Impact of GLP-1 Receptor Agonists in Gastrointestinal Endoscopy: An Updated Review
Abstract
1. Introduction
2. Clinical Guidelines
3. Clinical Studies
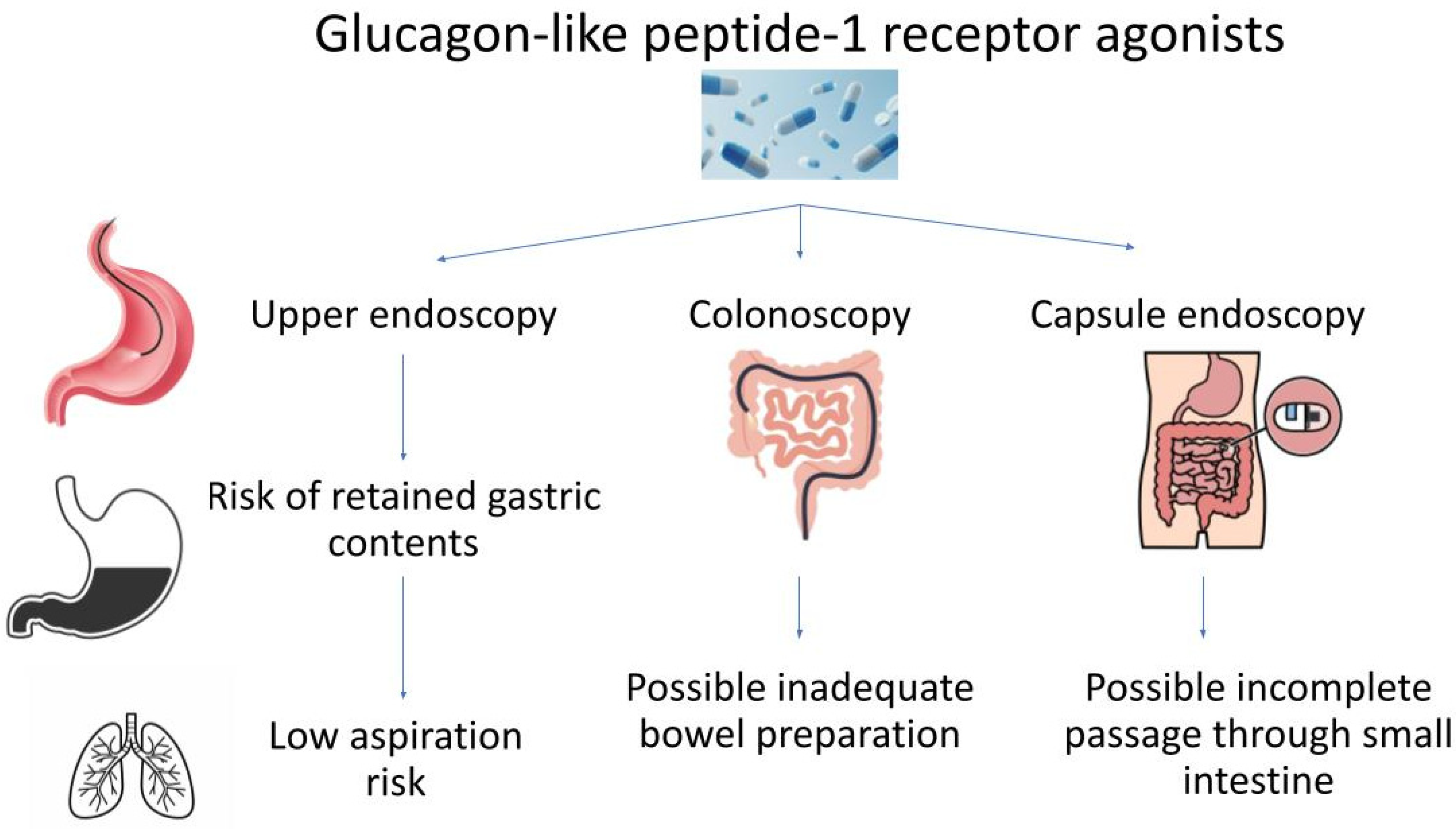
3.1. Upper Endoscopy
3.2. Colonoscopy
3.3. Capsule Endoscopy
3.4. Meta-Analyses
4. Controversy
5. Limitations and Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef] [PubMed]
- Qaseem, A.; Obley, A.J.; Shamliyan, T.; Hicks, L.A.; Harrod, C.S.; Crandall, C.J.; Clinical Guidelines Committee of the American College of Physicians; Balk, E.M.; Cooney, T.G.; Cross, J.T., Jr.; et al. Newer Pharmacologic Treatments in Adults With Type 2 Diabetes: A Clinical Guideline From the American College of Physicians. Ann. Intern. Med. 2024, 177, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Grunvald, E.; Shah, R.; Hernaez, R.; Chandar, A.K.; Pickett-Blakely, O.; Teigen, L.M.; Harindhanavudhi, T.; Sultan, S.; Singh, S.; Davitkov, P.; et al. AGA Clinical Practice Guideline on Pharmacological Interventions for Adults With Obesity. Gastroenterology 2022, 163, 1198–1225. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, J.H.; Kwon, J.; Nan, B.; Reikes, A. Trends in glucagon-like peptide 1 receptor agonist use, 2014 to 2022. J. Am. Pharm. Assoc. (2003) 2024, 64, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Arvanitakis, K.; Koufakis, T.; Popovic, D.; Maltese, G.; Mustafa, O.; Doumas, M.; Giouleme, O.; Kotsa, K.; Germanidis, G. GLP-1 Receptor Agonists in Obese Patients with Inflammatory Bowel Disease: From Molecular Mechanisms to Clinical Considerations and Practical Recommendations for Safe and Effective Use. Curr. Obes. Rep. 2023, 12, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Lupianez-Merly, C. Effects of GLP-1 and Other Gut Hormone Receptors on the Gastrointestinal Tract and Implications in Clinical Practice. Am. J. Gastroenterol. 2024, 119, 1028–1037. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kalas, M.A.; Dang, T.Q.; Galura, G.; Alvarado, L.; Dwivedi, A.K.; Deoker, A.; McCallum, R. Frequency of GLP-1 receptor agonists use in diabetic patients diagnosed with delayed gastric emptying and their demographic profile. J. Investig. Med. 2023, 71, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Hiramoto, B.; McCarty, T.R.; Lodhia, N.A.; Jenkins, A.; Elnaiem, A.; Muftah, M.; Flanagan, R.; Chan, W.W. Quantified Metrics of Gastric Emptying Delay by Glucagon-Like Peptide-1 Agonists: A Systematic Review and Meta-Analysis With Insights for Periprocedural Management. Am. J. Gastroenterol. 2024, 119, 1126–1140. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fujino, E.; Cobb, K.W.; Schoenherr, J.; Gouker, L.; Lund, E. Anesthesia Considerations for a Patient on Semaglutide and Delayed Gastric Emptying. Cureus 2023, 15, e42153. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Klein, S.R.; Hobai, I.A. Semaglutide, delayed gastric emptying, and intraoperative pulmonary aspiration: A case report. Can. J. Anaesth. 2023, 70, 1394–1396. [Google Scholar] [CrossRef] [PubMed]
