Barbara Ferreras, A.; Caicedo Toro, M.; Aznar de Legarra, M. Detection of residual pulmonary alterations with lung ultrasound and effects on postoperative pulmonary complications for patients with asymptomatic SARS-CoV-2 infection undergoing surgeries. BMC Anesthesiol. 2022, 22, 186. [Google Scholar] [CrossRef]
- Guinto, E.; Gerayeli, F.V.; Eddy, R.L.; Lee, H.; Milne, S.; Sin, D.D. Post-COVID-19 dyspnoea and pulmonary imaging: A systematic review and meta-analysis. Eur. Respir. Rev. 2023, 32, 220253. [Google Scholar] [CrossRef]
- Gargani, L.; Doveri, M.; D’Errico, L.; Frassi, F.; Bazzichi, M.L.; Delle Sedie, A.; Scali, M.C.; Monti, S.; Mondillo, S.; Bombardieri, S.; et al. Ultrasound lung comets in systemic sclerosis: A chest sonography hallmark of pulmonary interstitial fibrosis. Rheumatology 2009, 48, 1382–1387. [Google Scholar] [CrossRef]
- Manolescu, D.; Oancea, C.; Timar, B.; Traila, D.; Malita, D.; Birsasteanu, F.; Tudorache, V. Ultrasound mapping of lung changes in idiopathic pulmonary fibrosis. Clin. Respir. J. 2020, 14, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. Open Med. 2009, 3, e123–e130. [Google Scholar] [PubMed]
- Whiting, P.; Rutjes, A.W.; Reitsma, J.B.; Bossuyt, P.M.; Kleijnen, J. The development of QUADAS: A tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med. Res. Methodol. 2003, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Moses, L.E.; Shapiro, D.; Littenberg, B. Combining independent studies of a diagnostic test into a summary ROC curve: Data-analytic approaches and some additional considerations. Stat. Med. 1993, 12, 1293–1316. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Loke, T.K.; Earl, N.; Begbey, A.C.H.; Sharma, N.; Wakeham, N.R.; Sohn, H.M.; Greenslade, S.J.; Ince, E.; Davey, M.; Cox, K. Lung ultrasound as a tool for monitoring the interstitial changes in recently hospitalised patients with COVID-19 pneumonia—The COVIDLUS study. Respir. Med. 2023, 210, 107176. [Google Scholar] [CrossRef]
