Abstract
Background: Chronic obstructive pulmonary disease (COPD) is associated with subclinical atherosclerosis and endothelial dysfunction, which can be assessed non-invasively through flow-mediated dilation (FMD). In this study, we evaluated the potential impact of inhaled corticosteroid (ICS) therapy on FMD of COPD patients undergoing pulmonary rehabilitation (PR). Methods: Medical records of COPD patients undergoing FMD assessment upon admission to our Pulmonary Rehabilitation Unit were reviewed in this retrospective post hoc analysis. Results: A total of 46 patients with COPD (median age 71.5 years, 28.3% postmenopausal females) were included in the final analysis. Among these, 27 participants were currently receiving ICS therapy, while 19 were not. At baseline, the two groups showed no difference in the main clinical and functional variables. Similarly, no significant difference was observed in vascular reactivity parameters, with a median FMD of 3.12% (IQR: 2.23–4.45) in ICS users and 3.39% (IQR: 2.45–4.08) in ICS nonusers (p = 0.544). After PR, a significant improvement in the main rehabilitation and patient-reported outcomes was observed in all groups, with a significant improvement in FMD documented in both patients treated with steroids (from 3.12%; IQR: 2.23–4.45 to 4.77%; IQR: 3.25–5.63, p = 0.022) and in those who were not (from 3.39%; IQR: 2.45–4.08 to 5.04%; IQR: 3.98–6.06, p = 0.005). FMD changes were of comparable magnitude among groups. Conclusions: Our preliminary findings do not indicate a significant impact of medications containing ICS on the endothelial function of COPD patients, suggesting that the potential beneficial effect of PR on this surrogate marker of cardiovascular risk is independent of inhaled therapy.
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a respiratory disorder characterized by fixed airflow limitation, exercise intolerance, acute exacerbations, and a gradual progression leading to respiratory failure [1]. This condition, often caused by chronic exposure to lung irritants such as smoking and pollution, significantly impairs the quality of life and imposes a substantial burden on healthcare systems [2]. An increasing body of evidence has underscored that COPD patients are also at a higher risk of developing cardiovascular (CV) diseases, including myocardial infarction, stroke, and heart failure [3]. It has been suggested that this risk in COPD may not be solely attributable to the shared exposure to traditional CV risk factors, like smoking, obesity, and sedentary lifestyle [4]. Instead, immune-mediated inflammation and oxidative stress are thought to play a crucial role in the development and progression of atherosclerosis in this clinical setting, due to their harmful effects on the vascular endothelium [5]. Thus, the disruption of endothelial barrier integrity in both systemic and pulmonary circulation may be a common pathogenic mechanism of many COPD manifestations, including the increased CV risk [5,6].
Endothelial dysfunction is, in fact, the earliest stage of atherosclerosis [7], with several methods being proposed to assess this parameter, both invasively and non-invasively [8]. In this regard, flow-mediated dilation (FMD) has been considered as a reliable technique and a surrogate marker of CV risk [9]. FMD involves the use of ultrasound to measure the dilation of the brachial artery following a period of occlusion and subsequent release, reflecting the endothelium’s ability to respond to increased blood flow [10]. Using this methodology, prior investigations have hinted at the possibility of a beneficial effect of multidisciplinary rehabilitation on the endothelial function of individuals with COPD, thereby potentially improving their CV risk profile [11]. Multidisciplinary rehabilitation programs and other exercise-based interventions often include nutritional counseling, psychological support, and educational programs [12,13,14], all of which can somehow impact overall CV health. In particular, it has been proposed that physical exercise, a cornerstone component of these programs, may enhance endothelial function by positively impacting systemic inflammation and oxidative stress [15]. In this scenario, pharmacological therapies, such as triple therapy combining inhaled corticosteroids (ICSs) with long-acting bronchodilators, may further reduce the systemic inflammation that contributes to endothelial dysfunction and atherosclerosis in COPD patients, thus potentially reducing overall and CV mortality [16] while reducing the frequency of exacerbations [17]. Accordingly, meta-analytical findings indicate a substantial risk reduction in CV disease, including heart attacks and strokes, associated with the use of ICS-containing drugs in COPD patients [18].
Therefore, this study aims to investigate the potential impact of concomitant ICS therapy on the FMD of COPD patients undergoing in-hospital pulmonary rehabilitation (PR).
2. Methods
We designed a retrospective post hoc analysis of prospectively collected data. A detailed protocol for data collection has been reported elsewhere and part of the baseline data have been previously published [11]. In brief, medical records of COPD patients undergoing FMD assessment upon admission to the Pulmonary Rehabilitation Unit of Istituti Clinici Scientifici Maugeri IRCCS, Telese Terme, Italy, between August 2020 and September 2022 were reviewed in this observational study. The inclusion criteria were as follows: age ≥ 40 years; confirmed diagnosis of COPD dating back at least one year; no acute exacerbation in the 4 weeks before admission and stable maintenance inhalatory therapy for at least the prior 3 months; and complete baseline FMD data gathered at study entry. Exclusions were applied to patients with major CV comorbidities, ongoing malignancies, any respiratory disease other than COPD, history of asthma in adulthood, recent (<12 months) severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, or any history of severe-to-critical coronavirus disease 2019 (COVID-19) [9]. Where applicable, this study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [19] and complied with the Declaration of Helsinki by the World Medical Association. The Institutional Review Board of IRCCS Fondazione G. Pascale, Naples, Italy, reviewed and approved the protocol (No. 5.17OSS), and all participants provided written informed consent.