- Jalleh, R.J.; Rayner, C.K.; Hausken, T.; Jones, K.L.; Camilleri, M.; Horowitz, M. Gastrointestinal effects of GLP-1 receptor agonists: Mechanisms, management, and future directions. Lancet Gastroenterol. Hepatol. 2024, 9, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Kuo, B.; Nguyen, L.; Vaughn, V.M.; Petrey, J.; Greer, K.; Yadlapati, R.; Abell, T.L. ACG Clinical Guideline: Gastroparesis. Am. J. Gastroenterol. 2022, 117, 1197–1220. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rayner, C.K.; Watson, L.E.; Phillips, L.K.; Lange, K.; Bound, M.J.; Grivell, J.; Wu, T.; Jones, K.L.; Horowitz, M.; Ferrannini, E.; et al. Effects of Sustained Treatment With Lixisenatide on Gastric Emptying and Postprandial Glucose Metabolism in Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Care 2020, 43, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Jensterle, M.; Ferjan, S.; Ležaič, L.; Sočan, A.; Goričar, K.; Zaletel, K.; Janez, A. Semaglutide delays 4-hour gastric emptying in women with polycystic ovary syndrome and obesity. Diabetes Obes. Metab. 2023, 25, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Khan, R.J.; Grover, S.C. Measuring the Quality and Safety of Upper Endoscopy in Patients Taking Glucagon-like Peptide 1 Receptor Agonists. Gastroenterology, 2024, in press. [CrossRef] [PubMed]
- Mizubuti, G.B.; da Silva, L.M.; Silveira, S.Q.; Gilron, I.; Ho, A.M. Comment on: Association of glucagon-like peptide receptor 1 agonist therapy with the presence of gastric contents in fasting patients undergoing endoscopy under anesthesia care: A historical cohort study. Can. J. Anaesth. 2024, 71, 1172–1173. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Klapman, S.A.; Everett, L.L.; Kuo, B.; Hobai, I.A. In reply: Comment on: Association of glucagon-like peptide receptor 1 agonist therapy with the presence of gastric contents in fasting patients undergoing endoscopy under anesthesia care: A historical cohort study. Can. J. Anaesth. 2024, 71, 1174. [Google Scholar] [CrossRef] [PubMed]
- Raven, L.M.; Brown, C.; Greenfield, J.R. Considerations of delayed gastric emptying with peri-operative use of glucagon-like peptide-1 receptor agonists. Med. J. Aust. 2024, 220, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Sánchez, S.A.; Cedrón-Cheng, H.G. Gastroparesia severa asociada al uso de agonistas del receptor GLP-1 para bajar de peso [Severe gastroparesia associated with the use of GLP-1 receptor agonists for weight loss]. Rev. Gastroenterol. Peru 2024, 44, 71–74. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- American Society of Anesthesiologists Consensus-Based Guidance on Preoperative Management of Patients (Adults and Children) on Glucagon-like Peptide-1 (GLP-1) Receptor Agonists. Available online: https://www.asahq.org/about-asa/newsroom/news-releases/2023/06/american-society-of-anesthesiologists-consensus-based-guidance-on-preoperative (accessed on 1 September 2024).
- No Data to Support Stopping GLP-1 Agonists Prior to Elective Endoscopy. Available online: https://gastro.org/news/gi-multi-society-statement-regarding-glp-1-agonists-and-endoscopy/ (accessed on 1 September 2024).
