Omalizumab for the Treatment of Chronic Spontaneous Urticaria in Adults and Adolescents: An Eight-Year Real-Life Experience
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Population
2.2. Laboratory Methods
2.3. Statistical Analysis
3. Results
3.1. Study Population
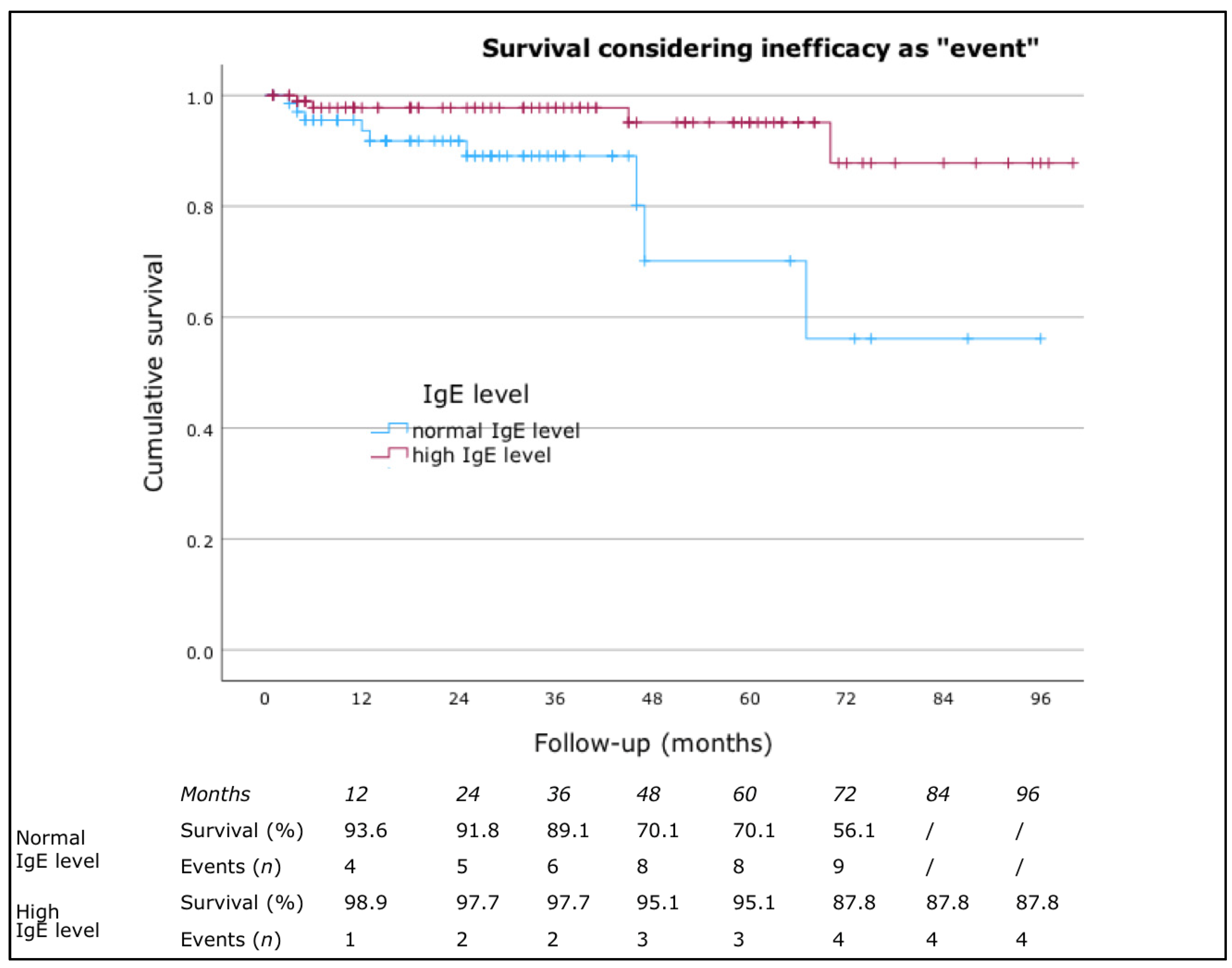
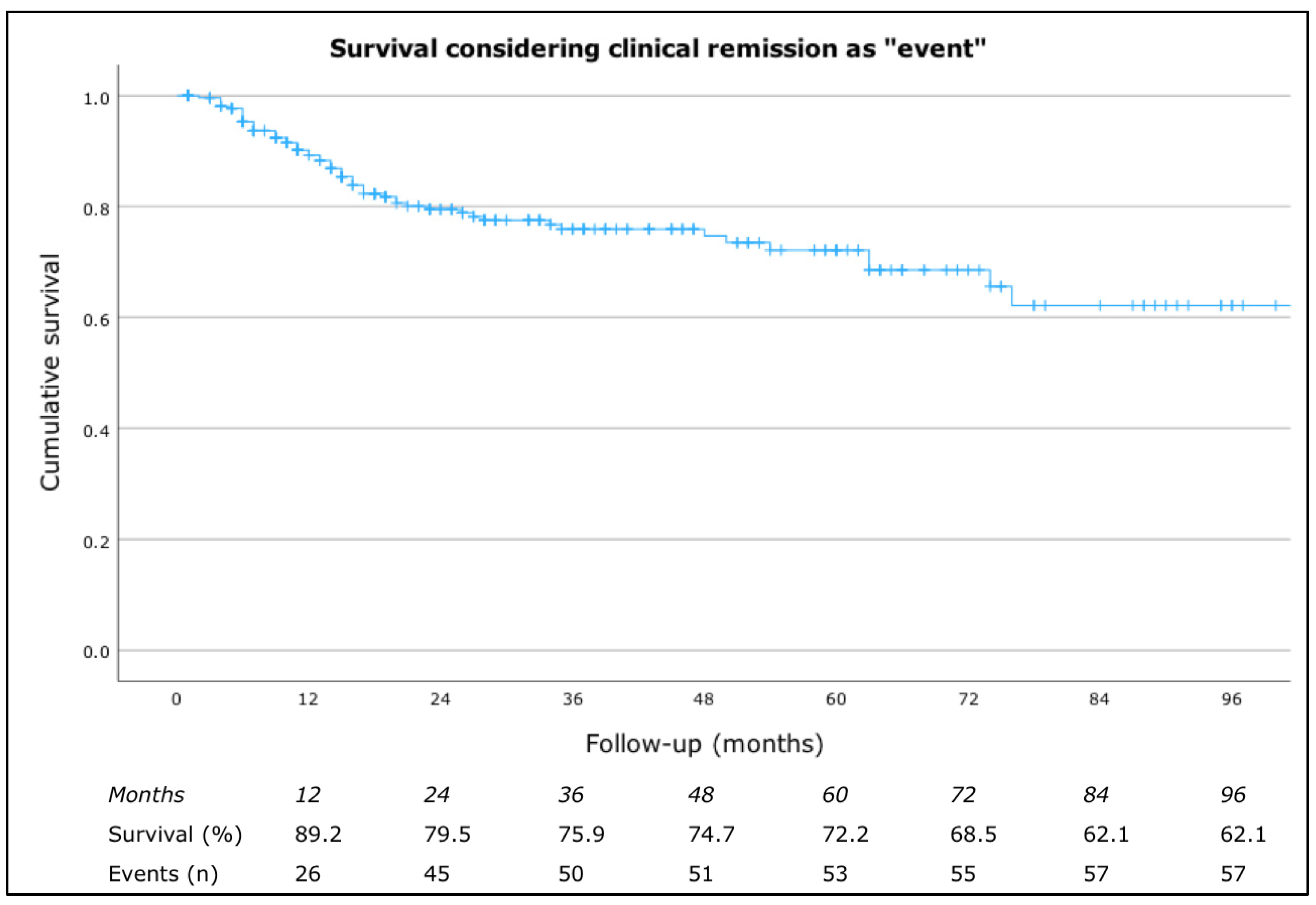
3.2. Clinical Response to Omalizumab
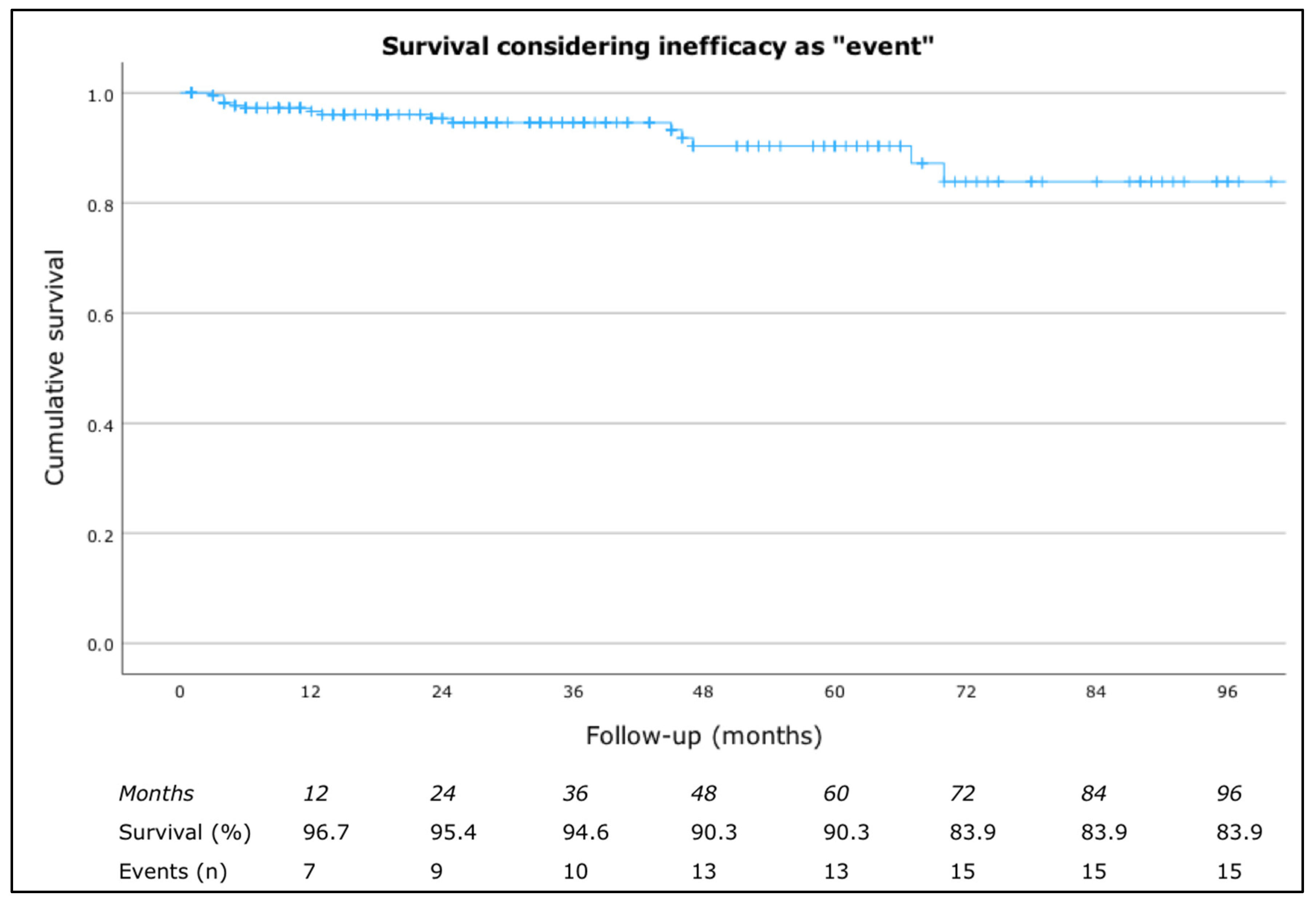
3.3. Drug Survival
3.4. Effectiveness in Adolescent
3.5. Safety
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zuberbier, T.; Abdul Latiff, A.H.; Abuzakouk, M.; Aquilina, S.; Asero, R.; Baker, D.; Ballmer-Weber, B.; Bangert, C.; Ben-Shoshan, M.; Bernstein, J.A.; et al. The international EAACI/GA2LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy 2022, 77, 734–766. [Google Scholar] [CrossRef] [PubMed]
- Zuberbier, T.; Grattan, C.; Maurer, M. (Eds.) Urticaria and Angioedema; Springer International: Berlin/Heidelberg, Germany, 2021; pp. 77–107. [Google Scholar]
- Weller, K.; Maurer, M.; Bauer, A.; Wedi, B.; Wagner, N.; Schliemann, S.; Kramps, T.; Baeumer, D.; Multmeier, J.; Hillmann, E.; et al. Epidemiology, comorbidities, and healthcare utilization of patients with chronic urticaria in Germany. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Kolkhir, P.; Giménez-Arnau, A.M.; Kulthanan, K.; Peter, J.; Metz, M.; Maurer, M. Urticaria. Nat. Rev. Dis. Primers 2022, 8, 61. [Google Scholar] [CrossRef]
- Greaves, M. Chronic urticaria. J. Allergy Clin. Immunol. 2000, 105, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Balp, M.M.; Halliday, A.C.; Severin, T.; Leonard, S.A.; Partha, G.; Kalra, M.; Marsland, A.M. Clinical Remission of Chronic Spontaneous Urticaria (CSU): A Targeted Literature Review. Dermatol. Ther. 2022, 12, 15–27. