Rapid and Robust Identification of Sepsis Using SeptiCyte RAPID in a Heterogeneous Patient Population
Abstract
:1. Introduction
2. Methods
2.1. Dataset and Comparator
2.2. Statistical Analyses
2.2.1. Conventional Statistical Tests
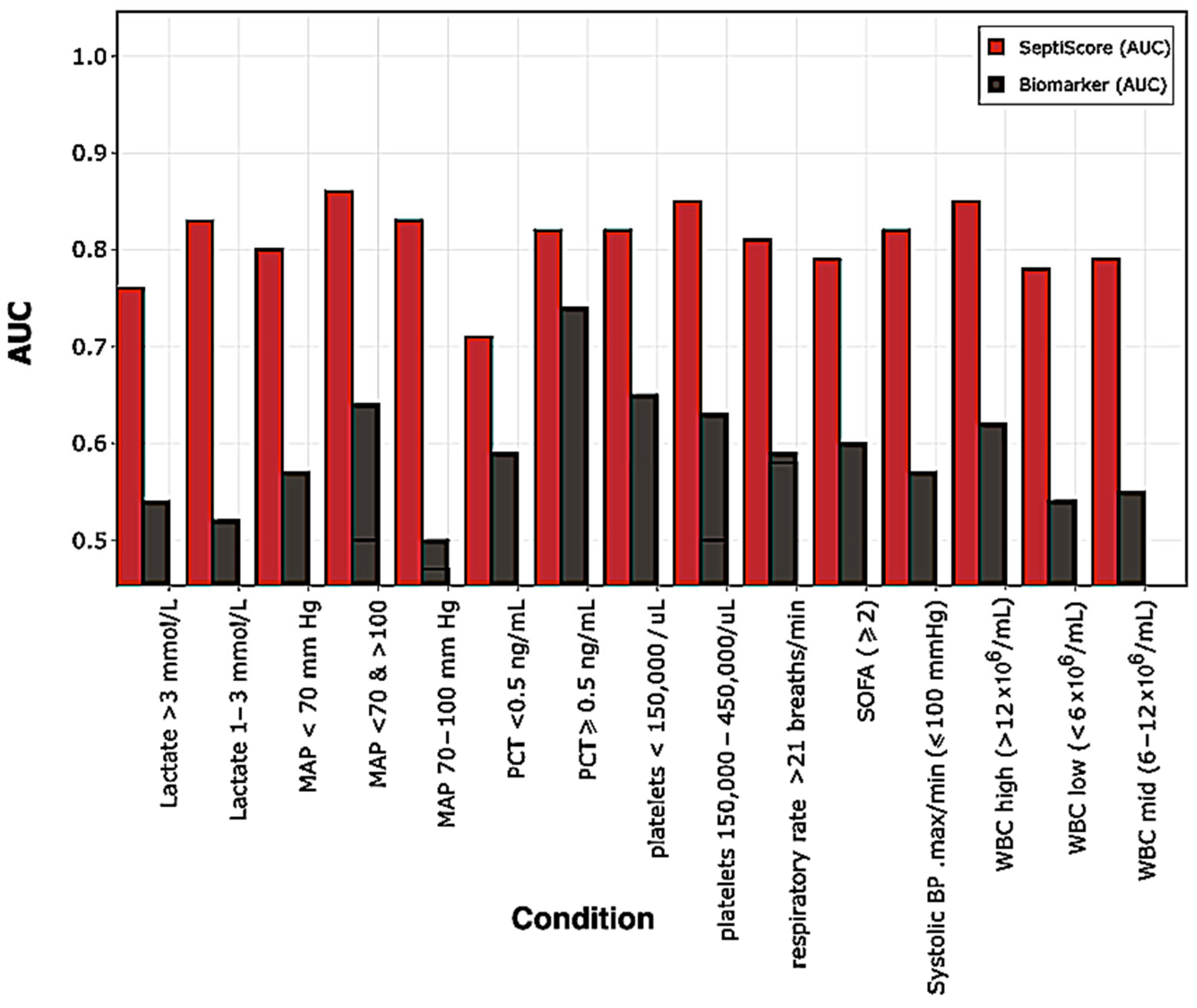
2.2.2. Receiver Operating Characteristic (ROC) Curve Analysis
2.3. Bioinformatics Analyses