- Hashash, J.G.; Thompson, C.C.; Wang, A.Y. AGA Rapid Clinical Practice Update on the Management of Patients Taking GLP-1 Receptor Agonists Prior to Endoscopy: Communication. Clin. Gastroenterol. Hepatol. 2024, 22, 705–707. [Google Scholar] [CrossRef] [PubMed]
- Duggan, E.W.; Carlson, K.; Umpierrez, G.E. Perioperative Hyperglycemia Management: An Update. Anesthesiology 2017, 126, 547–560. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shi, J.; Shen, H.; Gao, Q.; Mulmi Shrestha, S.; Tan, J.; Lu, T.; Yang, B. Evaluation of gastric emptying in patients with gastroparesis by three-dimensional ultrasound. Ann. Transl. Med. 2021, 9, 1343. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Silveira, S.Q.; da Silva, L.M.; de Campos Vieira Abib, A.; de Moura, D.T.H.; de Moura, E.G.H.; Santos, L.B.; Ho, A.M.; Nersessian, R.S.F.; Lima, F.L.M.; Silva, M.V.; et al. Relationship between perioperative semaglutide use and residual gastric content: A retrospective analysis of patients undergoing elective upper endoscopy. J. Clin. Anesth. 2023, 87, 111091. [Google Scholar] [CrossRef] [PubMed]
- Ushakumari, D.S.; Sladen, R.N. ASA Consensus-based Guidance on Preoperative Management of Patients on Glucagon-like Peptide-1 Receptor Agonists. Anesthesiology 2024, 140, 346–348. [Google Scholar] [CrossRef] [PubMed]
- Milder, D.A.; Milder, T.Y.; Liang, S.S.; Kam, P.C.A. Glucagon-like peptide-1 receptor agonists: A narrative review of clinical pharmacology and implications for peri-operative practice. Anaesthesia 2024, 79, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Stark, J.E.; Cole, J.L.; Ghazarian, R.N.; Klass, M.J. Impact of Glucagon-Like Peptide-1 Receptor Agonists (GLP-1RA) on Food Content During Esophagogastroduodenoscopy (EGD). Ann. Pharmacother. 2022, 56, 922–926. [Google Scholar] [CrossRef] [PubMed]
- Firkins, S.A.; Yates, J.; Shukla, N.; Garg, R.; Vargo, J.J.; Lembo, A.; Cleveland Clinic Obesity Medicine and Bariatric Endoscopy Working Group. Clinical Outcomes and Safety of Upper Endoscopy While on Glucagon-Like Peptide-1 Receptor Agonists. Clin. Gastroenterol. Hepatol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Anazco, D.; Fansa, S.; Hurtado, M.D.; Camilleri, M.; Acosta, A. Low Incidence of Pulmonary Aspiration During Upper Endoscopy in Patients Prescribed a Glucagon-Like Peptide 1 Receptor Agonist. Clin. Gastroenterol. Hepatol. 2024, 22, 1333–1335.e2. [Google Scholar] [CrossRef] [PubMed]
- Maselli, D.B.; Lee, D.; Bi, D.; Jirapinyo, P.; Thompson, C.C.; Donnangelo, L.L.; McGowan, C.E. Safe Continuation of Glucagon-like Peptide 1 Receptor Agonists at Endoscopy: A Case Series of 57 Adults Undergoing Endoscopic Sleeve Gastroplasty. Obes. Surg. 2024, 34, 2369–2374. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, D.; Taye, M.; Still, M.D.; McShea, S.; Satterfield, D.; Dove, J.T.; Wood, G.C.; Addissie, B.D.; Diehl, D.L.; Johal, A.S.; et al. Effects of glucagon-like peptide-1 receptor agonists on upper endoscopy in diabetic and nondiabetic patients. Gastrointest. Endosc. 2024, in press. [CrossRef] [PubMed]