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Toubi, E.; Kessel, A.; Avshovich, N.; Bamberger, E.; Sabo, E.; Nusem, D.; Panasoff, J. Clinical and laboratory parameters in predicting chronic urticaria duration: A prospective study of 139 patients. Allergy 2004, 59, 869–873. [Google Scholar] [CrossRef] [PubMed]
- Gonçalo, M.; Gimenéz-Arnau, A.; Al-Ahmad, M.; Ben-Shoshan, M.; Bernstein, J.A.; Ensina, L.F.; Fomina, D.; Galvàn, C.A.; Godse, K.; Grattan, C.; et al. The global burden of chronic urticaria for the patient and society. Br. J. Dermatol. 2021, 184, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.; Abuzakouk, M.; Bérard, F.; Canonica, W.; Oude Elberink, H.; Giménez-Arnau, A.; Grattan, C.; Hollis, K.; Knulst, A.; Lacour, J.P.; et al. The burden of chronic spontaneous urticaria is substantial: Real-world evidence from ASSURE-CSU. Allergy 2017, 72, 2005–2016. [Google Scholar] [CrossRef] [PubMed]
- Özkan, M.; Oflaz, S.B.; Kocaman, N.; Özşeker, F.; Gelincik, A.; Büyüköztürk, S.; Özkan, S.; Çolakoğlu, B. Psychiatric morbidity and quality of life in patients with chronic idiopathic urticaria. Ann. Allergy Asthma Immunol. 2007, 99, 29–33. [Google Scholar] [CrossRef]
- Maurer, M.; Weller, K.; Bindslev-Jensen, C.; Giménez-Arnau, A.; Bousquet, P.J.; Bousquet, J.; Canonica, G.W.; Church, M.K.; Godse, K.V.; Grattan, C.E.; et al. Unmet clinical needs in chronic spontaneous urticaria: A GA2LEN task force report. Allergy 2011, 66, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.P.; Giménez-Arnau, A.M.; Saini, S.S. Mechanisms of action that contribute to efficacy of omalizumab in chronic spontaneous urticaria. Allergy 2017, 72, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Asero, R.; Ferrucci, S.M.; Calzari, P.; Consonni, D.; Cugno, M. Thyroid Autoimmunity in CSU: A Potential Marker of Omalizumab Response? Int. J. Mol. Sci. 2023, 24, 7491. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schmetzer, O.; Lakin, E.; Topal, F.A.; Preusse, P.; Freier, D.; Church, M.K.; Maurer, M. IL-24 is a common and specific autoantigen of IgE in patients with chronic spontaneous urticaria. J. Allergy Clin. Immunol. 2018, 142, 876–882. [Google Scholar] [CrossRef]
- Grattan, C.E.; Francis, D.M.; Hide, M.; Greaves, M.W. Detection of circulating histamine releasing autoantibodies with functional properties of anti-IgE in chronic urticaria. Clin. Exp. Allergy 1991, 21, 695–704. [Google Scholar] [CrossRef]
- Hide, M.; Francis, D.M.; Grattan, C.E.; Hakimi, J.; Kochan, J.P.; Greaves, M.W. Autoantibodies against the high-affinity IgE receptor as a cause of histamine release in chronic urticaria. N. Engl. J. Med. 1993, 328, 1599–1604. [Google Scholar] [CrossRef]
- Asero, R.; Marzano, A.V.; Ferrucci, S.; Lorini, M.; Carbonelli, V.; Cugno, M. Co-occurrence of IgE and IgG autoantibodies in patients with chronic spontaneous urticaria. Clin. Exp. Immunol. 2020, 200, 242–249. [Google Scholar] [CrossRef]
- Maronese, C.A.; Ferrucci, S.M.; Moltrasio, C.; Lorini, M.; Carbonelli, V.; Asero, R.; Marzano, A.V.; Cugno, M. IgG and IgE Autoantibodies to IgE Receptors in Chronic Spontaneous Urticaria and Their Role in the Response to Omalizumab. J. Clin. Med. 2023, 12, 378. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maurer, M.; Kolkhir, P.; Pereira, M.P.; Siebenhaar, F.; Witte-Händel, E.; Bergmann, K.C.; Bonnekoh, H.; Buttgereit, T.; Fluhr, J.W.; Frischbutter, S.; et al. Disease modification in chronic spontaneous urticaria. Allergy 2024, 79, 2396–2413. [Google Scholar] [CrossRef] [PubMed]