2.3.1. Principal Components Analysis (PCA) + Hierarchical Clustering (HC)
2.3.2. k-Means Clustering
3. Results
3.1. Demographics
3.2. Comorbidities and Disease
3.3. Source and Type of Infection
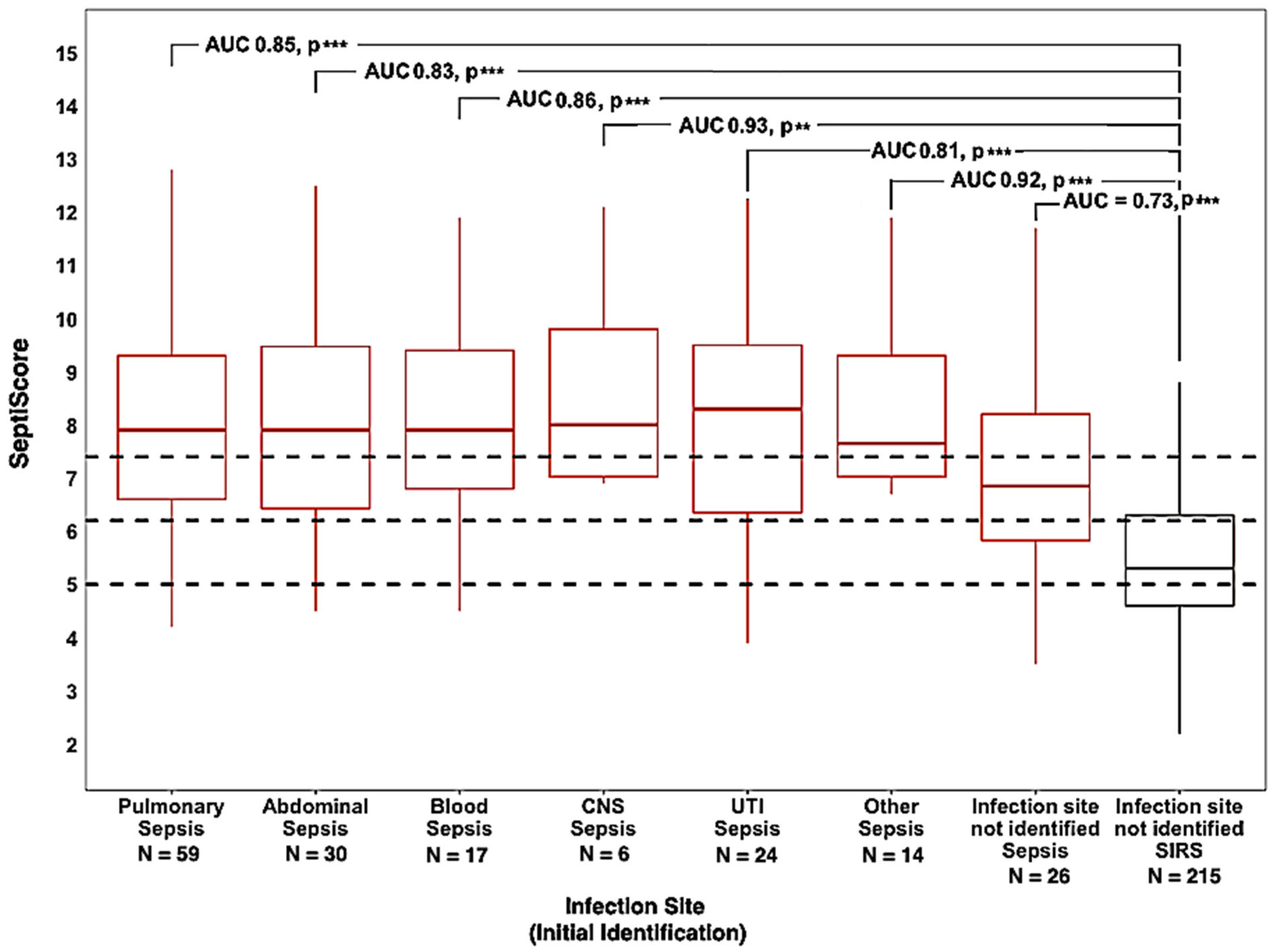
3.3.1. Infection Source
3.3.2. Infection Type
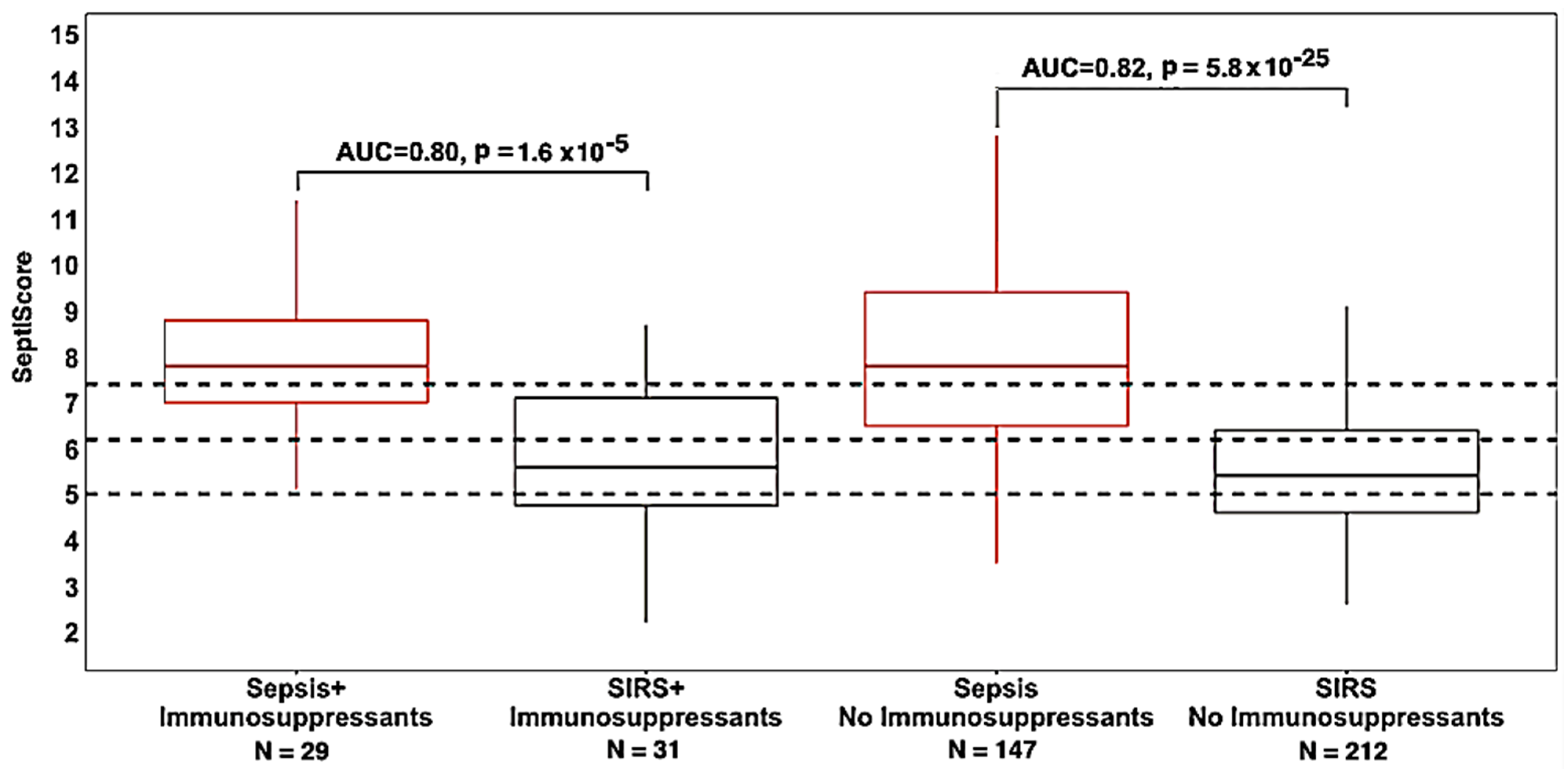
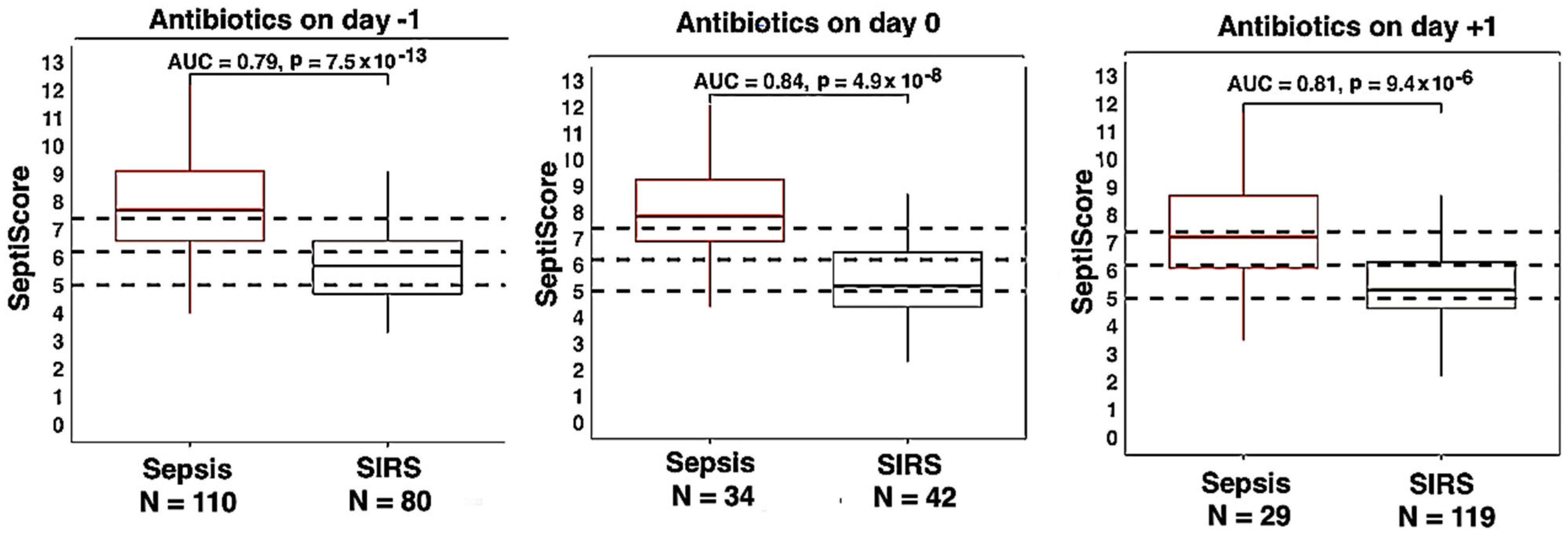