- Chapman, M.B.; Norwood, D.A.; Price, C.; Abdulhadi, B.; Kyanam Kabir Baig, K.; Ahmed, A.M.; Peter, S.; Routman, J.S.; Sánchez-Luna, S.A.; Duggan, E.W.; et al. Effects of glucagon-like peptide-1 receptor agonists on gastric mucosal visibility and retained gastric contents during EGD. Gastrointest. Endosc. 2024, in press. [CrossRef] [PubMed]
- Barlowe, T.S.; Anderson, C.; Sandler, R.S.; Subramaniam, D.; Muratore, A.; Buse, J.B.; Gouker, L.N.; Majithia, R.T.; Shaheen, N.J.; Stürmer, T.; et al. Glucagon-Like Peptide-1 Receptor Agonists Do Not Increase Aspiration During Upper Endoscopy in Patients With Diabetes. Clin. Gastroenterol. Hepatol. 2024, in press. [CrossRef] [PubMed]
- Jennifer, P.; Patrick, C.; Danny, I.; Ronald, T.; Jennifer, D.; Anders, W.; Rahul, K.; Lorenzo, O.; Firas, B.; Vahagn, A.; et al. GLP1-RA use prior to endoscopy is associated with low retained gastric contents: A multicenter cross-sectional analysis. Am. J. Gastroenterol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Garza, K.; Aminpour, E.; Shah, J.; Mehta, B.; Early, D.; Gyawali, C.P.; Kushnir, V. Glucagon-Like Peptide-1 Receptor Agonists Increase Solid Gastric Residue Rates on Upper Endoscopy Especially in Patients With Complicated Diabetes: A Case-Control Study. Am. J. Gastroenterol. 2024, 119, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Smith, M.R.; Mueller, A.L.; Klapman, S.A.; Everett, L.L.; Houle, T.; Kuo, B.; Hobai, I.A. Association of glucagon-like peptide receptor 1 agonist therapy with the presence of gastric contents in fasting patients undergoing endoscopy under anesthesia care: A historical cohort study. Can. J. Anaesth. 2024, 71, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Ghazanfar, H.; Javed, N.; Qasim, A.; Sosa, F.; Altaf, F.; Khan, S.; Mahasamudram, J.; Jyala, A.; Kandhi, S.D.; Shin, D.; et al. Is it necessary to stop glucagon-like peptide-1 receptor agonists prior to endoscopic procedure? A retrospective study. World. J. Gastroenterol. 2024, 30, 3221–3228. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tong, Y.; Huang, J.Q.; Chen, Y.; Tu, M.; Wang, W. Impact of glucagon-like peptide 1 receptor agonist liraglutide and dipeptidyl peptidase-4 inhibitor sitagliptin on bowel cleaning and gastrointestinal symptoms in type 2 diabetes. Front. Pharmacol. 2023, 14, 1176206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yao, R.; Gala, K.S.; Ghusn, W.; Abboud, D.M.; Wallace, F.K.; Vargas, E.J. Effect of Glucagon-Like Peptide-1 Receptor Agonists on Bowel Preparation for Colonoscopy. Am. J. Gastroenterol. 2024, 119, 1154–1157. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, Y.; Maeda, M.; Matsumura, M.; Shimizu, R.; Banba, N.; Aso, Y.; Yasu, T.; Harasawa, H. Effect of GLP-1 receptor agonist on gastrointestinal tract motility and residue rates as evaluated by capsule endoscopy. Diabetes Metab. 2017, 43, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Odah, T.; Vattikonda, A.; Stark, M.; Brahmbhatt, B.; Lukens, F.J.; Badurdeen, D.; Hashash, J.G.; Farraye, F.A. Glucagon-like Peptide-1 Receptor Agonists and Capsule Endoscopy in Patients with Diabetes: A Matched Cohort Study. Gastrointest. Endosc. 2024, in press. [CrossRef] [PubMed]