- Kolkhir, P.; Pogorelov, D.; Olisova, O.; Maurer, M. Autoimmune comorbidity in chronic spontaneous urticaria: A systematic review. Autoimmun. Rev. 2017, 16, 1196–1208. [Google Scholar] [CrossRef]
- Metz, M.; Maurer, M. Omalizumab in chronic urticaria. Curr. Opin. Allergy Clin. Immunol. 2012, 12, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Kolkhir, P.; Altrichter, S.; Asero, R.; Daschner, A.; Ferrer, M.; Giménez-Arnau, A.; Hawro, T.; Jakob, T.; Kinaciyan, T.; Kromminga, A.; et al. Autoimmune Diseases Are Linked to Type IIb Autoimmune Chronic Spontaneous Urticaria. Allergy Asthma Immunol. Res. 2021, 13, 545–559. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soegiharto, R.; Alizadeh Aghdam, M.; Sørensen, J.A.; van Lindonk, E.; Bulut Demir, F.; Porras, N.M.; Matsuo, Y.; Kiefer, L.; Knulst, A.C.; Maurer, M.; et al. Multinational Drug Survival Study of Omalizumab in Patients with Chronic Urticaria and Potential Predictors for Discontinuation. JAMA Dermatol. 2024, 160, 927–935. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weller, K.; Ohanyan, T.; Hawro, T.; Ellrich, A.; Sussman, G.; Koplowitz, J.; Gimenez-Arnau, A.M.; Peveling-Oberhag, A.; Staubach, P.; Metz, M.; et al. Total IgE levels are linked to the response of chronic spontaneous urticaria patients to omalizumab. Allergy 2018, 73, 2406–2408. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, W.; Yang, X.; Li, S.; Deng, S.; Wang, H.; Liu, W.; Ni, B.; Song, Z. Characteristics and Clinical Significance of Atopy in Chronic Spontaneous Urticaria: A Cross-Sectional Observational Study. Int. Arch. Allergy Immunol. 