2.1. Study Protocol
Within 24 h from admission, relevant data on medical history and pharmacological therapy were collected for all study participants. In line with the principles outlined in the official American Thoracic Society/European Respiratory Society (ATS/ERS) statement [20], all included patients participated in a personalized, exercise-based multidisciplinary PR program, undergoing clinical, laboratory, and functional assessments at admission (T0) and, again, after completing the rehabilitation program (T1).
2.2. Study Procedures
Whenever possible, study procedures were conducted at each time point, with participants in rooms maintained at a constant temperature of 23 °C. Under these conditions, fasting venous blood samples were drawn to assess various blood parameters, including creatinine (mg/dL), total cholesterol (mg/dL), triglycerides (mg/dL), glucose (mg/dL), and C-reactive protein (mg/dL). Following the recommendations of the European Society of Hypertension/European Society of Cardiology [21], systolic and diastolic blood pressure (SBP and DBP) were measured three times on separate occasions, with the average of these readings used as the final value.
Arterial oxygen tension (PaO2), arterial carbon dioxide tension (PaCO2), and power of hydrogen (pH) were measured in ambient air using a blood gas analyzer (ABL 825® FLEX BGA, Radiometer Medical Aps, Copenhagen, Denmark) according to a standardized protocol [22]. Spirometry was performed on all COPD patients using an automated system (Vmax® Encore, Vyasis Healthcare, Milan, Italy), in line with ATS/ERS guidelines [23,24]. Spirometry parameters, including forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC) values, were recorded as both absolute (liters) and percentages of predicted values (FEV1% and FVC%). The FEV1/FVC ratio was calculated as an indicator of airflow limitation.
To assess the impact of COPD on participants’ health, the COPD Assessment Test (CAT) [25] was utilized. Dyspnea severity was evaluated using the modified Medical Research Council (mMRC) dyspnea scale [26], while the Barthel index was used to measure daily living activities performance [27]. Additionally, the European Quality of Life (EuroQoL) scale was administered at each time point as a self-reported measure of health-related quality of life [28]. Functional exercise capacity was evaluated by measuring the six-minute walking distance (6MWD) in meters, following a predefined protocol [29].
2.3. Brachial Artery FMD Measurement
Brachial artery FMD was evaluated using ultrasound imaging, following the guidelines of the International Brachial Artery Reactivity Task Force [10]. A detailed protocol for this noninvasive vascular assessment has been described elsewhere [11]. In brief, measurements were taken after a 12 h fast and abstaining from smoking. Each examination was conducted after at least 10 min of rest in a supine position, with the use of a small head pillow being permitted and a blood pressure cuff placed on the forearm. A B-mode ultrasound machine (Vivid E95®, GE Healthcare, Chicago, IL, USA) and a linear vascular transducer with a 10 MHz frequency were used to image a longitudinal section of the brachial artery above the antecubital fossa. The brachial artery diameter (BAD) was measured at rest and following reactive hyperemia induced by forearm ischemia. To obtain a baseline image, the brachial artery was visualized for 1 min using pulsed Doppler before cuff inflation. The cuff was then inflated to 70 mmHg above the systolic pressure for 5 min. Following cuff deflation, BAD was registered for 4 min. The maximal post-occlusive diameter was used to calculate FMD as the percentage change in BAD during post-occlusive reactive hyperemia. The shear stress stimulus for FMD was determined by the shear rate area under the curve from cuff deflation to peak diameter (SRAUC), with shear rate calculated as (4 × blood velocity)/BAD. The total shear rate area under the curve (SRAUC-TOT) represented the complete post-occlusive reactive hyperemia over the 4 min following cuff deflation. Semi-automatic software (Cardiovascular Suite® Version 3, FMD studio, QUIPU Srl, Pisa, Italy) was used to analyze the ultrasound images in real-time, thus calculating all vascular reactivity parameters. All recorded scans were independently analyzed by another ultrasound operator under blinded conditions to ensure strict quality control.
2.4. Pulmonary Rehabilitation
The interdisciplinary PR program for patients with COPD adhered to the latest ATS/ERS guidelines [20]. During the baseline assessment, a thorough evaluation of the extra-pulmonary features and comorbidities of COPD patients was conducted. This evaluation guided the application of various treatments, including physical exercise training, occupational therapy, dietary counseling, psychosocial counseling, and education. Physical exercise training was the cornerstone of the program. It involved exercises designed to strengthen muscle groups in the upper and lower extremities, flexibility exercises, treadmill walking, and stationary cycling. Supervised incremental aerobic exercise was planned, aimed at achieving at least 30 min of continuous activity at 50–75% of the maximal load [30]. Throughout the exercise sessions, pulse oximetry, blood pressure, and heart rate were continuously monitored. The daily activities lasted between 2 and 3 h, spread across 6 days per week, over a total period of 5 weeks.