- Ramos Hernández, C.; Tilve Gomez, A.; Sánchez Fernández, A.; Cordovilla, R.; Núñez Ares, A.; Ordoñez Gómez, P.; Wangüemert Pérez, A.; Castro Anón, O.; González Ramírez, J.; Valdivia Salas, M.; et al. Multicentre study on the accuracy of lung ultrasound in the diagnosis and monitoring of respiratory sequelae in the medium and long term in patients with COVID-19. Front. Med. 2023, 10, 1199666. [Google Scholar] [CrossRef]
- Russo, G.; Flor, N.; Casella, F.; Ippolito, S.; Leidi, F.; Casazza, G.; Radovanovic, D.; Vezzulli, F.; Santus, P.; Cogliati, C. Lung ultrasound in the follow-up of severe COVID-19 pneumonia: Six months evaluation and comparison with CT. Intern. Emerg. Med. 2022, 17, 2261–2268. [Google Scholar] [CrossRef]
- Zimna, K.; Sobiecka, M.; Wakuliński, J.; Wyrostkiewicz, D.; Jankowska, E.; Szturmowicz, M.; Tomkowski, W.Z. Lung Ultrasonography in the Evaluation of Late Sequelae of COVID-19 Pneumonia-A Comparison with Chest Computed Tomography: A Prospective Study. Viruses 2024, 16, 905. [Google Scholar] [CrossRef]
- Islam, N.; Ebrahimzadeh, S.; Salameh, J.P.; Kazi, S.; Fabiano, N.; Treanor, L.; Absi, M.; Hallgrimson, Z.; Leeflang, M.M.; Hooft, L.; et al. Thoracic imaging tests for the diagnosis of COVID-19. Cochrane Database Syst. Rev. 2021, 3, CD013639. [Google Scholar] [CrossRef] [PubMed]
- Simion, C.; Campello, E.; Boccatonda, A.; Tormene, D.; Spiezia, L.; Dalla Valle, F.; Sartori, M.; Perin, N.; Forestan, C.; Simioni, P. POST-discharge thromboprophylaxis in patients with COVID-19: A single-center experience. Intern. Emerg. Med. 2023, 18, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Perrone, T.; Falaschi, F.; Meloni, F.; Ballesio, A.; Sabatini, U.; Lenti, M.V.; Melazzini, F.; Lettieri, S.; Novati, S.; Cutti, S.; et al. A mid-term follow-up with a lung ultrasonographic score correlates with the severity of COVID-19 acute phase. Intern. Emerg. Med. 2023, 18, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Boccatonda, A.; D’Ardes, D.; Rossi, I.; Grignaschi, A.; Lanotte, A.; Cipollone, F.; Guagnano, M.T.; Giostra, F. Platelet Count in Patients with SARS-CoV-2 Infection: A Prognostic Factor in COVID-19. J. Clin. Med. 2022, 11, 4112. [Google Scholar] [CrossRef]
