Abstract
Background: Cannabis use is associated with an increased risk of coronary heart disease (CHD), including angina pectoris (AP), and myocardial infarction (MI). However, it is not clear whether cannabis use is an independent risk factor of AP and/or MI, because cannabis is often smoked together with tobacco. We investigated whether cannabis is an independent risk factor of MI and whether this risk is similar in cannabis smokers, cannabis vapers, and those who use cannabis edibles. Methods: A systematic review was performed, according to the PRISMA guidelines and using Medline (PubMed), Embase, and Google Scholar as databases. Results: Twenty-two eligible papers were identified. After adjustment for concurrent tobacco use, cannabis smoking remained significantly associated with incidents of MI, with aORs ranging between 1.03 and 5.24, and particularly high aORs in the younger age group. In never-tobacco smokers, frequent cannabis smoking was also associated with a significant MI risk (aOR = 1.88). Frequent and current cannabis use in any form other than smoking (e.g., vaping, but mostly ingestion) was not associated with a significantly increased cardiovascular risk (frequent use: aOR = 1.00 ns; current use: aOR = 1.31 ns). Conclusions: Like tobacco smoking, cannabis smoking may independently provoke MI. Vaping and ingestion of cannabis might be less harmful, probably because absence of combustion prevents exposure to certain toxins in cannabis smoke, including carbon monoxide.
Keywords:
cannabis; marijuana; myocardial infarction; coronary heart disease; smoking; vaping; edibles; carbon monoxide 1. Introduction
Cannabis is the most widely-used internationally regulated drug in the US, with nearly 22.2 million users each month, 38% of high school students reporting cannabis use [1], and more than half of the adult US population having tried cannabis [2]. In the Behavioural Risk Factor Surveillance System (BRFSS, a yearly telephone survey), the prevalence of last-month cannabis use in the US increased from 10.0% (95% CI: 9.4–10.7%) in 2017 to 13.4% (95% CI: 12.8–12.0%) in 2019 [3]. Smoking cannabis was the most prevalent primary route of administration (76.3%), but cannabis vaping (11.3%) and oral consumption of cannabis (edibles; 12.4% to nearly 30%) are emerging [4,5,6,7]. Especially among youngsters, cannabis vaping and cannabis edibles have become popular. For example, past-month pooled prevalence of adolescent cannabis vaping in the US and Canada has increased 5-fold from 1.6% in 2013–2016 to 8.4% in 2019–2020 for all cannabis users [8,9]. Among US Grade 12 students, 35% and 40% reported vaping and eating cannabis, respectively, in the past 12 months in 2018 [10,11].
Apart from the pulmonary and cancer risk, cannabis smoking is known to be associated with an increased risk of coronary heart disease (CHD), also known as ischemic heart disease, including angina pectoris and myocardial infarction (MI) [12,13,14]. In the 1960s and 1970s, numerous studies documented that tobacco smoking poses a significant risk of CHD, including higher rates of MI and sudden death from CHD, especially in young and middle-aged men and heavy smokers in general [15,16,17,18,19,20]. For instance, a case-controlled study reported a relative risk (RR) of MI of 2.8 (95% CI: 2.0–4.0) for current smokers compared to never-smokers aged 30 to 54 years [20].
In 1975, Aronow & Cassidy suggested that co-use of cannabis and tobacco more often precipitates coronary syndromes (including acute MI) in patients with coronary history compared to those who only smoke tobacco [21]. In 2011, cannabis smoking was added to the list of the potential triggers for MI [22], which was followed by numerous studies showing substantial evidence for an association of frequent cannabis smoking with a higher risk of MI and CHD [23,24,25,26,27]. For instance, an odds ratio of 5.03 (95% CI: 3.5–7.3) was calculated for developing MI after cannabis use in teenagers [28]. The latter study also showed that MI occurs in healthy cannabis users who have a low incidence of cardiac risk factors (e.g., smoking, diabetes).
A wealth of information is available about cannabis-induced MI, but it is not clear whether cannabis use is independently associated with MI. It cannot be excluded that the MI risk of cannabis use is limited to cannabis smoking or that the presumed association is heavily biased by (concurrent) tobacco smoking [29]. We hypothesize that cannabis-related MI is primarily driven by cannabis use in smoked form. Therefore, the aim of the current systematic review is to outline the independent risk of smoked cannabis for CHD, in particular MI. In addition, this review explores the MI risk of non-smoked cannabis, e.g., the risk of vaping or eating cannabis.
2. Methods
2.1. Search Strategy
A systematic literature review was performed on 20 July 2024, according to the PRISMA guidelines. Utilizing several databases (Medline (PubMed), Embase, and Google Scholar), a comprehensive search was performed for literature concerning the association between cannabis use and myocardial infarction. Keywords, including ‘myocardial infarction’, ‘acute coronary syndrome’, and ‘heart attack’, were employed in addition to various synonyms for cannabis. These keywords were used individually and in combination to select eligible studies. Eligible studies included randomized controlled trials, review articles, meta-analyses, observational cohort studies, observational cross-sectional studies, and case reports referring only to smoking cannabis. Studies in samples with clear co-morbidity for MI, like diabetes, were excluded.
2.2. Screening of Records and Data Extraction
The selection of eligible studies was independently conducted by JvA and WvdB in two rounds. Initially, 724 studies were identified and, after removing duplicates, 604 articles remained. One study could not be retrieved. Of the remaining 603 studies, titles and abstracts were screened to determine eligibility based on the inclusion and exclusion criteria outlined above to identify potentially relevant studies. In a second round, the full texts of 68 studies were comprehensively reviewed to check their eligibility. Finally, 28 studies (including six case reports and one systematic review) were eligible, of which 22 are presented in Table 1. The single case studies on vaping/eating cannabis were selected from the reviews retrieved and briefly described in a separate paragraph (3.9). Figure 1 illustrates the PRISMA flow chart for the identification, screening, and inclusion of the studies. Here, we refer to the ‘Supplement’ for the search string and PRISMA checklist.

Table 1.
Studies on cannabis-related MI. Outcome was either adjusted for concomitant tobacco use, tobacco smokers were compared with non-tobacco smokers, or the study was performed on never-tobacco smokers.

Figure 1.
PRISMA flow diagram.
2.3. Risk of Bias and Quality Assessment
Critical Appraisal Tools (CAT) checklists, developed by the Joanna Briggs Institute (JBI) [47], were used to assess the risk of bias in all included studies. Since this review included studies with different designs, the following appropriate JBI CAT checklists were selected [48]: CAT for observational, analytical cross-sectional studies, CAT for systematic reviews, CAT for observational cohort studies, and CAT for case reports.
The two authors (JvA and WvdB) independently appraised the studies. Any disagreement between them was discussed until a consensus for each score was reached. The items of the JBI CAT checklist were rated as either yes bias (Y), no bias (N), bias unclear (U), or bias not applicable (N/A). As recommended by the JBI manual [47,49], the scoring system and cut-off points to determine high, moderate, or low risk of bias, were decided according to Algarni et al. [50] and agreed by the authors JvA and WvdB, before starting the critical appraisal.