2024; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Litovsky, J.; Hacard, F.; Tétart, F.; Boccon-Gibod, I.; Soria, A.; Staumont-Sallé, D.; Doutre, M.S.; Amsler, E.; Mansard, C.; Dezoteux, F.; et al. Urticaria Study Group of French Dermatology Society. Omalizumab Drug Survival in Chronic Urticaria: A Retrospective Multicentric French Study. J. Allergy Clin. Immunol Pract. 2023, 11, 3752–3762.e2. [Google Scholar] [CrossRef] [PubMed]
- Spekhorst, L.S.; van den Reek, J.M.P.A.; Knulst, A.C.; Röckmann, H. Determinants of omalizumab drug survival in a long-term daily practice cohort of patients with chronic urticaria. Allergy 2019, 74, 1185–1187. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.S.; Bindslev-Jensen, C.; Maurer, M.; Grob, J.J.; Baskan, E.B.; Bradley, M.S.; Canvin, J.; Rahmaoui, A.; Georgiou, P.; Alpan, O.; et al. Efficacy and Safety of Omalizumab in Patients with Chronic Idiopathic/Spontaneous Urticaria who Remain Symptomatic on H1 Antihistamines: A Randomized, Placebo-Controlled Study. J. Investig. Dermatol. 2015, 135, 67–75. [Google Scholar] [CrossRef]

| Overall Population (n) | 296 |
|---|---|
| Sex, n (%) | |
| - Male | 104 (35.1) |
| - Female | 192 (64.9) |
| CSU onset age, mean (SD) | 42.7 (17.0) |
| - ≥18-year-old, n (%) | 291 (97.2) |
| - <18-year-old, n (%) | 8 (2.8) |
| Baseline age, mean (SD) | 46.9 (16.5) |
| - ≥18-year-old, n (%) | 290 (98.0) |
| - <18-year-old, n (%) | 6 (2.0) |
| CSU duration before starting omalizumab, median (Q1–Q3) | 24.1 (9.7–68.4) |
| Angioedema, n (%) | 100 (33.8) |
| Inducible urticaria, n (%) | 66 (22.3) |
| missing | 23 (7.8) |
| Atopic comorbidities, n (%) | 130 (43.9) |