2.5. Statistical Analysis
Statistical analysis was performed using SPSS 29.0 (IBM, Chicago, IL, USA) with statistical significance set at p < 0.05. In brief, the Shapiro–Wilk test was employed to assess the normality of the distribution of continuous variables. In case of a Gaussian distribution, continuous variables were expressed as means ± standard deviation (SD), while in case of a skewed non-Gaussian distribution, they were reported as medians (interquartile range, IQR). Within-group comparisons between the two pre-specified time points were carried out with a paired Student’s t-test in case of normally distributed variables, and with a Wilcoxon Rank Test in case of skewed distributions. Comparisons between independent groups were performed using the unpaired Student’s t-test for normally distributed variables, and the Mann–Whitney U test for non-normally distributed variables. Categorical variables were reported as relative frequencies and compared using Pearson’s chi-squared test or Fisher’s exact test, as appropriate.
3. Results
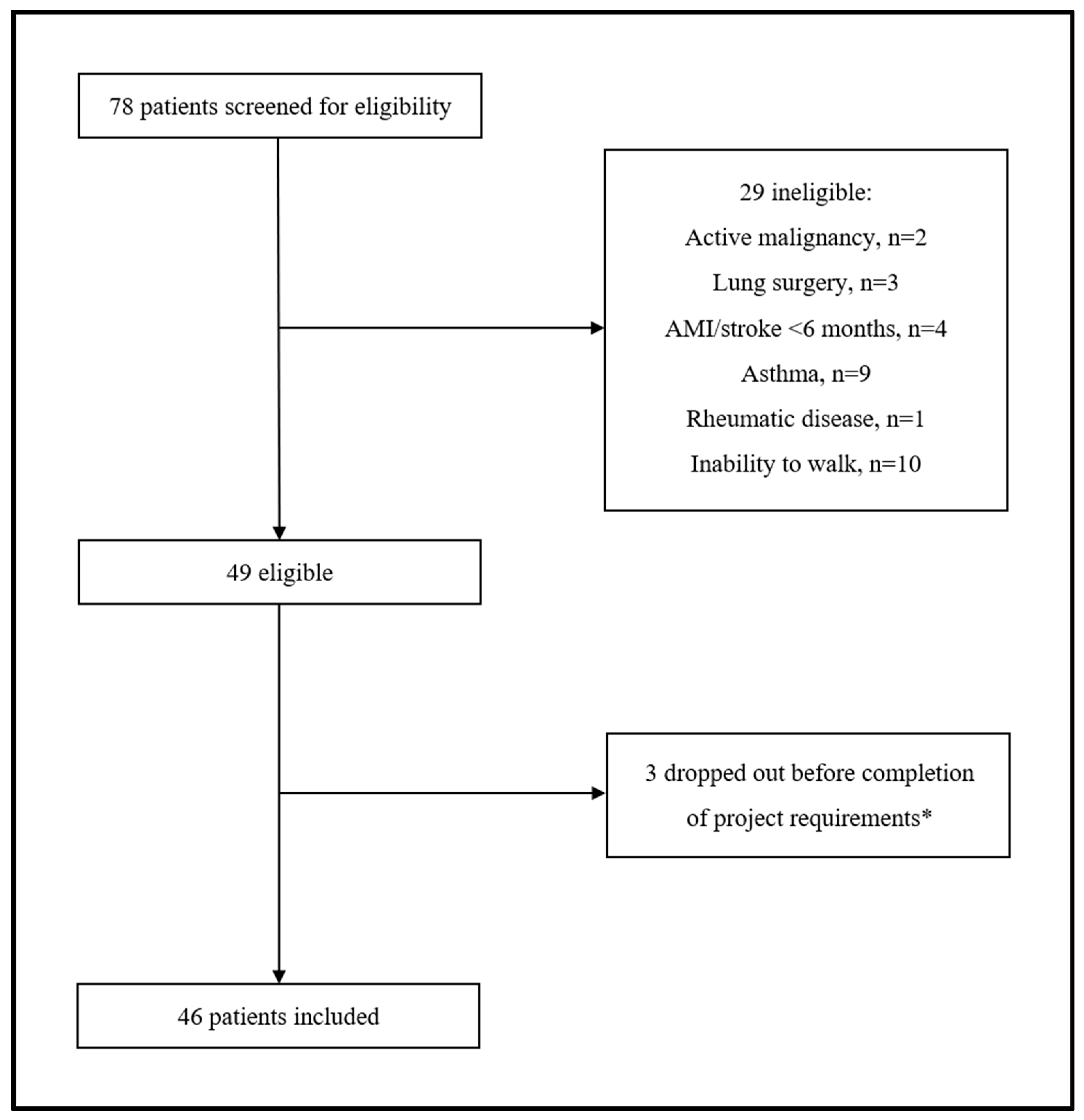
A total of 78 participants with COPD were screened for eligibility. Of them, 29 (37.2%) were excluded for various reasons related to the inclusion/exclusion criteria of our protocol. Among the 49 eligible patients, 3 (6.1%) withdrew before completion of the minimum project requirements. Therefore, 46 patients with COPD (median age 71.5 years, 28.3% postmenopausal females) were included in the final analysis (Figure 1).