Quality assessment scores for each study were based on the calculated percentage of affirmative responses to the total number of questions. If a criterion was considered not applicable, this point was deducted from the overall score. Studies with a JBI score > 66% were at low risk of bias, scores between 33 and 66% were at moderate risk of bias, and studies with a JBI score less than 33% were deemed at a high risk of bias [50].
3. Results
3.1. Search Results
Figure 1 shows the PRISMA diagram of the identification, screening, and inclusion of the 28 eligible studies.
3.2. Summary of Studies
The articles selected for this review are summarised in Table 1, and detail the sample characteristics, main outcome(s), and literature reference. Studies were classified as (a) studies not adjusting for tobacco use, (b) studies adjusting for tobacco use, (c) studies in non-tobacco users, and (d) studies that included edibles. The total set of 28 eligible studies consists of eighteen cross-sectional investigations [4,23,26,42,43,44,45], three observational cohort studies [21,30,38], one systematic review [46], and six single case studies not mentioned in Table 1.
3.3. Risk of Bias
The quality score and assigned risk of bias is represented in Table 2 for all 28 studies. Of the studies, 20 studies were at low risk of bias [4,23,24,32,33,34,36,37,38,39,40,41,43,44,45,51,52,53,54,55], three studies were at moderate risk of bias [21,30,46], and five studies were at high risk of bias [26,31,35,42,56]. Five out of six subjects in the case studies scored low risk of bias and one subject scored a high risk of bias. Tobacco smoking status was, however, unknown in three cases, two cases were smokers, and one case was a non-smoker. The appraisal data for each study is represented in Table S1 of the Supplement.

Table 2.
Quality score and risk of bias in the eligible studies using the JBI Critical Appraisal Tool.
3.4. Studies Not Adjusted for Tobacco Smoking
Numerous reviews have previously outlined the cardiovascular implications of cannabis use [12,58,59,60,61,62,63,64,65,66,67,68,69] (for an overview see [70]). Table 1 summarizes the findings of “studies adjusted for tobacco smoking” included in the current systematic literature search.
Importantly, in a prospective cohort study, Aronow and Cassidy showed, in 10 patients with chronic but stable angina, that smoking one cannabis cigarette significantly decreased the exercise time until angina (by 48–50%) more than either smoking placebo cannabis (8.6%) or smoking one high-nicotine cigarette (23%) [21]. Another important study is the case-cross-over study by Mittleman et al. who examined the self-reported acute effects of cannabis in over 3800 patients and found a 4.8-fold increase in the RR for MI in the first hour after smoking cannabis. In the second hour after smoking, the RR decreased to 1.7 and was no longer significant, suggesting a substantial decrease in cardiac effects in the second hour after cannabis use. The study also found a RR of 3.2 for smoking cannabis in the absence of other potential MI triggers (e.g., regular exertion, cocaine use). Cannabis users were more likely to be current cigarette smokers (68% versus 32%), but the MI risk data were not controlled for combined tobacco-cannabis smoking [32]. Finally, a systemic review of 46 papers also showed in 14 cases that the time from last cannabis use to the onset of MI symptoms is relatively short, i.e., usually within 5 h [71].
In fully adjusted multivariable models, using cross-sectional data from five two-year cycles, between 2009 and 2018, from the National Health and Nutrition Examination Survey representing almost 1000 middle-aged adults, it was shown that, compared to never use, a history of monthly cannabis use was not associated with MI (OR = 0.78;), but the risk of cannabis use in the past month for MI was threefold greater compared to no use within the past month (OR = 2.98) [33].
In summary, abundant evidence is available for up to a 4.8 times higher risk of MI in cannabis smokers with a relatively high risk of an MI within the first two hours after smoking cannabis.
3.5. Studies Adjusting for Tobacco Use
The results of the longitudinal, multi-centre CARDIA study, in 5115 young adults in 1985–1986, on the development of CVD over time showed that, after adjustment for tobacco smoking (and many other potential confounders), neither cumulative lifetime nor recent cannabis use were significantly associated with incidents of CVD, transient ischemic attacks or coronary heart disease [34].
A large population-based study using sample patients (2011–2016) with a history of cannabis abuse, including age- and sex-matched cannabis naive controls, investigated the 3-year cumulative incidence of MI as primary outcome. Incidents of MI were significantly higher in the cannabis use group than in controls with a relative risk (RR) of 2.53. After adjustment for a number of confounders, including a greater predilection for tobacco in the cannabis abuse group, cannabis abuse was still significantly associated with incidents of MI (aOR = 1.72) [35].
A retrospective study utilizing the Nationwide Inpatient Sample (NIS) database analysed the trends of MI in young cannabis users aged 18–49 yrs. from 2007 to 2018, i.e., a period in which legalization of cannabis for medical and recreational purposes took place in some states in the US [36]. Cannabis use was reported by 230,497 hospitalized patients (28%). The incidence of acute MI (AMI) among cannabis users consistently increased from 2.36% in 2007 to 6.55% in 2018. Tobacco smoking was identified as an independent risk factor for AMI among cannabis users with an odds ratio (OR) of 2.38, indicating an added risk of MI by tobacco-cannabis co-use. Other results from the NIS showed that the prevalence of coronary artery disease (CAD) was slightly but significantly higher in patients with versus patients without a history of cannabis use (5% vs. 4.6%, p < 0.0001); however, the positive associations between cannabis use and rates of CAD were no longer significant after adjustment for a variety of confounders, including co-morbidity and tobacco use [37].
In another large retrospective study, using the NIS database, which included patients with and without a history of cannabis use, hospitalized MI patients showed a small but significant increased risk of MI for those who used cannabis even after adjustment for tobacco smoking (aOR = 1.03; p < 0.001) [24].
In a prospective study of HIV-infected men (aged 40–60 years), long-term heavy (daily or weekly) cannabis users compared to cannabis non-users had an increased risk of cardiovascular events, including MI, heart failure, and angina pectoris, after controlling for tobacco use (aOR = 2.5; p = 0.016). The risk of cannabis/tobacco co-use for cardiovascular events was additive (OR = 4.8; p < 0.01) [38].
Using data from the 2017 BRFSS, a retrospective cross-sectional study was conducted to study the association between cannabis use and self-reported doctor-confirmed cardiovascular disease which included myocardial infarction, angina, coronary heart disease, or a stroke [39]. Remarkably, there was a negative association between cannabis use and cardiovascular disease (OR = 0.65) but statistical significance was lost after adjustment for smoking and other variables [39].
In a retrospective study of hospitalized patients between 2012 and 2014, the risk of MI was compared between the cannabis urine positive group (n = 3638) and the cannabis urine negative group (n = 10,852) [40]. In the >37 yrs. age group, no difference in the risk of MI was observed between the two groups (p = 0.48). However, in the 18–36 age group, the risk of MI was considerably higher in the cannabis urine positive group (OR = 2.84). Both tobacco use and cocaine use were found to be significantly higher in cannabis users compared to cannabis non-users. After adjustment for confounding by tobacco smoking and cocaine use, the risk in the 18–36 age cannabis positive group of MI increased almost twofold (OR = 2.84 → OR = 5.24), whereas the risk between the cannabis users and non-users remained the same in the 37–54 age group (OR = 1.11).