- Mafort, T.T.; Rufino, R.; da Costa, C.H.; da Cal, M.S.; Monnerat, L.B.; Litrento, P.F.; Parra, L.L.Z.; Marinho, A.; Lopes, A.J. One-month outcomes of patients with SARS-CoV-2 infection and their relationships with lung ultrasound signs. Ultrasound J. 2021, 13, 19. [Google Scholar] [CrossRef]
- Buonsenso, D.; Morello, R.; Mariani, F.; De Rose, C.; Cortese, R.; Vetrugno, L.; Valentini, P. Role of Lung Ultrasound in the Follow-Up of Children with Previous SARS-CoV-2 Infection: A Case-Control Assessment of Children with Long COVID or Fully Recovered. J. Clin. Med. 2023, 12, 3342. [Google Scholar] [CrossRef]
- Dearing, E.; Rempfer, E.; Frasure, S.E.; Akselrod, H.; Dobbs, J.E.; Poon, A.N.; Salazar, J.E.; Prajapati, D.; Boniface, K.S. Point-of-Care Ultrasound of Post-acute COVID-19 Syndrome: A Prospective Cohort Study. Cureus 2023, 15, e42569. [Google Scholar] [CrossRef]
- Demi, L.; Mento, F.; Di Sabatino, A.; Fiengo, A.; Sabatini, U.; Macioce, V.N.; Robol, M.; Tursi, F.; Sofia, C.; Di Cienzo, C.; et al. Lung Ultrasound in COVID-19 and Post-COVID-19 Patients, an Evidence-Based Approach. J. Ultrasound Med. 2022, 41, 2203–2215. [Google Scholar] [CrossRef]
- Eman, G.; Synn, S.; Galen, B.; Shah, R.; Nauka, P.; Hope, A.A.; Congdon, S.; Islam, M. Thoracic Ultrasound in COVID-19: Use of Lung and Diaphragm Ultrasound in Evaluating Dyspnea in Survivors of Acute Respiratory Distress Syndrome from COVID-19 Pneumonia in a Post-ICU Clinic. Lung 2023, 201, 149–157. [Google Scholar] [CrossRef]
- Gräger, S.; Pfirschke, R.; Lorenz, M.; Vilser, D.; Krämer, M.; Mentzel, H.J.; Glutig, K. Lung ultrasound in children and adolescents with long-term effects of COVID-19: Initial results. Front. Pediatr. 2023, 11, 1112881. [Google Scholar] [CrossRef]