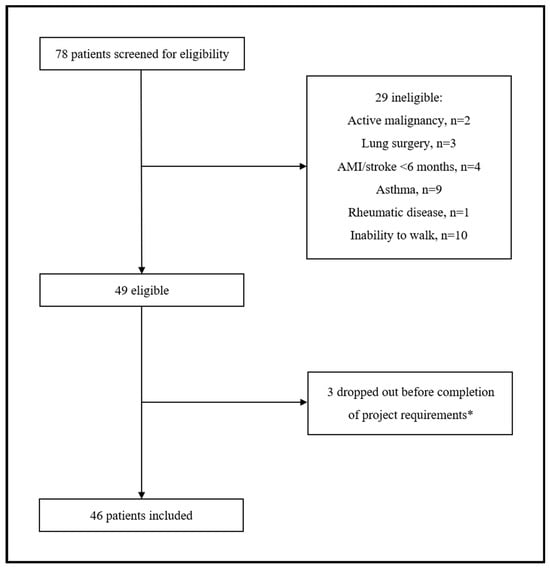
Figure 1.
Flow chat of study participants. * Three patients transferred to another hospital as unable to attend/complete the rehabilitation program.
All 46 participants were frequent exacerbators and, according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines [31], were classified in Group E. Out of these, 27 were currently receiving ICS therapy, while 19 were not. The main clinical and functional characteristics of the two groups and changes from baseline (T0) to completion of the PR program (T1) are reported in Table 1.

Table 1.
Main clinical and functional characteristics of chronic obstructive pulmonary disease (COPD) patients undergoing pulmonary rehabilitation, stratified and compared by inhaled corticosteroid (ICS) therapy.
At baseline, the two groups showed no difference in the main clinical and functional variables, except for a lower proportion of males among ICS users (p = 0.025) and higher peripheral saturation of oxygen (p = 0.022), as well as arterial oxygen partial pressure (p = 0.012). Similarly, no significant difference was observed between the two groups with regards to vascular reactivity parameters, with a median FMD of 3.12% (IQR: 2.23–4.45) in ICS users and 3.39% (IQR: 2.45–4.08) in ICS nonusers (p = 0.544) at T0.
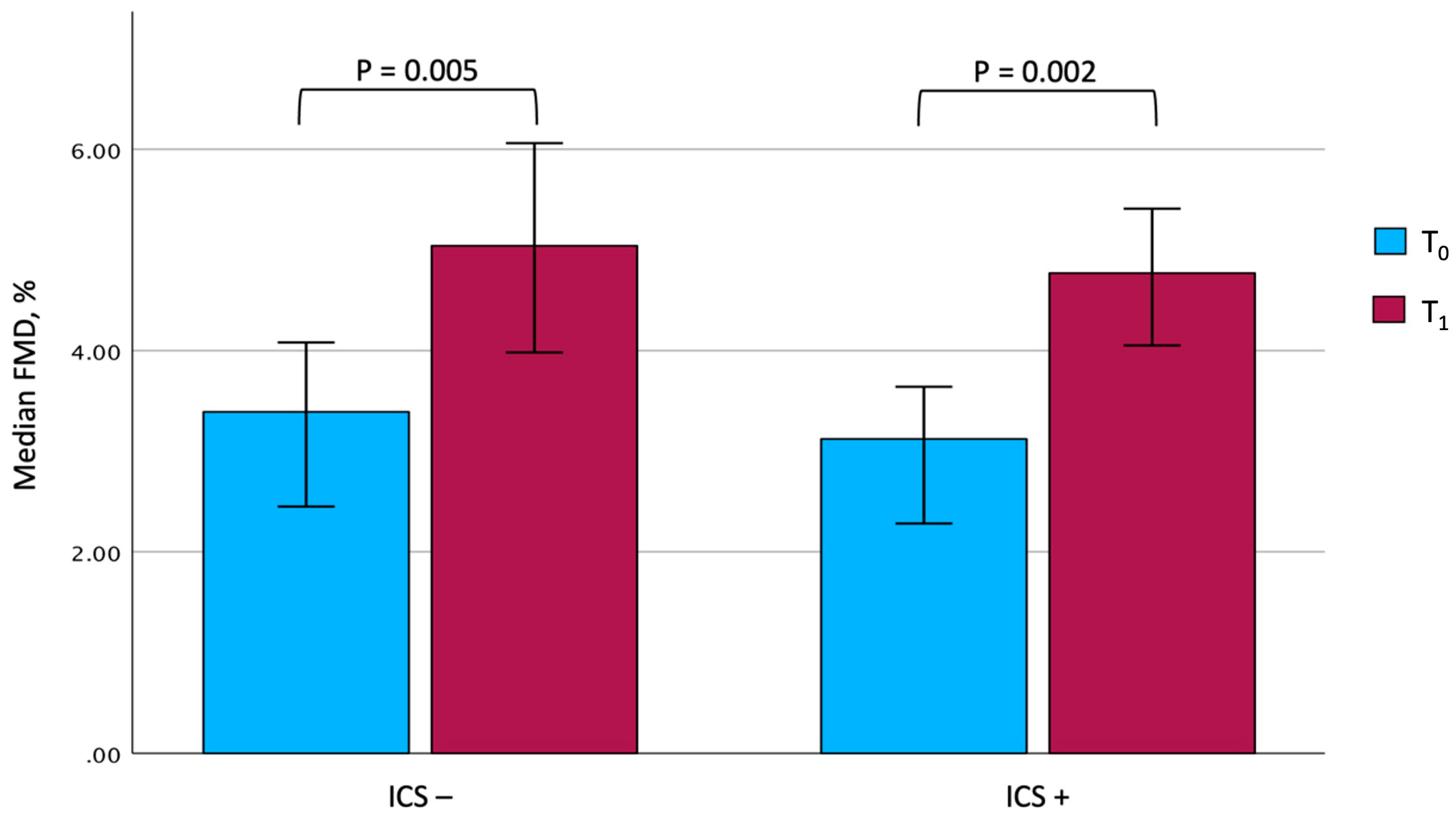
At the end of the rehabilitation program, due to technical issues, complete information on vascular reactivity could be gathered for only 25 out of 27 patients under ICS and 15 out of 19 patients not taking them. Although some differences were observed between the two groups, it is interesting to note that both showed a significant improvement in the main rehabilitation and patient-reported outcomes. In particular, the 6MWD significantly improved after PR (from 166.0 ± 73.1 m to 242.1 ± 88.8 m, p < 0.001, among ICS users, and from 166.5 ± 74.4 m to 246.4 ± 79.1 m, p < 0.001, among ICS nonusers), with comparable mean changes between the two groups (81.8 ± 42.9 m in ICS users vs. 79.9 ± 54.0 m in ICS nonusers, p = 0.900). Similarly, a significant improvement