Using the data the Third National Health and Examination Survey (NHES), the effect of cannabis on CHD was investigated, using the cardiac infarction and/or injury score (CIIS), in 900 ever-cannabis users of whom 538 were subjects with myocardial injury [41]. Adjusted for potential confounders, including tobacco smoking, ever-cannabis use had 43% increased odds of myocardial injury compared to never users (OR = 1.43) [41].
The results of a National Health and Nutrition Examination Survey (NHANES) study showed a significant OR of ever- versus never-cannabis use for coronary artery disease of 1.90. Similar significant ORs were observed for current cannabis users (OR = 1.98) and heavy cannabis users (OR = 1.99) [42]. These results were consistent in subgroups stratified by tobacco smoking status, amongst other factors.
In summary, adjustment for tobacco smoking often reduced the risk of cannabis use for MI, but, generally, cannabis smoking remained significantly associated with incidents MI with aORs up to 2.84. The risk of cardiovascular events, which includes MI, is particularly high in the younger age group and was reported to be additive in cannabis/tobacco co-use. Tobacco smoking proved to be an independent risk factor for MI among cannabis users.
3.6. Studies in Non-Tobacco Users
Using the NIS (2007–2014) database, national trends in hospitalizations for major cardiovascular events, including MI, among young non-tobacco smoking cannabis users were assessed [23]. As compared to non-cannabis users, the rate of hospital admission for MI among 0.7 million (1.3%) current/former cannabis users (no abuse of other substances) was 0.23% compared to 0.14% in non-cannabis users (p < 0.001) [23].
The association between cannabis use disorder (CUD) and major adverse cardiac and cerebrovascular events was investigated in an NIS 2019 subsample of older (>65 yrs.) patients without a tobacco use disorder [43]. The prevalence of CUD in this subsample was 0.3% (n = 28,535). Compared to the non-CUD cohort, the CUD cohort reported a higher risk of major adverse cardiac and cerebrovascular events (MACCE: OR 1.20, 95% CI 1.11–1.29, p < 0.001). Of the patients with CUD and at risk of cardiovascular disease (CVD), 13.9% reported MACCE. In this group, the CUD subgroup had higher unadjusted rates of MI than the non-CUD group (7.6% vs. 6.0%) [43].
Data from the BRFSS 2016–2020 was used to assess the association of cannabis use in the past 30 days in a general population subsample of never-tobacco smokers (primary cannabis smoking) with self-reported MI (18–74 years). For daily cannabis users in the general population, the aOR for MI was 1.25 (95% CI, 1.07–1.46) and a similar negative effect of daily cannabis use on MI was shown in never-tobacco smokers (aOR = 1.49; 95% CI, 1.03–2.15) [44].
In summary: the rate of MI and hospital admission for MI was higher among non-tobacco smoking current/former cannabis users compared to non-cannabis users. Thus, it seems that cannabis-tobacco co-use does not fully explain the increased cardiovascular risk of cannabis smoking.
3.7. Vaping Cannabis
Cigarettes and joints are burned at a temperature of 500–600 °C. In vaping the cannabinoids are heated at around 170 °C to 210 °C and not combusted. Smoking cannabis thus produces significantly higher CO and CO-haemoglobin concentrations 0.25–6 h post-dose compared to vaporization suggesting that vaporized cannabis might be safer than smoked cannabis in terms of its cardiovascular risk. Another advantage of vaporization is that less toxic pyrolytic compounds are produced at vaping temperatures below 200 °C [72]. Except for some case studies [51,52,56], no studies are available on larger cohorts of cannabis vapers and their possible risk of MI, suitable for the current review.
In summary, vaping cannabis is presumably associated with a lower risk of MI, but solid data endorsing this assumption is not available.
3.8. Edibles
Apart from some case studies (cf. Table 1), four studies [4,26,45,46] have addressed the risk of MI in a larger sample of users who had orally ingested cannabis or cannabis-like substances as an edible or beverage.
Using pooled data from the 2017 and 2018 cohorts of the BRFSS, Ladha et al. [4] assessed the association between any recent cannabis use and a history of MI among 4610 recent cannabis users. The rates were adjusted for a large variety of confounders, including concomitant tobacco use. A history of MI was significantly associated with smoking cannabis as a primary method of administration (aOR 2.01), but not significantly with vaporizing cannabis (aOR = 2.26) or other forms of cannabis use, including edibles (aOR = 2.36). However, these latter non-significant findings may result from insufficient power due to small samples: cannabis vapers (n = 431), or other non-smoking routes of administration (n = 539), compared to cannabis smokers (n = 3640).
Monte et al. (2019) [45] stratified ED visits between 2014 and 2016 that were at least partially due to cannabis use into 2329 related to cannabis smoking (90.7%) and 238 related to cannabis ingestion (9.3%). ED admissions due to cannabis-related cardiovascular symptoms were significantly more frequent in cannabis ingesting users than in cannabis smokers: 8.0% versus 3.1% of the admissions, with severe adverse cardiovascular events, including myocardial infarction and ventricular dysrhythmia, occurring in both groups (no numbers presented). Many more visits attributable to edible cannabis were, however, due to acute psychiatric symptoms (18.0%) and intoxications (48%).
The pooled 2016–2018 data from the BRFSS was used to evaluate the association between cannabis and reported cardiovascular disease among US adults who never smoked cigarettes [26]. The aOR of frequent cannabis use (all modalities of use, including edibles) for MI or coronary artery disease was 1.88 when compared with non-cannabis users, while the risk for frequent cannabis-only smokers was very similar (aOR = 2.07). Importantly, across all age groups, cannabis use in any form other than smoking (mostly oral ingestion) was not significantly associated with cardiovascular disease: current non-smoking cannabis use (aOR = 1.31) and frequent non-smoking cannabis use (aOR = 1.00) [26]. This shows that cannabis use in any form other than smoking (mostly oral ingestion) was not significantly associated with cardiovascular disease, but the sample was rather small (n = 786) and therefore the study had insufficient power to detect such an association if it existed.
A systematic review and meta-analysis of clinical trials performed with dronabinol (2.5–5 mg) and nabilone (1–3 mg), orally administered on a daily base for 4 to 16 weeks, showed no adverse cardiovascular effects, but again the power to detect such effects were probably too small [46].
3.9. Case Reports on Cannabis Edibles and Vaping
A 17-year-old male (tobacco smoking unknown) with no prior medical, cardiac or substance abuse history suffered from chest pain 3–4 h after vaping cannabis. The patient was diagnosed with elevated troponin and ST-segment elevations, pointing to cardiac ischemia [51]. Following cannabis vaping, a 26-year-old male habitual tobacco smoker suffered acute chest pain the next morning. Clinical investigation showed rising troponin levels (from 8.3 to 14.6 ng/L) indicating myocardial infarction [52]. A 70-year-old cannabis naïve male vaped several times, in close succession, a 65% THC concentrate which resulted, within minutes of use, in chest pain, palpitations, and a STEMI progressing to a fatal cardiac arrest [56]. A male tobacco smoker (70-year-old) consumed a cannabis lollipop at a dose of 70 mg (7-fold the recommended dose) which elicited MI [53]. Ingestion of cannabis formulated as a burger by a 55-year-old non-smoking male resulted in elevated troponin I (0.167 ng/mL), chest pain, and NSTEMI [54]. Finally, the ingestion of 600 mg of cannabis, plus an unknown amount of inhaled cannabis, resulted in a 27-year-old male (tobacco smoking unknown) with a high troponin level (peaked > 270,000 pg/mL; normal range: 0–40 pg/mL), STEMI, and a subsequent fatal cerebrovascular accident [55].