- Gurbani, N.; Acosta-Sorensen, M.; Díaz-Pérez, D.; Figueira-Goncalves, J.M.; Ramallo-Fariña, Y.; Trujillo-Castilla, J.L. Clinical outcomes and lung ultrasound findings in COVID-19 follow up: Calm comes after the storm? Respir. Med. Res. 2022, 82, 100907. [Google Scholar] [CrossRef] [PubMed]
- Indolfi, C.; Klain, A.; Dinardo, G.; D’Addio, E.; Ferrara, S.; Decimo, F.; Ciprandi, G.; Tosca, M.A.; Miraglia Del Giudice, M. COVID-19 Pediatric Follow-Up: Respiratory Long COVID-Associated Comorbidities and Lung Ultrasound Alterations in a Cohort of Italian Children. Children 2024, 11, 166. [Google Scholar] [CrossRef] [PubMed]
- Calamai, I.; Greco, M.; Finazzi, S.; Savi, M.; Vitiello, G.; Garbero, E.; Spina, R.; Montisci, A.; Mongodi, S.; Bertolini, G. Thoracic UltrasONOgraphy Reporting: The TUONO Study. J. Clin. Med. 2022, 11, 7126. [Google Scholar] [CrossRef] [PubMed]
- Demi, L.; Wolfram, F.; Klersy, C.; De Silvestri, A.; Ferretti, V.V.; Muller, M.; Miller, D.; Feletti, F.; Wełnicki, M.; Buda, N.; et al. New International Guidelines and Consensus on the Use of Lung Ultrasound. J. Ultrasound Med. 2023, 42, 309–344. [Google Scholar] [CrossRef]

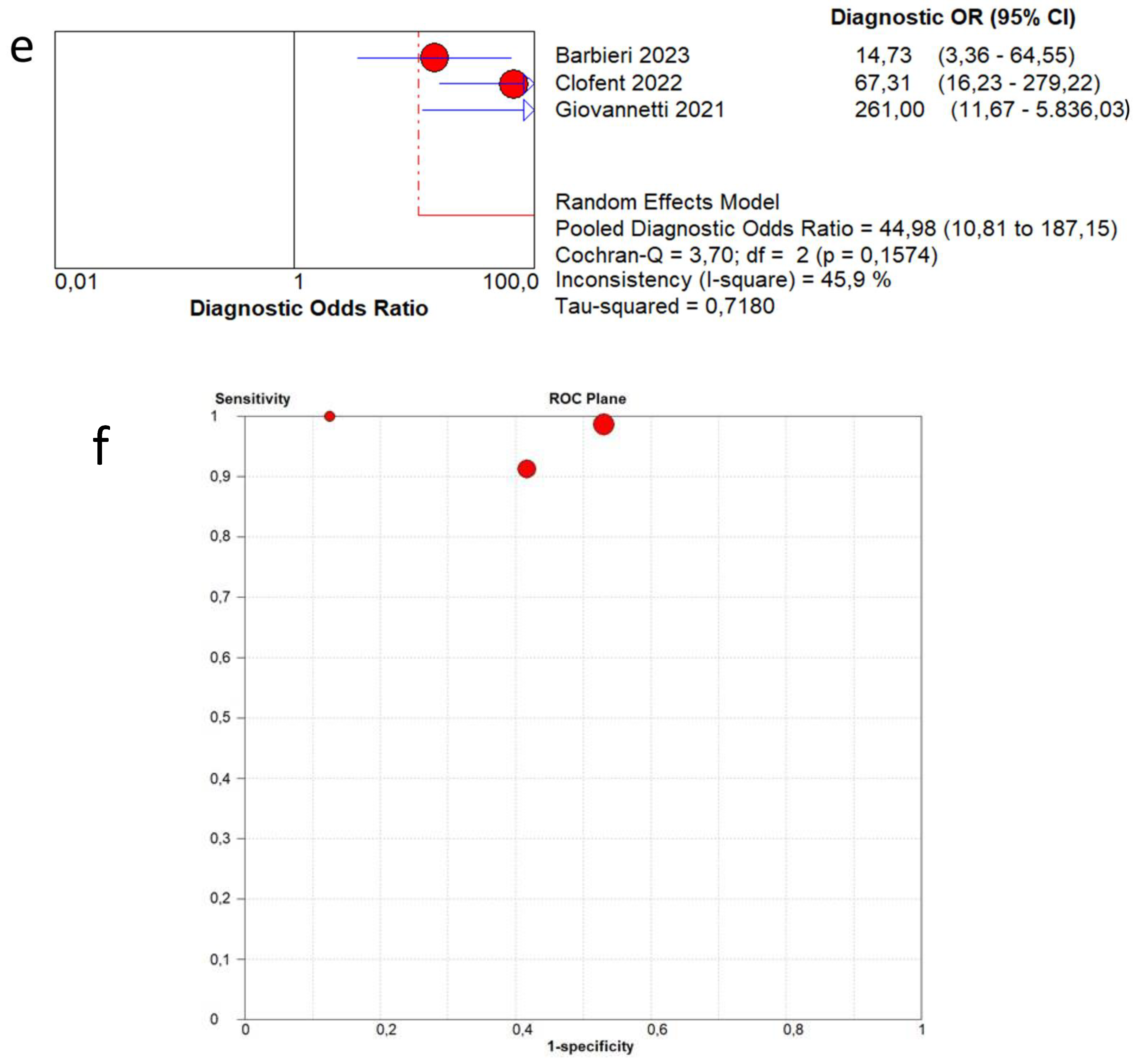
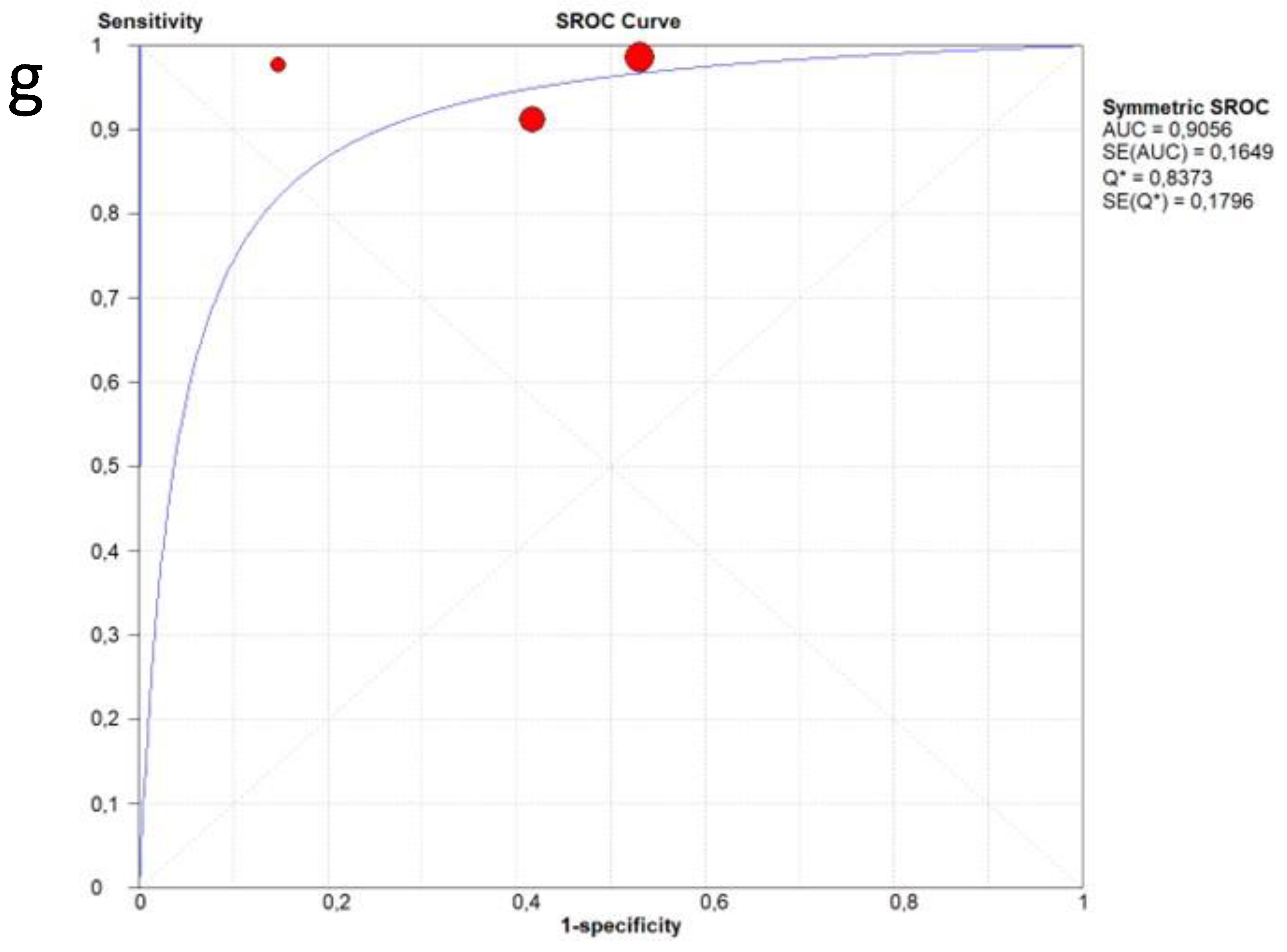


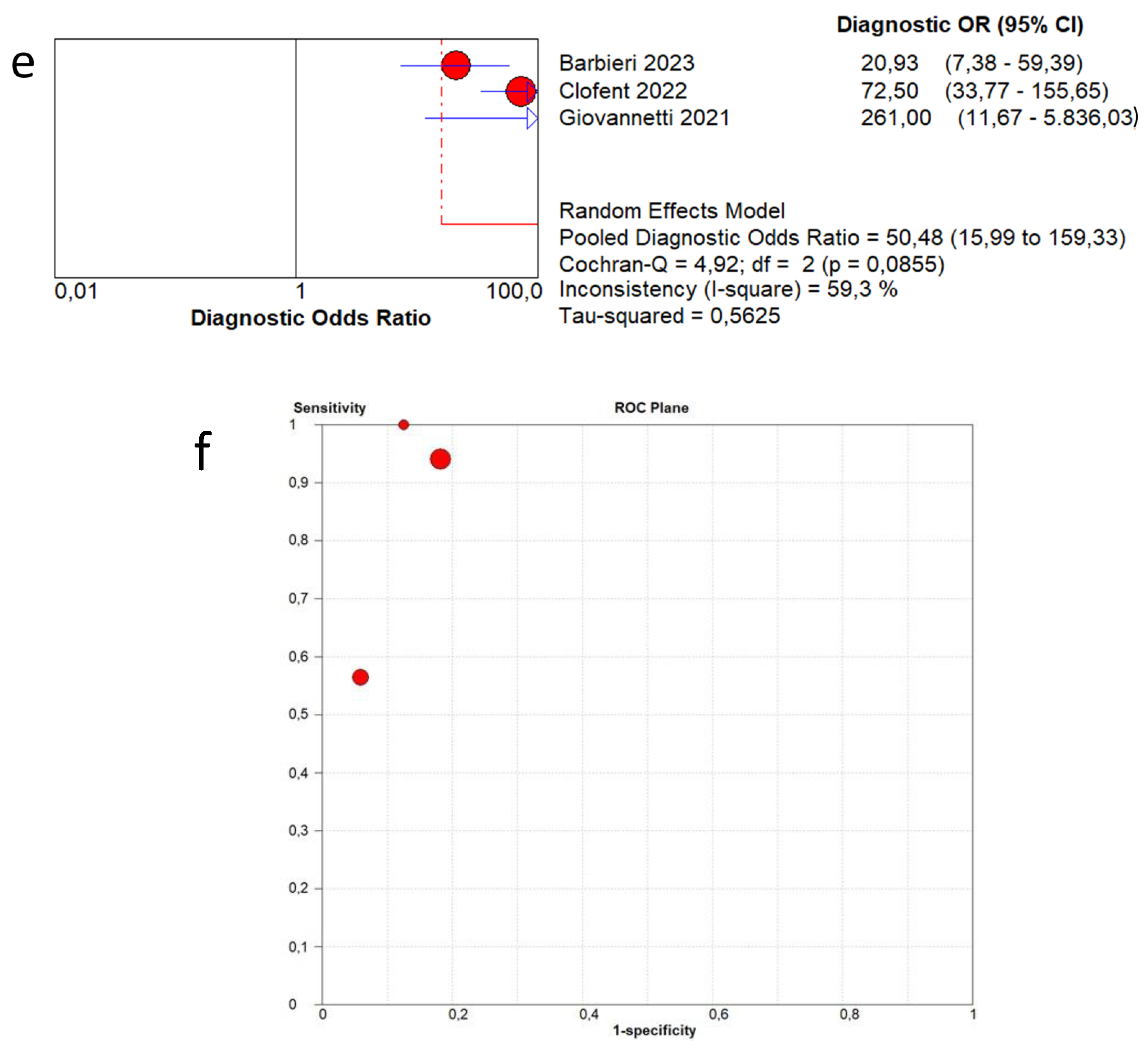


| Author | Year | Study Design | Patients | Age | Follow-Up Timing | Male | Healthy Patients | Pathological Patients | Standard Reference | LUS Scheme | LUS Interpretation | Probe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Barbieri et al. [13] | 2023 | Prospective | 220 | 62.2 | 3 months | 62.1% | 197 | 23 | CT | 16 areas | Score 0 only A-lines or less than 3 separated B-lines; score 1 in case of 3 or more B-lines or coalescent B-lines occupying ≤ 50% of the screen; score 2 for coalescent B-lines occupying > 50% of the screen; and score 3 for consolidation. A final LUS score, achieved from the sum of all values obtained within the 16 areas can range from 0 to 48. | Convex probes (frequency 2.5–5 MHz) |