| missing | 15 (5.1) |
| Hashimoto thyroiditis, n (%) | 52 (17.6) |
| missing | 13 (4.4) |
| Dermatological disease, n (%) | |
| - Vitiligo | 2 (0.6) |
| - Prurigo nodularis | 1 (0.3) |
| Other comorbidities, n (%) | |
| - Rheumatoid arthritis | 1 (0.3) |
| - Familiar colon polyposis | 1 (0.3) |
| - Coeliac disease | 1 (0.3) |
| - Diverticulosis | 1 (0.3) |
| - Cardiovascular disease | 1 (0.3) |
| - Psychiatric disease | 3 (0.9) |
| - Familiar Mediterranean Fever | 1 (0.3) |
| - Autoimmune atrophic gastritis | 1 (0.3) |
| - HBV | 1 (0.3) |
| - HCV | 1 (0.3) |
| - Hypertension | 1 (0.3) |
| - Lupus | 3 (0.9) |
| - Multiple myeloma | 1 (0.3) |
| - Sacroiliitis HLA B27+ | 1 (0.3) |
| - Sjogren syndrome | 1 (0.3) |
| - Chronic pyelonephritis | 1 (0.3) |
| IgE level, n (%) | |
| - High IgE level | 123 (41.6) |
| - Normal IgE level | 93 (31.4) |
| missing | 8 (27.0) |
| Previous therapies, n (%) | |
| - Bilastine | 167 (56.4) |
| - Ebastine | 93 (31.4) |
| - Levocetirizine | 36 (12.2) |
| - Cetirizine | 72 (24.3) |
| - Rupatadine | 47 (15.8) |
| - Fexofenadine | 19 (6.4) |
| - Idroxizine | 11 (3.7) |
| - Oxatomide | 2 (0.7) |
| UAS7 at baseline, median (Q1–Q3) | 22.0 (22.0–28.0) |
| Clinical Response Categories | |||||
|---|---|---|---|---|---|
| Inducible Urticaria | NR | ER | LR | PR | Total |
| No, n (%) | 20 (9.9) | 90 (45.8) | 40 (19.7) | 50 (24.6) | 200 (100.0) |
| Yes, n (%) | 5 (7.6) | 19 (28.8) | 24 (36.4) | 18 (27.3) | 66 (100.0) |
| p = 0.021, χ2 = 9.692 | |||||
| Clinical Response Categories | |||||
|---|---|---|---|---|---|
| Hashimoto’s Thyroiditis | NR | ER | LR | PR | Total |
| No, n (%) | 15 (6.6) | 98 (43.0) | 58 (25.4) | 57 (25.0) | 228 (100.0) |