Figure 2 summarizes the main findings on the relationship between cannabis use and the risk for MI or coronary artery disease.
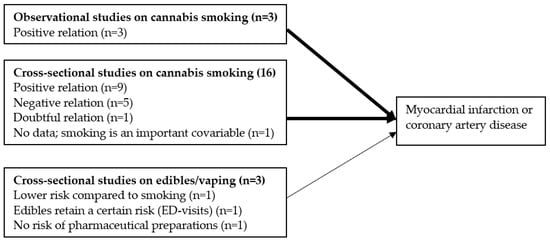
Figure 2.
Main findings on the relationship between cannabis use and the risk for MI (number of studies between parenthesis). The thinner arrow reflects the smaller effect size of vaping/edibles compared to smoking cannabis.
4. Discussion
This systematic review shows a consistent association of cannabis smoking with an increased risk of MI. Next to tobacco smoking, cannabis smoking seems to retain a certain risk of CHD which may, amongst others, be related to the formation of carbon monoxide (CO) upon smoking. However, this effect is not (fully) explained by the cardiovascular effects of (concurrent) tobacco use. The association between other forms of cannabis use (vaping, edibles) and MI was weaker or non-significant, but this may be partly due to small sample sizes with insufficient power to detect existing effects.
The precise mechanism behind MI provoked by cannabis (and tobacco) smoking is still not fully known, but likely involves carbon monoxide (CO) combined with THC-induced tachycardia and a pro-coagulant effect by polycyclic aromatic hydrocarbons (PHAs) in cannabis smoke, THC or both, akin to the mechanisms observed in tobacco smokers (CO, cardiac stimulation by nicotine and PHA-formation). Overall, blood supply to the heart muscle is insufficient which elicits myocardial ischemia via a mismatch between oxygen supply and demand [73]. Compared to administration of cannabis via vaporization or ingestion, smoking cannabis acutely and dose-dependently increases carbon monoxide (CO) and carboxyhaemoglobin (COHb) concentrations [74,75]. The generation of CO via combustion and subsequent formation of carboxyhaemoglobin in the blood seems to be the main culprit in CHD due to a reduction of oxygen delivery to the myocardium. For example, Aronow and Cassidy (1974) showed that, in patients with chronic but stable angina pectoris, the exercise time to the onset of angina symptoms was decreased by about 48% after smoking a single cannabis cigarette, compared with a decrease of only 9% after smoking a placebo cigarette [30]. Moreover, smoking one cannabis cigarette increased the COHb level significantly more than smoking one high-nicotine cigarette [21]. Finally, the topography of smoking of tobacco and cannabis is quite different: compared to tobacco smoking, cannabis is smoked with 60% larger puff volumes and a 4-fold longer breath-hold time, which may lead to higher exposure levels (internal dose) resulting in a nearly 5-fold increase in COHb levels after cannabis smoking compared to tobacco smoking [76].
The negative impact of tobacco on the MI risk of cannabis smoking may be even more persistent than just described, because smokers are, in addition to CO, repeatedly exposed to a large number of toxic compounds other than CO. These compounds represent, in addition to e.g., lung cancer, a major risk of progressive coronary thrombosis known to be implicated in the pathology of myocardial infarction [77,78]. PHAs found in the tar fraction of both cigarette and cannabis smoke [79] have been shown, at least in experimental models, to accelerate atherosclerosis [80,81]. This pathological element therefore applies to tobacco smoking, as well as cannabis smoking. Moreover, blood platelets are responsive to cannabinoids which explains the pro-coagulant effect of THC [82] an important factor in (coronary) atherosclerosis. Though the data indicate an MI crisis is provoked within 1–2 h of cannabis smoking [32], part of the cannabis-use-related cardiovascular incidents, including atherosclerotic damage and MI, may be due to long-term cannabis use. Such prolonged cannabis use may, like prolonged tobacco smoking, amongst other factors, lead to platelet aggregation, and atherosclerosis progression [83], components known to increase the risk of MI. Collectively, the data show that the precise mechanism of MI induced by both cannabis smoking and tobacco smoking remains to be resolved, including whether THC or other cannabis smoke components have an indirect negative impact on the risk of MI via atherogenesis.
Natural cannabis contains over 60 compounds with varying pharmacological activity [84]. At first glance, it seems, therefore, attractive to include synthetic cannabinoid (SC) use in a review on the MI risk in cannabis users, because SC are single compounds (THC-analogues). However, in contrast to the partial agonist THC (i.e., cannabis) at the CB1 and CB2 receptors, SC are full agonists with a 5–80 times higher potency, which may account for their greater toxicities [85] and the relatively high number of serious incidents, including fatal incidents compared to cannabis [86,87,88]. Another argument to exclude SC from this study is the very low prevalence of SC-use compared to the use of natural cannabis, leading to low numbers of MI cases.
It is not clear yet whether the MI risk in cannabis vapers and people who orally ingest cannabis is really (much) smaller than in cannabis smokers. However, edible cannabis, which avoids exposure to cardiotoxic CO, might be a safer administration route, as suggested by the absence of significant associations with cardiovascular events in some studies. Similarly, vaping leads to virtually no expired CO and is therefore presumably less harmful than smoking cannabis and may offer a safer alternative to smoking, although further research is needed to clarify its cardiovascular effects, especially considering reported cases of MI following vaping (cf. Table 1), although it is not clear whether these observations were biassed by concomitant tobacco smoking or co-morbidities. However, vaping and edibles are popular among younger, healthy men with no cardiovascular risk factors, which may lead to an underestimation of the MI risk. This further implies that it cannot be excluded that the MI patient population using both routes of cannabis administration will change to include older individuals in the future. Indicative of the lower harm of non-smoking forms of cannabis use are the results of a Canadian study from 2012 on cannabis attributable harm showing that, if only non-smoked forms of cannabis were consumed, at least two-thirds of deaths attributable to cannabis use that year would have been avoided [89].
5. Conclusions
It is concluded that cannabis use, certainly in smoked form, may have deleterious cardiovascular effects, including provocation of (acute) MI. Therefore, patients with known coronary artery disease should refrain from cannabis use, while those seeking the euphoric effects of THC should consider vaporizers to avoid exposure to the harmful components generated via combustion of either cannabis or tobacco (e.g., when formulated in a joint), including CO [90]. High quality prospective studies are essential to further elucidate the role of cannabis, in particular smoking versus vaporizing and edibles, in precipitating acute coronary syndrome and other cardiovascular problems.