| Clofent et al. [14] | 2022 | Prospective | 352 | 56.0 | 2−5 months | 57.7% | 198 | 154 | CT | 12 areas | B-line score by summing 1 point for each thoracic area with pathological B lines (score range, 0 to 12). | 2–5 MHz convex transducer |
| Giovannetti et al. [15] | 2021 | Prospective | 38 | 60.6 | 3 months | 71.1% | 16 | 22 | CT | 12 areas | Score 0 as normal pattern, A-lines or <3 B-lines; 1 as moderate loss, ≥3 B-lines; 2 as severe loss, coalescent B-lines; 3 as complete loss, white lung and/or lung consolidations. The total LUS score was the sum of the points from each lobe and ranges from 0 to 36 points. | Linear array probe (MHz 7.5–10) |
| Author | Year | Study Design | Patients | Age | Follow-Up Timing | Male | Patients with Lung Damage Resolution | Patients with Persistent Lung Damage | Standard Reference | LUS Scheme | Interpretation | Probe | Findings |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ramos Hernandez et al. [38] | 2023 | Observational and prospective multicentre study | 233 | 62.4 | 3 ± 1 and 12 ± 1 months after hospital discharge | 64.4% | 65 | 168 | CT/X-ray | 14 areas | (0) = A lines; (1) = at least 3 vertical hyperechogenic artifacts; (2) = B lines tended toward coalescence; (3) = area of white lung or consolidation of the lung parenchyma. Altered LUS results were defined as all those with a lung score ≥ 1 while normal LUS findings were those with a score of 0 without the presence of artifacts in the pleural line. | 2–5 MHz convex probe | The mean LUS score was 5.3 (SD = 5.1). LUS showed a sensitivity of 89.7%, specificity of 50%, and PPV of 90%. In the second visit, LUS showed a significant improvement compared to the first visit (5.8 ± SD 5.2 vs. 2.1 ± SD 3.8; p = 0.001). The sensitivity of LUS at this visit was 76%, specificity was 74%, PPV was 93%, and the AUC was 0.74. |