in FMD could be documented in both patients treated with steroids (from 3.12%; IQR: 2.23–4.45 to 4.77%; IQR: 3.25–5.63, p = 0.022) and in those who were not (from 3.39%; IQR: 2.45–4.08 to 5.04%; IQR: 3.98–6.06, p = 0.005), with changes of comparable magnitude (Figure 2). In contrast, no significant changes in BAD, SRAUC, and SRAUC-TOT were documented in both groups. After completing PR, it is remarkable that ICS users showed a significantly higher SRAUC compared with nonusers (p = 0.016).
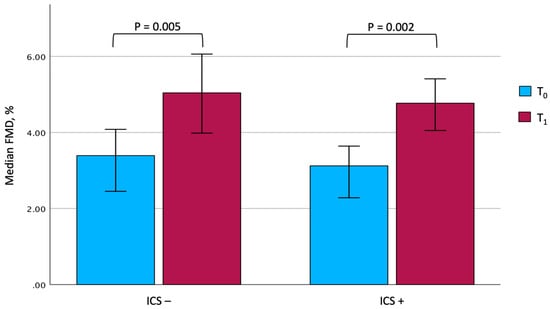
Figure 2.
Changes in FMD values in the two groups. The comparison between the two groups at T0 and T1 was not significant. Data are expressed as median and 95% confidence interval. Abbreviations: FMD, flow-mediated dilation. T0, baseline. T1, follow-up. ICS, inhaled corticosteroids.
4. Discussion
Albeit preliminary, our findings do not suggest a significant impact of ICS-containing medications on the endothelial function of COPD patients, while indicating that the potential beneficial effect of rehabilitation on this CV risk marker may occur independently of the prescribed therapy.
This report thus contributes to a scientific landscape that consistently shows that COPD is burdened by an elevated prevalence of CV comorbidities, which account for 30% of its excess mortality [32]. The latest GOLD document stressed the crucial importance of preventing acute exacerbations [31], which have been linked to an increase in CV risk in such patients [33]. In this regard, a mounting body of evidence has been showing that triple inhaled therapy, comprising ICS combined with long-acting bronchodilators, is more efficient than dual therapy with long-acting bronchodilators alone in reducing the frequency of exacerbations [17], thus improving symptoms and reducing both overall and CV mortality [16]. Such benefits deriving from the use of triple therapy in COPD patients could be explained by the presence of ICSs, which reduce bronchial inflammation and decrease the amount of circulant reactive oxide species (ROS), which have been linked to endothelial dysfunction [34].