| Yes, n (%) | 10 (19.6) | 16 (31.4) | 8 (15.7) | 17 (33.3) | 51 (100.0) |
| p = 0.007, χ2 = 12.037 | |||||
| Clinical Response Categories | |||||
|---|---|---|---|---|---|
| IgE Level | NR | ER | LR | PR | Total |
| Normal level, n (%) | 12 (13.0) | 29 (31.5) | 30 (32.6) | 21 (22.8) | 92 (100.0) |
| High level, n (%) | 7 (5.8) | 57 (47.1) | 27 (22.3) | 30 (24.8) | 121 (100.0) |
| p = 0.039, χ2 = 8.385 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calzari, P.; Chiei Gallo, A.; Barei, F.; Bono, E.; Cugno, M.; Marzano, A.V.; Ferrucci, S.M. Omalizumab for the Treatment of Chronic Spontaneous Urticaria in Adults and Adolescents: An Eight-Year Real-Life Experience. J. Clin. Med. 2024, 13, 5610. https://doi.org/10.3390/jcm13185610
Calzari P, Chiei Gallo A, Barei F, Bono E, Cugno M, Marzano AV, Ferrucci SM. Omalizumab for the Treatment of Chronic Spontaneous Urticaria in Adults and Adolescents: An Eight-Year Real-Life Experience. Journal of Clinical Medicine. 2024; 13(18):5610. https://doi.org/10.3390/jcm13185610
Chicago/Turabian StyleCalzari, Paolo, Alessandra Chiei Gallo, Francesca Barei, Eleonora Bono, Massimo Cugno, Angelo Valerio Marzano, and Silvia Mariel Ferrucci. 2024. "Omalizumab for the Treatment of Chronic Spontaneous Urticaria in Adults and Adolescents: An Eight-Year Real-Life Experience" Journal of Clinical Medicine 13, no. 18: 5610. https://doi.org/10.3390/jcm13185610
APA StyleCalzari, P., Chiei Gallo, A., Barei, F., Bono, E., Cugno, M., Marzano, A. V., & Ferrucci, S. M. (2024). Omalizumab for the Treatment of Chronic Spontaneous Urticaria in Adults and Adolescents: An Eight-Year Real-Life Experience. Journal of Clinical Medicine, 13(18), 5610. https://doi.org/10.3390/jcm13185610