| Loke et al. [37] | 2023 | Prospective | 21 | 52.1 | Days 0 (D0), 41 (D41) and 83 (D83) | 61.9% | - | - | CT | 12 areas | (0) = A lines; (1) = vertical hyperechogenic artifacts; (2) = B lines tended toward coalescence; (3) = consolidation of the lung parenchyma. | Portable LUS probe | The mean LUS scores of patients on D41 and D83 were significantly lower, as compared to D0 of the study (2.9 ± 2.1 [D41] and 1.3 ± 1.3 [D83]) vs. 10.7 ± 3.3 [D0]; p < 0.001). |
| Russo et al. [39] | 2022 | Single centre, prospective observational study | 74 | 65.0 | 6 months | 73% | 24 | 50 | CT | 12 areas | (0) = A-line pattern; <3 B lines can be present. (1) = at least 3 B lines in at least one scan of the region; the B lines are well separated and do not merge one in the other. Small subpleural consolidations ≤1 cm diameter and irregular pleural line. (2) = multiple, converging B-lines (white lung) in at least one scan of the region. Small subpleural consolidations ≤ 1 cm diameter and irregular pleural line. (3) = at least one consolidation with major vs. > 1 cm in at least one scan of the region. | Convex probe | Lung abnormalities were detected in 69.4%, with a median LUS score of 2 (IQR 0–5.25). When compared to LUS during hospitalization, a decrease in total score greater than 50% was observed in 76% of patients after 6 months. ROC showed an AUC of 0.85 (95% CI 0.76–0.93). LUS score < 2 can rule out fibrotic-like changes with a sensitivity of 0.92 (95% CI 0.73–0.99) and a specificity of 0.60 (95% CI 0.45–0.74). |
| Zimna et al. [40] | 2024 | Prospective observational study | 72 | 58.0 | 3 months | 62% | - | - | CT | 12 areas | 0 = Regular and continuous pleural line, A-lines; 1 = Irregular or broken pleural line, or consolidation ≤2.5 mm, or ≤3 B-lines; 2 = consolidation >2.5 mm ≤10 mm, or >3 B-lines; 3 = consolidation >10 mm, or pleural effusion, or coalescence B-lines, or “white lung” image. | Linear array transducer (3–13 MHz) | Fibrotic changes were observed in 41.6% of the patients. LUS score was significantly higher in patients with radiological evidence of fibrosis compared to those without (p = 0.000002, and