Endothelial dysfunction is considered the earliest pathogenetic mechanism of CV disease [7], representing a marker of subclinical atherosclerosis [9] and even an attractive therapeutic target [35]. In our previous study [11], we demonstrated that endothelial function assessed by FMD could significantly and consistently improve in COPD patients after an intensive PR program, independently of most traditional CV risk factors, except for hypercholesterolemia. Given the evidence of CV risk reduction associated with steroid therapy in COPD [18], we hypothesized that ICS may somehow impact the FMD of COPD patients undergoing PR. The fact that our results indicate otherwise suggests that the positive effects of PR on endothelial function and, potentially, on CV risk, may also be independent of steroid therapy. Numerous studies have previously documented the positive impact of rehabilitation and exercise-based approaches on endothelial function, arterial stiffness, and various surrogate markers of CV risk, not only in respiratory diseases, but also in other clinical settings [36,37,38,39]. Therefore, it could be hypothesized that the effect of steroids might have been somewhat “diluted” by an exercise-based intervention, which has already demonstrated its strength in improving endothelial function. In other words, ICS may not simply provide additional support in terms of CV risk reduction in adjunct to PR. Another aspect to consider when interpreting the results of our post hoc analysis is the specific population enrolled, characterized by a group of COPD patients with severe disease and a high number of exacerbations per year. This might lead us to believe that our results are not generalizable to all stages of the disease and that the inflammatory state of our hyper-selected population may account for the above results. It is important to highlight that, beyond the effects of steroids on CV risk during rehabilitation, our findings also indicate that steroids do not impact baseline FMD values. Given the small sample size, we believe this does not contradict the epidemiological evidence showing a reduction in CV risk associated with steroids in COPD [18], but rather aligns with meta-analytic data suggesting that, at the very least, steroids do not worsen this risk [40].
Some important limitations of this retrospective post hoc analysis should be outlined, in addition to those of the original protocol that have been extensively described elsewhere [11]. Firstly, our analyses were conducted after data collection and may not align with the original study design, which can introduce biases. Thus, uncontrolled confounding factors may influence our results and, as the analysis was not pre-specified, no formal sample size calculation was performed. Moreover, there was a high risk of Type I errors due to multiple comparisons, which could have led to false positives or negatives. Finally, the findings of our analyses may not be generalizable beyond the study sample due to its specific context and limitations.
5. Conclusions
Overall, our results suggest that ICSs do not have a significant impact on endothelial function in patients with COPD and, most importantly, they indicate that the potential positive effect of PR on this CV risk marker is not influenced by concurrent steroid therapy. While acknowledging the preliminary nature of these findings and the considerable limitations of our analysis, our results do not rule out the potential CV benefits of corticosteroids, but rather suggest that, at the very least, triple inhaler therapy does not have a negative effect on CV risk. Furthermore, our findings reinforce the potential effectiveness of exercise-based interventions in improving the CV risk profile of COPD patients, while indicating that the potential beneficial effect of rehabilitation on endothelial function may occur independently of the prescribed therapy.
Author Contributions
P.A. and M.M. conceived and designed the study, interpreted the results, and drafted the manuscript. C.C. drafted the manuscript and performed statistical analysis. C.M. (Claudia Merola) and C.L. acquired clinical data. C.M. (Costantino Mancusi), M.C. and M.G.M. interpreted results and performed critical revisions. M.M. supervised the project. C.C. takes responsibility for integrity of the data and for accuracy of data analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by the “Ricerca Corrente” funding scheme of the Ministry of Health, Italy.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the local Institutional Review Board (No. 5.17OSS) on 19 April 2017.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding authors upon reasonable request due to privacy/ethical restrictions.
Acknowledgments
The authors would like to thank Anna Ciullo and Anna Lanzillo for technical support.
Conflicts of Interest
M.M. reports receiving grants for his institution from AstraZeneca and GlaxoSmithKline, as well as payments or honoraria for presentations or educational events from GlaxoSmithKline, Chiesi, and Damor Farmaceutici. All of these are unrelated to the current manuscript.