6. Limitations
Tobacco smoking is commonly known to be associated with MI. To examine the independent impact of cannabis use on MI, confounding by tobacco smoking must therefore be eliminated. However, tobacco smoking history is often not addressed, nor were data routinely adjusted for concomitant tobacco smoking as a confounder. Unfortunately, tobacco-cannabis co-use among cannabis users is very common (see e.g. [91]), which leads to small sample sizes of non-tobacco smoking cannabis users. The low number also applies to cannabis vapers and those who ingest cannabis (edibles), which may result in null findings (assuming differences between cannabis use modalities exist).
The high variability in the many compounds that natural cannabis contains, including the variation in THC-concentration and the emerging number of cannabis-containing products, may result in large differences in exposure among cannabis users. This variation is not or is poorly addressed in the selected studies and could, therefore, not be studied in this review as separate predictors of myocardial infarction.
Many papers suffer from variability in design and some from poor quality which hinder proper evaluation of the association between cannabis use and MI. Most studies refer to ‘cannabis smoking’ without specifying the form (joint, spliff, or blunt), the frequency of use, and amount of cannabis per session, and the THC-concentration, making it impossible to study the dose-relation between cannabis use in MI. In some studies, angina pectoris was used as the main outcome instead of MI, making results less comparable. In addition, risk factors for MI, like co-morbidities, and use of substances other than cannabis were mostly not addressed or described.
Finally, most of the eligible studies were performed in the US, which may have skewed our results because of major differences in cannabis consumption between the US and Europe. This could be considered a limitation. Although we used PubMed, Embase, and Google Scholar, which account for 93% of published literature on a given search [92], we were not able to retrieve more eligible studies from either Europe or Australia.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm13185620/s1, Search string used; Table S1: Appraisal according to Critical Appraisal Tools (CAT) checklists developed by the Joanna Briggs Institute (JBI); Table S2: PRISMA 2020 Checklist.
Author Contributions
The following contributions were provided: Conceptualization, J.v.A.; Methodology, J.v.A.; Investigation, W.v.d.B. and J.v.A.; Writing—Original Draft Preparation, W.v.d.B. and J.v.A.; Writing—Review & Editing, W.v.d.B. and J.v.A.; Supervision, W.v.d.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare that they have no affiliations with or involvement in any organization or entity with any financial or non-financial interest in the subject matter or materials discussed in this manuscript that could inappropriately influence (bias) their work.
References
- CDC. Centers for Disease Control and Prevention, Centers for Disease Control and Prevention (CDC). What You Need to Know about Marijuana Use in Teens. 2017. Available online: https://stacks.cdc.gov/view/cdc/43947 (accessed on 9 August 2024).
- SAMHSA. Substance Abuse and Mental Health Services Administration (SAMHSA), Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health, 2021 and 2022. 2022. Available online: https://www.samhsa.gov/data/sites/default/files/reports/rpt42728/NSDUHDetailedTabs2022/NSDUHDetailedTabs2022/NSDUHDetTabsSect1pe2022.htm (accessed on 9 August 2024).
- Boakye, E.; Obisesan, O.H.; Uddin, S.I.; El-Shahawy, O.; Dzaye, O.; Osei, A.D.; Benjamin, E.J.; Stokes, A.C.; Robertson, R.M.; Bhatnagar, A. Cannabis vaping among adults in the United States: Prevalence, trends, and association with high-risk behaviors and adverse respiratory conditions. Prev. Med. 2021, 153, 106800. [Google Scholar] [CrossRef] [PubMed]
- Ladha, K.S.; Mistry, N.; Wijeysundera, D.N.; Clarke, H.; Verma, S.; Hare, G.M.T.; Mazer, C.D. Recent cannabis use and myocardial infarction in young adults: A cross-sectional study. Can. Med. Assoc. J. 2021, 193, E1377–E1384. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.C.; Chan, G.C.; Wadsworth, E.; Stjepanović, D.; Chiu, V.; Chung, J.Y.; Sun, T.; Connor, J.; Leung, J.; Gartner, C. Trends and socio-demographic differences of cannabis vaping in the USA and Canada. Int. J. Environ. Res. Public Health 2022, 19, 14394. [Google Scholar] [CrossRef] [PubMed]
- Schauer, G.L.; King, B.A.; Bunnell, R.E.; Promoff, G.; McAfee, T.A. Toking, vaping, and eating for health or fun: Marijuana use patterns in adults, US, 2014. Am. J. Prev. Med. 2016, 50, 1–8. [Google Scholar] [CrossRef]
- Steigerwald, S.; Wong, P.O.; Cohen, B.E.; Ishida, J.H.; Vali, M.; Madden, E.; Keyhani, S. Smoking, vaping, and use of edibles and other forms of marijuana among US adults. Ann. Intern. Med. 2018, 169, 890–892. [Google Scholar] [CrossRef]
- Lim, C.C.; Sun, T.; Leung, J.; Chung, J.Y.; Gartner, C.; Connor, J.; Hall, W.; Chiu, V.; Stjepanović, D.; Chan, G.C. Prevalence of adolescent cannabis vaping: A systematic review and meta-analysis of US and Canadian studies. JAMA Pediatr. 2022, 176, 42–51. [Google Scholar] [CrossRef]
- Lim, C. Emerging Trends of Cannabis Vaping in the USA and Canada: Prevalence, Correlates, and Online Presence. Thesis. 2023. Available online: https://tinyurl.com/y6zzd4rm (accessed on 9 August 2024).
- Patrick, M.E.; Miech, R.A.; Kloska, D.D.; Wagner, A.C.; Johnston, L.D. Trends in marijuana vaping and edible consumption from 2015 to 2018 among adolescents in the US. JAMA Pediatr. 2020, 174, 900–902. [Google Scholar] [CrossRef]
- Wadsworth, E.; Craft, S.; Calder, R.; Hammond, D. Prevalence and use of cannabis products and routes of administration among youth and young adults in Canada and the United States: A systematic review. Addict. Behav. 2022, 129, 107258. [Google Scholar] [CrossRef]
- DeFilippis, E.M.; Bajaj, N.S.; Singh, A.; Malloy, R.; Givertz, M.M.; Blankstein, R.; Bhatt, D.L.; Vaduganathan, M. Marijuana use in patients with cardiovascular disease: JACC review topic of the week. J. Am. Coll. Cardiol. 2020, 75, 320–332. [Google Scholar] [CrossRef]
- Frost, L.; Mostofsky, E.; Rosenbloom, J.I.; Mukamal, K.J.; Mittleman, M.A. Marijuana use and long-term mortality among survivors of acute myocardial infarction. Am. Heart J. 2013, 165, 170–175. [Google Scholar] [CrossRef]
- Mukamal, K.J.; Maclure, M.; Muller, J.E.; Mittleman, M.A. An exploratory prospective study of marijuana use and mortality following acute myocardial infarction. Am. Heart J. 2008, 155, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.T.; Dawber, T.R.; Kannel, W.B.; Kinch, S.H.; Kahn, H.A. The relationship of cigarette smoking to coronary heart disease: The second report of the combined experience of the Albany, NY, and Framingham, Mass, studies. JAMA 1964, 190, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Aronow, W.S. Effect of cigarette smoking and of carbon monoxide on coronary heart disease. Chest 1976, 70, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, C.D.; Rosenman, R.H.; Zyzanski, S.J. Cigarette smoking: Its relationship to coronary heart disease and related risk factors in the Western Collaborative Group Study. Circulation 1968, 38, 1140–1155. [Google Scholar] [CrossRef] [PubMed]
- Doll, R.; Hill, A.B. Mortality in relation to smoking: Ten years’ observations of British doctors. Br. Med. J. 1964, 1, 1399. [Google Scholar] [CrossRef] [PubMed]
- Lushniak, B.D.; Samet, J.M.; Pechacek, T.F.; Norman, L.A.; Taylor, P.A. The Health Consequences of Smoking. 50 Years of Progress: A Report of the Surgeon General. 2014. Available online: https://www.ncbi.nlm.nih.gov/books/NBK179276/ (accessed on 7 August 2024).