- Facciorusso, A.; Ramai, D.; Dhar, J.; Samanta, J.; Chandan, S.; Gkolfakis, P.; Crinò, S.F.; Maida, M.; Anderloni, A.; Boskoski, I.; et al. Effects of Glucagon-Like Peptide-1 Receptor Agonists on Upper Gastrointestinal Endoscopy: A Meta-Analysis. Clin. Gastroenterol. Hepatol. 2024, in press. [CrossRef] [PubMed]
- Joshi, G.P. Anesthetic Considerations in Adult Patients on Glucagon-Like Peptide-1 Receptor Agonists: Gastrointestinal Focus. Anesth. Analg. 2024, 138, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Aharon, A.; Singer, P. The paracetamol absorption test: A useful addition to the enteral nutrition algorithm? Clin. Nutr. 2000, 19, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Madsbad, S. Review of head-to-head comparisons of glucagon-like peptide-1 receptor agonists. Diabetes Obes. Metab. 2016, 18, 317–332. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Facciorusso, A. The influence of diabetes in the pathogenesis and the clinical course of hepatocellular carcinoma: Recent findings and new perspectives. Curr. Diabetes Rev. 2013, 9, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Crinò, S.F.; Napoleon, B.; Facciorusso, A.; Lakhtakia, S.; Borbath, I.; Caillol, F.; Do-Cong Pham, K.; Rizzatti, G.; Forti, E.; Palazzo, L.; et al. Endoscopic Ultrasound-guided Radiofrequency Ablation Versus Surgical Resection for Treatment of Pancreatic Insulinoma. Clin. Gastroenterol. Hepatol. 2023, 21, 2834–2843.e2. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Straus Takahashi, M.; Eyileten Postula, C.; Buccino, V.R.; Muscatiello, N. Efficacy of hemostatic powders in upper gastrointestinal bleeding: A systematic review and meta-analysis. Dig. Liver Dis. 2019, 51, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Mangiavillano, B.; Moon, J.H.; Crinò, S.F.; Larghi, A.; Pham, K.D.; Teoh, A.Y.B.; Paduano, D.; Lee, Y.N.; Yoo, H.W.; Shin, I.S.; et al. Safety and efficacy of a novel electrocautery-enhanced lumen-apposing metal stent in interventional EUS procedures (with video). Gastrointest. Endosc. 2022, 95, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Ramai, D.; Gkolfakis, P.; Khan, S.R.; Papanikolaou, I.S.; Triantafyllou, K.; Tringali, A.; Chandan, S.; Mohan, B.P.; Adler, D.G. Comparative efficacy of different methods for difficult biliary cannulation in ERCP: Systematic review and network meta-analysis. Gastrointest. Endosc. 2022, 95, 60–71.e12. [Google Scholar] [CrossRef] [PubMed]
- Boicean, A.; Prisca, D.; Bratu, D.G.; Bacila, C.I.; Tanasescu, C.; Chicea, R.; Fleaca, S.R.; Birsan, S.A.; Ichim, C.; Mohor, C.I.; et al. Uncommon Presentation of Gastric Duplication Cyst with Left-Sided Portal Hypertension: A Case Report and Literature Review. Diagnostics 2024, 14, 675. [Google Scholar] [CrossRef]
| Reference | Age (y) | Male % | Race/Ethnicity % | BMI (kg/m2) | DM % | GLP-1 RA Dose | GLP-1 RA Route | Findings |
|---|---|---|---|---|---|---|---|---|