References
- Christenson, S.A.; Smith, B.M.; Bafadhel, M.; Putcha, N. Chronic obstructive pulmonary disease. Lancet 2022, 399, 2227–2242. [Google Scholar] [CrossRef] [PubMed]
- Boesing, M.; Ottensarendt, N.; Luthi-Corridori, G.; Leuppi, J.D. The Management of Acute Exacerbations in COPD: A Retrospective Observational Study and Clinical Audit. J. Clin. Med. 2023, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Curkendall, S.M.; Lanes, S.; de Luise, C.; Stang, M.R.; Jones, J.K.; She, D.; Goehring, E., Jr. Chronic obstructive pulmonary disease severity and cardiovascular outcomes. Eur. J. Epidemiol. 2006, 21, 803–813. [Google Scholar] [CrossRef]
- Cuttica, M.J.; Colangelo, L.A.; Dransfield, M.T.; Bhatt, S.P.; Rana, J.S.; Jacobs, D.R., Jr.; Thyagarajan, B.; Sidney, S.; Lewis, C.E.; Liu, K.; et al. Lung Function in Young Adults and Risk of Cardiovascular Events Over 29 Years: The CARDIA Study. J. Am. Heart Assoc. 2018, 7, e010672. [Google Scholar] [CrossRef] [PubMed]
- Green, C.E.; Turner, A.M. The role of the endothelium in asthma and chronic obstructive pulmonary disease (COPD). Respir. Res. 2017, 18, 20. [Google Scholar] [CrossRef]
- Ambrosino, P.; Lupoli, R.; Iervolino, S.; De Felice, A.; Pappone, N.; Storino, A.; Di Minno, M.N.D. Clinical assessment of endothelial function in patients with chronic obstructive pulmonary disease: A systematic review with meta-analysis. Intern. Emerg. Med. 2017, 12, 877–885. [Google Scholar] [CrossRef]
- Davignon, J.; Ganz, P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004, 109, III27–III32. [Google Scholar] [CrossRef] [PubMed]
- Ambrosino, P.; Bachetti, T.; D’Anna, S.E.; Galloway, B.; Bianco, A.; D’Agnano, V.; Papa, A.; Motta, A.; Perrotta, F.; Maniscalco, M. Mechanisms and Clinical Implications of Endothelial Dysfunction in Arterial Hypertension. J. Cardiovasc. Dev. Dis. 2022, 9, 136. [Google Scholar] [CrossRef]
- Matsuzawa, Y.; Kwon, T.G.; Lennon, R.J.; Lerman, L.O.; Lerman, A. Prognostic Value of Flow-Mediated Vasodilation in Brachial Artery and Fingertip Artery for Cardiovascular Events: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2015, 4, e002270. [Google Scholar] [CrossRef]
- Corretti, M.C.; Anderson, T.J.; Benjamin, E.J.; Celermajer, D.; Charbonneau, F.; Creager, M.A.; Deanfield, J.; Drexler, H.; Gerhard-Herman, M.; Herrington, D.; et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J. Am. Coll. Cardiol. 2002, 39, 257–265. [Google Scholar] [CrossRef]
- Ambrosino, P.; Di Minno, M.N.D.; D’Anna, S.E.; Formisano, R.; Pappone, N.; Mancusi, C.; Molino, A.; Motta, A.; Maniscalco, M. Pulmonary rehabilitation and endothelial function in patients with chronic obstructive pulmonary disease: A prospective cohort study. Eur. J. Intern. Med. 2023, 116, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Rochester, C.L.; Alison, J.A.; Carlin, B.; Jenkins, A.R.; Cox, N.S.; Bauldoff, G.; Bhatt, S.P.; Bourbeau, J.; Burtin, C.; Camp, P.G.; et al. Pulmonary Rehabilitation for Adults with Chronic Respiratory Disease: An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2023, 208, e7–e26. [Google Scholar] [CrossRef] [PubMed]
- Ambrosino, P.; Fuschillo, S.; Papa, A.; Di Minno, M.N.D.; Maniscalco, M. Exergaming as a Supportive Tool for Home-Based Rehabilitation in the COVID-19 Pandemic Era. Games Health J. 2020, 9, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.T.; Lewthwaite, H.; Paquet, C.; Cafarella, P.; Frith, P. Pulmonary Rehabilitation with and without a Cognitive Behavioral Intervention for Breathlessness in People Living with Chronic Obstructive Pulmonary Disease: Randomized Controlled Trial. J. Clin. Med. 2023, 12, 7286. [Google Scholar] [CrossRef]
- Munoz Montiel, A.; Ruiz-Esteban, P.; Domenech Del Rio, A.; Valdivielso, P.; Sanchez Chaparro, M.A.; Olveira, C. The effect of pulmonary rehabilitation on cardiovascular risk, oxidative stress and systemic inflammation in patients with COPD. Respir. Med. 2024, 232, 107740. [Google Scholar] [CrossRef]
- Chen, H.; Deng, Z.X.; Sun, J.; Huang, Q.; Huang, L.; He, Y.H.; Ma, C.; Wang, K. Association of Inhaled Corticosteroids With All-Cause Mortality Risk in Patients With COPD: A Meta-analysis of 60 Randomized Controlled Trials. Chest 2023, 163, 100–114. [Google Scholar] [CrossRef]
- Papi, A.; Vestbo, J.; Fabbri, L.; Corradi, M.; Prunier, H.; Cohuet, G.; Guasconi, A.; Montagna, I.; Vezzoli, S.; Petruzzelli, S.; et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): A double-blind, parallel group, randomised controlled trial. Lancet 2018, 391, 1076–1084. [Google Scholar] [CrossRef]