- Kaufman, D.W.; Helmrich, S.P.; Rosenberg, L.; Miettinen, O.S.; Shapiro, S. Nicotine and carbon monoxide content of cigarette smoke and the risk of myocardial infarction in young men. N. Engl. J. Med. 1983, 308, 409–413. [Google Scholar] [CrossRef]
- Aronow, W.S.; Cassidy, J. Effect of smoking marihuana and of a high-nicotine cigarette on angina pectoris. Clin. Pharmacol. Ther. 1975, 17, 549–554. [Google Scholar] [CrossRef]
- Nawrot, T.S.; Perez, L.; Künzli, N.; Munters, E.; Nemery, B. Public health importance of triggers of myocardial infarction: A comparative risk assessment. Lancet 2011, 377, 732–740. [Google Scholar] [CrossRef]
- Desai, R.; Fong, H.K.; Shah, K.; Kaur, V.P.; Savani, S.; Gangani, K.; Damarlapally, N.; Goyal, H. Rising trends in hospitalizations for cardiovascular events among young cannabis users (18–39 years) without other substance abuse. Medicina 2019, 55, 438. [Google Scholar] [CrossRef]
- Desai, R.; Patel, U.; Sharma, S.; Amin, P.; Bhuva, R.; Patel, M.S.; Sharma, N.; Shah, M.; Patel, S.; Savani, S. Recreational marijuana use and acute myocardial infarction: Insights from nationwide inpatient sample in the United States. Cureus 2017, 9, e1816. [Google Scholar] [CrossRef]
- Patel, R.S.; Manocha, P.; Patel, J.; Patel, R.; Tankersley, W.E. Cannabis use is an independent predictor for acute myocardial infarction related hospitalization in younger population. J. Adolesc. Health 2020, 66, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Patel, S.; Paulraj, S.; Chaudhuri, D. Association of marijuana use and cardiovascular disease: A behavioral risk factor surveillance system data analysis of 133,706 US adults. Am. J. Med. 2021, 134, 614–620.e1. [Google Scholar] [CrossRef] [PubMed]
- Desai, R.; Shamim, S.; Patel, K.; Sadolikar, A.; Kaur, V.P.; Bhivandkar, S.; Patel, S.; Savani, S.; Mansuri, Z.; Mahuwala, Z. Primary causes of hospitalizations and procedures, predictors of in-hospital mortality, and trends in cardiovascular and cerebrovascular events among recreational marijuana users: A five-year nationwide inpatient assessment in the United States. Cureus 2018, 10, e3195. [Google Scholar] [CrossRef] [PubMed]
- Ramphul, K.; Aggarwal, S.; Verma, R.; Lohana, P.; Sombans, S.; Ramphul, Y.; Mejias, S.G.; Kumar, D.; Kwansa, N.A.; Pekyi-Boateng, P.K. Acute myocardial infarction among teenagers in the United States between 2016 and 2020: A retrospective analysis from the National Inpatient Sample. Arch. Med. Sciences. Atheroscler. Dis. 2023, 8, e177. [Google Scholar] [CrossRef]
- NAS. National Academies of Sciences (NAS). The National Academies Collection. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. 2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK423845/ (accessed on 8 August 2024).
- Aronow, W.S.; Cassidy, J. Effect of marihuana and placebo-marihuana smoking on angina pectoris. N. Engl. J. Med. 1974, 291, 65–67. [Google Scholar] [CrossRef]
- Jouanjus, E.; Lapeyre-Mestre, M.; Micallef, J. Cannabis use: Signal of increasing risk of serious cardiovascular disorders. J. Am. Heart Assoc. 2014, 3, e000638. [Google Scholar] [CrossRef]
- Mittleman, M.A.; Lewis, R.A.; Maclure, M.; Sherwood, J.B.; Muller, J.E. Triggering myocardial infarction by marijuana. Circulation 2001, 103, 2805–2809. [Google Scholar] [CrossRef] [PubMed]
- Corroon, J.; Grant, I.; Allison, M.A.; Bradley, R. Associations between monthly cannabis use and myocardial infarction in middle-aged adults: NHANES 2009 to 2018. Am. J. Cardiol. 2023, 204, 226–233. [Google Scholar] [CrossRef]
- Reis, J.P.; Auer, R.; Bancks, M.P.; Goff, D.C., Jr.; Lewis, C.E.; Pletcher, M.J.; Rana, J.S.; Shikany, J.M.; Sidney, S. Cumulative lifetime marijuana use and incident cardiovascular disease in middle age: The Coronary Artery Risk Development in Young Adults (CARDIA) study. Am. J. Public Health 2017, 107, 601–606. [Google Scholar] [CrossRef]
- Chami, T.; Kim, C.H. Cannabis abuse and elevated risk of myocardial infarction in the young: A population-based study. Mayo Clin. Proc. 2019, 94, 1647–1649. [Google Scholar] [CrossRef]
- Sandhyavenu, H.; Patel, H.P.; Patel, R.H.; Desai, R.; Patel, A.A.; Patel, B.A.; Patel, J.; Zahid, S.; Khan, S.U.; Deshmukh, A. Rising trend of acute myocardial infarction among young cannabis users: A 10-year nationwide gender and race stratified analysis. Int. J. Cardiol. Cardiovasc. Risk Prev. 2023, 16, 200167. [Google Scholar] [CrossRef] [PubMed]
- Kalla, A.; Krishnamoorthy, P.M.; Gopalakrishnan, A.; Figueredo, V.M. Cannabis use predicts risks of heart failure and cerebrovascular accidents: Results from the National Inpatient Sample. J. Cardiovasc. Med. 2018, 19, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, D.R.; Dutta, A.; Mukerji, S.S.; Holman, A.; Uno, H.; Gabuzda, D. Marijuana use impacts midlife cardiovascular events in HIV-infected men. Clin. Infect. Dis. 2017, 65, 626–635. [Google Scholar] [CrossRef]
- Jivanji, D.; Mangosing, M.; Mahoney, S.P.; Castro, G.; Zevallos, J.; Lozano, J. Association between marijuana use and cardiovascular disease in US adults. Cureus 2020, 12, e11868. [Google Scholar] [CrossRef] [PubMed]
- Karki, N.; Sapkota, B.; Magar, S.R.; Muhammad, A.; Paudel, B.M.; Chernek, P.; Afshar, M.; Bhandari, M.; Bella, J.N. Relationship between marijuana use and hospitalization for acute coronary syndrome. Cureus 2022, 14, 84. [Google Scholar] [CrossRef] [PubMed]
- Skipina, T.M.; Upadhya, B.; Soliman, E.Z. Cannabis use and electrocardiographic myocardial injury. Am. J. Cardiol. 2021, 151, 100–104. [Google Scholar] [CrossRef]
- Skipina, T.M.; Patel, N.; Upadhya, B.; Soliman, E.Z. Cannabis use is associated with prevalent coronary artery disease. Am. J. Med. Sci. 2022, 364, 304–308. [Google Scholar] [CrossRef]