| [25] | 50.8 | 51.5 | - | 26.2 | 9.4 | - | Subcutaneous | RGCs in the GLP-1 RA vs. control group (24.2% vs. 5.1%, p < 0.001), only 1 aspiration event in the GLP-1 RA group |
| [28] | 65 | 88.5 | - | 33 | 97.5 | - | Subcutaneous | RGCs in the GLP-1 RA vs. control group (6.8% vs. 1.7%, p = 0.08) |
| [29] | 60.9 | 35.8 | - | 35.2 | 76.7 | - | Subcutaneous or oral | RGCs (9.4%), aspiration (0.1%) |
| [30] | - | - | - | - | - | - | - | 4.8 aspiration cases per 10,000 endoscopies |
| [31] | 44 | 10.5 | - | 40.1 | 35.1 | Semaglutide 0.25–2.4 mg/week Liraglutide 0.6–3 mg/day Dulaglutide 0.75–4.5 mg/week Tirzepatide 2.5–15 mg/week | Subcutaneous | No cases of RGCs or pulmonary aspiration |
| [32] | 54 | 41 | White 91 Hispanic 5 Black 2 | 30.7 | 18 | - | - | RGCs in the GLP-1 RA vs. control group (13.6% vs. 2.3%, p < 0.0001), only 1 aspiration event in the control group |
| [33] | 53.94 | 29.8 | White 60.1 Black 39.9 | 35.96 | 85.7 | - | - | RGCs in the GLP-1 RA vs. control group (13.1% vs. 4.8%, p = 0.025) |
| [34] | 56 | 45 | - | - | 100 | - | - | Aspiration in the GLP-1 RA, dipeptidyl peptidase 4 inhibitor, and chronic opioid users (0.05% vs. 0.07% vs. 0.11%) |
| [35] | 60.7 | 42.3 | Caucasian 53.9 African American 19.6 Hispanic 17.5 Asian 3.1 | - | 82.5 | - | Subcutaneous or oral | RGCs (8.6%) |
| [36] | 61.5 | 50.5 | - | 32.45 | 88 | - | Subcutaneous or oral | RGCs in the GLP-1 RA vs. control group (14% vs. 4%, p < 0.01), no aspiration events |
| [37] | 61.3 | 42.5 | - | 34 | 47 | - | - | RGCs in the GLP-1 RA vs. control group (18.7% vs. 4.9%, p = 0.004), 1 aspiration event in the GLP-1 RA group vs. 0 in the control group |
| [38] | 60 | 63.1 | - | - | 85.6 | - | - | RGCs in the regular diet vs. clear liquid/low-residue diet groups (10% vs. 1.5%, p = 0.03), no aspiration events |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, S.; Chandan, S.; Dahiya, D.S.; Aswath, G.; Ramai, D.; Maida, M.; Anderloni, A.; Muscatiello, N.; Facciorusso, A. Impact of GLP-1 Receptor Agonists in Gastrointestinal Endoscopy: An Updated Review. J. Clin. Med. 2024, 13, 5627. https://doi.org/10.3390/jcm13185627
Singh S, Chandan S, Dahiya DS, Aswath G, Ramai D, Maida M, Anderloni A, Muscatiello N, Facciorusso A. Impact of GLP-1 Receptor Agonists in Gastrointestinal Endoscopy: An Updated Review. Journal of Clinical Medicine. 2024; 13(18):5627. https://doi.org/10.3390/jcm13185627
Chicago/Turabian StyleSingh, Sahib, Saurabh Chandan, Dushyant Singh Dahiya, Ganesh Aswath, Daryl Ramai, Marcello Maida, Andrea Anderloni, Nicola Muscatiello, and Antonio Facciorusso. 2024. "Impact of GLP-1 Receptor Agonists in Gastrointestinal Endoscopy: An Updated Review" Journal of Clinical Medicine 13, no. 18: 5627. https://doi.org/10.3390/jcm13185627
APA StyleSingh, S., Chandan, S., Dahiya, D. S., Aswath, G., Ramai, D., Maida, M., Anderloni, A., Muscatiello, N., & Facciorusso, A. (2024). Impact of GLP-1 Receptor Agonists in Gastrointestinal Endoscopy: An Updated Review. Journal of Clinical Medicine, 13(18), 5627. https://doi.org/10.3390/jcm13185627