- Gadhvi, K.; Kandeil, M.; Raveendran, D.; Choi, J.; Davies, N.; Nanchahal, S.; Wing, O.; Quint, J.; Whittaker, H. Inhaled Corticosteroids and Risk of Cardiovascular Disease in Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Regression. Chronic Obstr. Pulm. Dis. 2023, 10, 317–327. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Spruit, M.A.; Singh, S.J.; Garvey, C.; ZuWallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.; et al. An official American Thoracic Society/European Respiratory Society statement: Key concepts and advances in pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Quanjer, P.H.; Tammeling, G.J.; Cotes, J.E.; Pedersen, O.F.; Peslin, R.; Yernault, J.C. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur. Respir. J. Suppl. 1993, 16, 5–40. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef]
- Wanger, J.; Clausen, J.L.; Coates, A.; Pedersen, O.F.; Brusasco, V.; Burgos, F.; Casaburi, R.; Crapo, R.; Enright, P.; van der Grinten, C.P.; et al. Standardisation of the measurement of lung volumes. Eur. Respir. J. 2005, 26, 511–522. [Google Scholar] [CrossRef]
- Karloh, M.; Fleig Mayer, A.; Maurici, R.; Pizzichini, M.M.M.; Jones, P.W.; Pizzichini, E. The COPD Assessment Test: What Do We Know So Far? A Systematic Review and Meta-Analysis About Clinical Outcomes Prediction and Classification of Patients Into GOLD Stages. Chest 2016, 149, 413–425. [Google Scholar] [CrossRef]
- Mahler, D.A.; Wells, C.K. Evaluation of clinical methods for rating dyspnea. Chest 1988, 93, 580–586. [Google Scholar] [CrossRef]
- Mahoney, F.I.; Barthel, D.W. Functional Evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Nord, E. EuroQol: Health-related quality of life measurement. Valuations of health states by the general public in Norway. Health Policy 1991, 18, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Hernandes, N.A.; Wouters, E.F.; Meijer, K.; Annegarn, J.; Pitta, F.; Spruit, M.A. Reproducibility of 6-minute walking test in patients with COPD. Eur. Respir. J. 2011, 38, 261–267. [Google Scholar] [CrossRef]
- Maltais, F.; LeBlanc, P.; Jobin, J.; Berube, C.; Bruneau, J.; Carrier, L.; Breton, M.J.; Falardeau, G.; Belleau, R. Intensity of training and physiologic adaptation in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1997, 155, 555–561. [Google Scholar] [CrossRef]
- Global Initiative for Chronic Obstructive Lung Disease. 2024 GOLD Report. Available online: https://goldcopd.org/2024-gold-report/ (accessed on 6 June 2024).
- McGarvey, L.P.; John, M.; Anderson, J.A.; Zvarich, M.; Wise, R.A.; Committee, T.C.E. Ascertainment of cause-specific mortality in COPD: Operations of the TORCH Clinical Endpoint Committee. Thorax 2007, 62, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.M.; Ryu, M.H.; Carey, V.J.; Kinney, G.L.; Hokanson, J.E.; Dransfield, M.T.; Hersh, C.P.; Silverman, E.K.; Dagger, C.O.I. Chronic Obstructive Pulmonary Disease Exacerbations Increase the Risk of Subsequent Cardiovascular Events: A Longitudinal Analysis of the COPDGene Study. J. Am. Heart Assoc. 2024, 13, e033882. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Aramouni, K.; Assaf, R.; Parenti, A.; Orekhov, A.; Yazbi, A.E.; Pintus, G.; Eid, A.H. Oxidative Stress-Induced Endothelial Dysfunction in Cardiovascular Diseases. Front Biosci. (Landmark Ed.) 2022, 27, 105. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, C.K.; Yi, M.; Lui, K.O.; Huang, Y. Targeting endothelial dysfunction and inflammation. J. Mol. Cell Cardiol. 2022, 168, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Legallois, D.; Belin, A.; Nesterov, S.V.; Milliez, P.; Parienti, J.J.; Knuuti, J.; Abbas, A.; Tirel, O.; Agostini, D.; Manrique, A. Cardiac rehabilitation improves coronary endothelial function in patients with heart failure due to dilated cardiomyopathy: A positron emission tomography study. Eur. J. Prev. Cardiol. 2016, 23, 129–136. [Google Scholar] [CrossRef]
- Guo, Y.; Ledesma, R.A.; Peng, R.; Liu, Q.; Xu, D. The Beneficial Effects of Cardiac Rehabilitation on the Function and Levels of Endothelial Progenitor Cells. Heart Lung Circ. 2017, 26, 10–17. [Google Scholar] [CrossRef][Green Version]
- Kourek, C.; Briasoulis, A.; Karatzanos, E.; Zouganeli, V.; Psarra, K.; Pratikaki, M.; Alevra-Prokopiou, A.; Skoularigis, J.; Xanthopoulos, A.; Nanas, S.; et al. The Effects of a Cardiac Rehabilitation Program on Endothelial Progenitor Cells and Inflammatory Profile in Patients with Chronic Heart Failure of Different Severity. J. Clin. Med. 2023, 12, 6592. [Google Scholar] [CrossRef]
- Cavero-Redondo, I.; Saz-Lara, A.; Martinez-Garcia, I.; Bizzozero-Peroni, B.; Diaz-Goni, V.; Diez-Fernandez, A.; Moreno-Herraiz, N.; Pascual-Morena, C. Comparative Effect of Two Types of Physical Exercise for the Improvement of Exercise Capacity, Diastolic Function, Endothelial Function and Arterial Stiffness in Participants with Heart Failure with Preserved Ejection Fraction (ExIC-FEp Study): Protocol for a Randomized Controlled Trial. J. Clin. Med. 2023, 12, 3535. [Google Scholar] [CrossRef]
- Jing, X.; Li, Y.; Xu, J. Risk of Cardiovascular Events Associated with Inhaled Corticosteroid Treatment in Patients with Chronic Obstructive Pulmonary Disease: A Meta-Analysis. Can. Respir. J. 2018, 2018, 7097540. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).