- Mondal, A.; Dadana, S.; Parmar, P.; Mylavarapu, M.; Dong, Q.; Butt, S.R.; Kali, A.; Bollu, B.; Desai, R. Association of cannabis use disorder with major adverse cardiac and cerebrovascular events in older non-tobacco users: A population-based analysis. Med. Sci. 2024, 12, 13. [Google Scholar] [CrossRef]
- Jeffers, A.M.; Glantz, S.; Byers, A.L.; Keyhani, S. Association of cannabis use with cardiovascular outcomes among US adults. J. Am. Heart Assoc. 2024, 13, e030178. [Google Scholar] [CrossRef]
- Monte, A.A.; Shelton, S.K.; Mills, E.; Saben, J.; Hopkinson, A.; Sonn, B.; Devivo, M.; Chang, T.; Fox, J.; Brevik, C. Acute illness associated with cannabis use, by route of exposure: An observational study. Ann. Intern. Med. 2019, 170, 531–537. [Google Scholar] [CrossRef]
- Bajtel, Á.; Kiss, T.; Tóth, B.; Kiss, S.; Hegyi, P.; Vörhendi, N.; Csupor-Löffler, B.; Gede, N.; Hohmann, J.; Csupor, D. The safety of dronabinol and nabilone: A systematic review and meta-analysis of clinical trials. Pharmaceuticals 2022, 15, 100. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Stone, J.C.; Aromataris, E.; Klugar, M.; Sears, K.; Leonardi-Bee, J.; Barker, T.H. Assessing the risk of bias of quantitative analytical studies: Introducing the vision for critical appraisal within JBI systematic reviews. JBI Evid. Synth. 2023, 21, 467–471. [Google Scholar] [CrossRef]
- Ma, L.-L.; Wang, Y.-Y.; Yang, Z.-H.; Huang, D.; Weng, H.; Zeng, X.-T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Mil. Med. Res. 2020, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K.; et al. Systematic reviews of etiology and risk. In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; JBI: Adelaide, Australia, 2020; Chapter 7; Available online: https://synthesismanual.jbi.global (accessed on 9 August 2024).
- Algarni, M.; Hadi, M.A.; Yahyouche, A.; Mahmood, S.; Jalal, Z. A mixed-methods systematic review of the prevalence, reasons, associated harms and risk-reduction interventions of over-the-counter (OTC) medicines misuse, abuse and dependence in adults. J. Pharm. Policy Pract. 2021, 14, 76. [Google Scholar] [CrossRef] [PubMed]
- Schreier, M.D.; Williams, C.; Ma, T.M. Cardiac ischemia associated with marijuana use in an adolescent. Cureus 2020, 12, e9661. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Alqaisi, S. Myopericarditis associated with marijuana intake: A case report and literature review. Cureus 2023, 15, e39413. [Google Scholar] [CrossRef]
- Saunders, A.; Stevenson, R.S. Marijuana lollipop-induced myocardial infarction. Can. J. Cardiol. 2019, 35, 229.e1–e3. [Google Scholar] [CrossRef]
- Kariyanna, P.T.; Yadav, R.; Yadav, V.; Jayarangaiah, A.; Srinivasan, M.; Chandrakumar, H.P.; McFarlane, I.M. Atrioventricular nodal reentrant tachycardia triggered by edible marijuana: A case report and review of the literature. Am. J. Med. Case Rep. 2020, 8, 123–127. [Google Scholar] [CrossRef]
- Lavertue, S.M.; Signorella, S.; Baig, R.; Emerson, A.; Joyce, J.; Zhang, R. Death after marijuana use in a 27-year-old male: A case report. Adv. Clin. Med. Res. Healthc. Deliv. 2023, 3, 8. [Google Scholar] [CrossRef]
- Hendrickson, R.G.; McKeown, N.J.; Kusin, S.G.; Lopez, A.M. Acute cannabis toxicity in older adults. Toxicol. Commun. 2020, 4, 67–70. [Google Scholar] [CrossRef]
- Tirkey, N.K.; Gupta, S. Acute antero-inferior wall ischaemia with acute ischaemic stroke caused by oral ingestion of cannabis in a young male. J. Assoc. Physicians India 2016, 64, 93–94. [Google Scholar] [PubMed]
- Franz, C.A.; Frishman, W.H. Marijuana use and cardiovascular disease. Cardiol. Rev. 2016, 24, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Jouanjus, E.; Raymond, V.; Lapeyre-Mestre, M.; Wolff, V. What is the current knowledge about the cardiovascular risk for users of cannabis-based products? A systematic review. Curr. Atheroscl Rep. 2017, 19, 26. [Google Scholar] [CrossRef]
- Pacher, P.; Steffens, S.; Haskó, G.; Schindler, T.H.; Kunos, G. Cardiovascular effects of marijuana and synthetic cannabinoids: The good, the bad, and the ugly. Nat. Rev. Cardiol. 2018, 15, 151–166. [Google Scholar] [CrossRef]
- Thomas, G.; Kloner, R.A.; Rezkalla, S. Adverse cardiovascular, cerebrovascular, and peripheral vascular effects of marijuana inhalation: What cardiologists need to know. Am. J. Cardiol. 2014, 113, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Saluja, S.; Kumar, A.; Agrawal, S.; Thind, M.; Nanda, S.; Shirani, J. Cardiovascular complications of marijuana and related substances: A review. Cardiol. Ther. 2018, 7, 45–59. [Google Scholar] [CrossRef]
- Latif, Z.; Garg, N. The impact of marijuana on the cardiovascular system: A review of the most common cardiovascular events associated with marijuana use. J. Clin. Med. 2020, 9, 1925. [Google Scholar] [CrossRef]
- Wang, A.; Dey, S.; Subhan, S.; Patel, J.; Frishman, W.H.; Aronow, W.S. Cardiovascular effects of cannabinoids. Cardiol. Rev. 2023, 95, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.R.; Bing, M.L.; Moulin, A.K.; Elder, J.W.; Rominski, R.T.; Summers, P.J.; Laurin, E.G. Cannabis use and acute coronary syndrome. Clin. Toxicol. 2019, 57, 831–841. [Google Scholar] [CrossRef]
- Theerasuwipakorn, N.; Prechawat, S.; Chokesuwattanaskul, R.; Siranart, N.; Marsukjai, A.; Thumtecho, S.; Rungpradubvong, V. Cannabis and adverse cardiovascular events: A systematic review and meta-analysis of observational studies. Toxicol. Rep. 2023, 10, 537–543. [Google Scholar] [CrossRef]
- Chetty, K.; Lavoie, A.; Deghani, P. A literature review of cannabis and myocardial infarction. What clinicians may not be aware of. CJC Open 2021, 3, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Gandhi, A.B.; Antony, I.; Alexander, J.; Hisbulla, M.; Kannichamy, V.; Kaleem, I.; Mishra, V.; Khan, S. Role of cannabis in the incidence of myocardial infarction: A review. Cureus 2020, 12, e11097. [Google Scholar] [CrossRef]