p = 0.000000, in the two-follow-up timing, respectively). The mean ultrasound score in I e for the group exhibiting fibrotic features was 19.4 ± 5.7 points, whereas, for those without these features, it was 11 ± 6.6 points, and in II e, these scores were 16 ± 5.3 points and 6 ± 2.7 points, respectively. All patients with an ultrasound score below 9 points showed near complete regression of lung lesions on chest CT. The optimal LUS score for the detection of more than 10% of GGOs was 13 points, which combined the highest sensitivity of 0.964 and lowest false-positive rate of 0.262 (specificity 0.738; NPV 0.904; PPV 0.89; AUC: 0.94. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boccatonda, A.; D’Ardes, D.; Tallarico, V.; Guagnano, M.T.; Cipollone, F.; Schiavone, C.; Piscaglia, F.; Serra, C. Role of Lung Ultrasound in the Detection of Lung Sequelae in Post-COVID-19 Patients: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 5607. https://doi.org/10.3390/jcm13185607
Boccatonda A, D’Ardes D, Tallarico V, Guagnano MT, Cipollone F, Schiavone C, Piscaglia F, Serra C. Role of Lung Ultrasound in the Detection of Lung Sequelae in Post-COVID-19 Patients: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2024; 13(18):5607. https://doi.org/10.3390/jcm13185607
Chicago/Turabian StyleBoccatonda, Andrea, Damiano D’Ardes, Viola Tallarico, Maria Teresa Guagnano, Francesco Cipollone, Cosima Schiavone, Fabio Piscaglia, and Carla Serra. 2024. "Role of Lung Ultrasound in the Detection of Lung Sequelae in Post-COVID-19 Patients: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 13, no. 18: 5607. https://doi.org/10.3390/jcm13185607
APA StyleBoccatonda, A., D’Ardes, D., Tallarico, V., Guagnano, M. T., Cipollone, F., Schiavone, C., Piscaglia, F., & Serra, C. (2024). Role of Lung Ultrasound in the Detection of Lung Sequelae in Post-COVID-19 Patients: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 13(18), 5607. https://doi.org/10.3390/jcm13185607