- Pasha, A.K.; Clements, C.Y.; Reynolds, C.A.; Lopez, M.K.; Lugo, C.A.; Gonzalez, Y.; Shirazi, F.M.; Abidov, A. Cardiovascular effects of medical marijuana: A systematic review. Am. J. Med. 2021, 134, 182–193. [Google Scholar] [CrossRef]
- Weresa, J.; Pędzińska-Betiuk, A.; Mińczuk, K.; Malinowska, B.; Schlicker, E. Why do marijuana and synthetic cannabimimetics induce acute myocardial infarction in healthy young people? Cells 2022, 11, 1142. [Google Scholar] [CrossRef]
- Patel, R.S.; Kamil, S.H.; Bachu, R.; Adikey, A.; Ravat, V.; Kaur, M.; Tankersley, W.E.; Goyal, H. Marijuana use and acute myocardial infarction: A systematic review of published cases in the literature. Trends Cardiovasc. Med. 2020, 30, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Pomahacova, B.; Van der Kooy, F.; Verpoorte, R. Cannabis smoke condensate III: The cannabinoid content of vaporised Cannabis sativa. Inhal. Toxicol. 2009, 21, 1108–1112. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.T. Cardiovascular system effects of marijuana. J. Clin. Pharmacol. 2002, 42, 58S–63S. [Google Scholar] [CrossRef]
- Newmeyer, M.N.; Swortwood, M.J.; Abulseoud, O.A.; Huestis, M.A. Subjective and physiological effects, and expired carbon monoxide concentrations in frequent and occasional cannabis smokers following smoked, vaporized, and oral cannabis administration. Drug Alcohol. Depend. 2017, 175, 67–76. [Google Scholar] [CrossRef]
- Azorlosa, J.L.; Greenwald, M.K.; Stitzer, M.L. Marijuana smoking: Effects of varying puff volume and breathhold duration. J. Pharmacol. Exp. Ther. 1995, 272, 560–569. [Google Scholar]
- Wu, T.-C.; Tashkin, D.P.; Djahed, B.; Rose, J.E. Pulmonary hazards of smoking marijuana as compared with tobacco. N. Engl. J. Med. 1988, 318, 347–351. [Google Scholar] [CrossRef]
- Ambrose, J.A.; Barua, R.S. The pathophysiology of cigarette smoking and cardiovascular disease: An update. J. Am. Coll. Cardiol. 2004, 43, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Thomas, A.C. Plaque fissuring--the cause of acute myocardial infarction, sudden ischaemic death, and crescendo angina. Br. Heart J. 1985, 53, 363. [Google Scholar] [CrossRef]
- Tomar, R.S.; Beaumont, J.; Hsieh, J.C.Y. Evidence on the Carcinogenicity of Marijuana Smoke. California Environmental Protection Agency. Sacremento (CA). 2009. Available online: http://oehha.ca.gov/media/downloads/proposition-65/chemicals/finalmjsmokehid.pdf (accessed on 9 August 2024).
- Penn, A.; Snyder, C. Arteriosclerotic plaque development is ‘promoted’ by polynuclear aromatic hydrocarbons. Carcinogenesis 1988, 9, 2185–2189. [Google Scholar] [CrossRef] [PubMed]
- Mallah, M.A.; Mallah, M.A.; Liu, Y.; Xi, H.; Wang, W.; Feng, F.; Zhang, Q. Relationship between polycyclic aromatic hydrocarbons and cardiovascular diseases: A systematic review. Front. Public Health 2021, 9, 763706. [Google Scholar] [CrossRef] [PubMed]
- Deusch, E.; Kress, H.G.; Kraft, B.; Kozek-Langenecker, S.A. The procoagulatory effects of delta-9-tetrahydrocannabinol in human platelets. Anesth. Analg. 2004, 99, 1127–1130. [Google Scholar] [CrossRef]
- Subramaniam, V.N.; Menezes, A.R.; DeSchutter, A.; Lavie, C.J. The cardiovascular effects of marijuana: Are the potential adverse effects worth the high? Mo. Med. 2019, 116, 146. [Google Scholar]
- Choo, E.K.; Emery, S.L. Clearing the haze: The complexities and challenges of research on state marijuana laws. Ann. N. Y. Acad. Sci. 2017, 1394, 55–73. [Google Scholar] [CrossRef]
- Alzu’bi, A.; Almahasneh, F.; Khasawneh, R.; Abu-El-Rub, E.; Baker, W.B.; Al-Zoubi, R.M. The synthetic cannabinoids menace: A review of health risks and toxicity. Eur. J. Med. Res. 2024, 29, 49. [Google Scholar] [CrossRef]
- Waugh, J.; Najafi, J.; Hawkins, L.; Hill, S.L.; Eddleston, M.; Vale, J.A.; Thompson, J.P.; Thomas, S.H. Epidemiology and clinical features of toxicity following recreational use of synthetic cannabinoid receptor agonists: A report from the United Kingdom National Poisons Information Service. Clin. Toxicol. 2016, 54, 512–518. [Google Scholar] [CrossRef]
- Elhelaly, H.; Salah Eldin, H. Comparative study between acute toxicity of natural cannabis and synthetic cannabinoids. Ain Shams J. Forens. Med. Clin. Toxicol. 2022, 38, 103–109. [Google Scholar] [CrossRef]
- de Oliveira, M.C.; Vides, M.C.; Lassi, D.L.S.; Torales, J.; Ventriglio, A.; Bombana, H.S.; Leyton, V.; Périco, C.d.A.-M.; Negrão, A.B.; Malbergier, A. Toxicity of synthetic cannabinoids in K2/spice: A systematic review. Brain Sci. 2023, 13, 990. [Google Scholar] [CrossRef] [PubMed]
- Imtiaz, S.; Shield, K.D.; Roerecke, M.; Cheng, J.; Popova, S.; Kurdyak, P.; Fischer, B.; Rehm, J. The burden of disease attributable to cannabis use in Canada in 2012. Addiction 2016, 111, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L. Managing cannabis use in patients with cardiovascular disease. Can. J. Cardiol. 2019, 35, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Hindocha, C.; Brose, L.S.; Walsh, H.; Cheeseman, H. Cannabis use and co-use in tobacco smokers and non-smokers: Prevalence and associations with mental health in a cross-sectional, nationally representative sample of adults in Great Britain, 2020. Addiction 2021, 116, 2209–2219. [Google Scholar] [CrossRef]
- Bramer, W.M.; Rethlefsen, M.L.; Kleijnen, J.; Franco, O.H. Optimal database combinations for literature searches in systematic reviews: A prospective exploratory study. Syst. Rev. 2017, 6, 245. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).