3.4. Therapeutic Interventions
3.5. Phenotypic Subgrouping
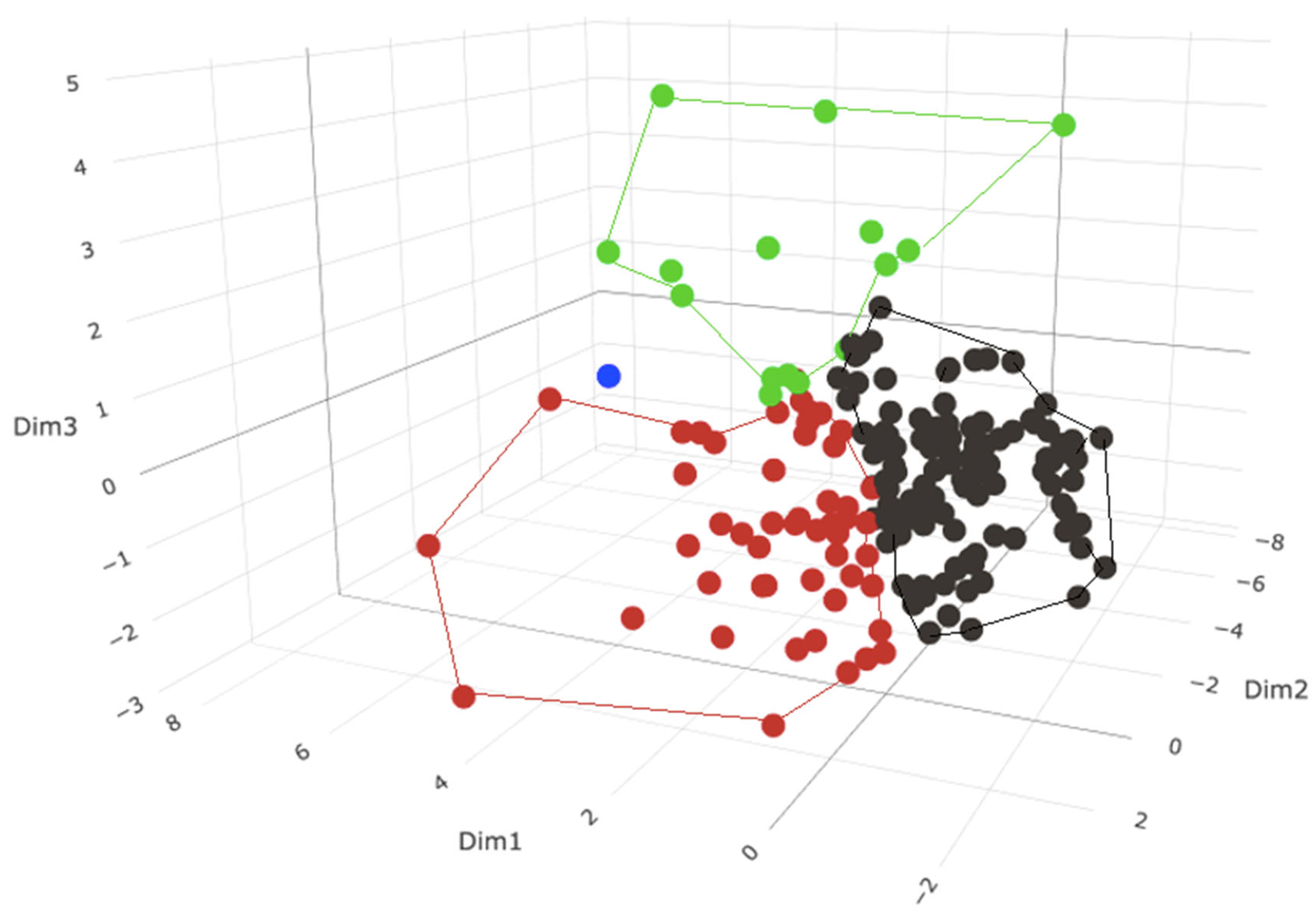
3.5.1. Phenotypic Subgrouping of the Septic Patients (N = 176) by PCA/HC
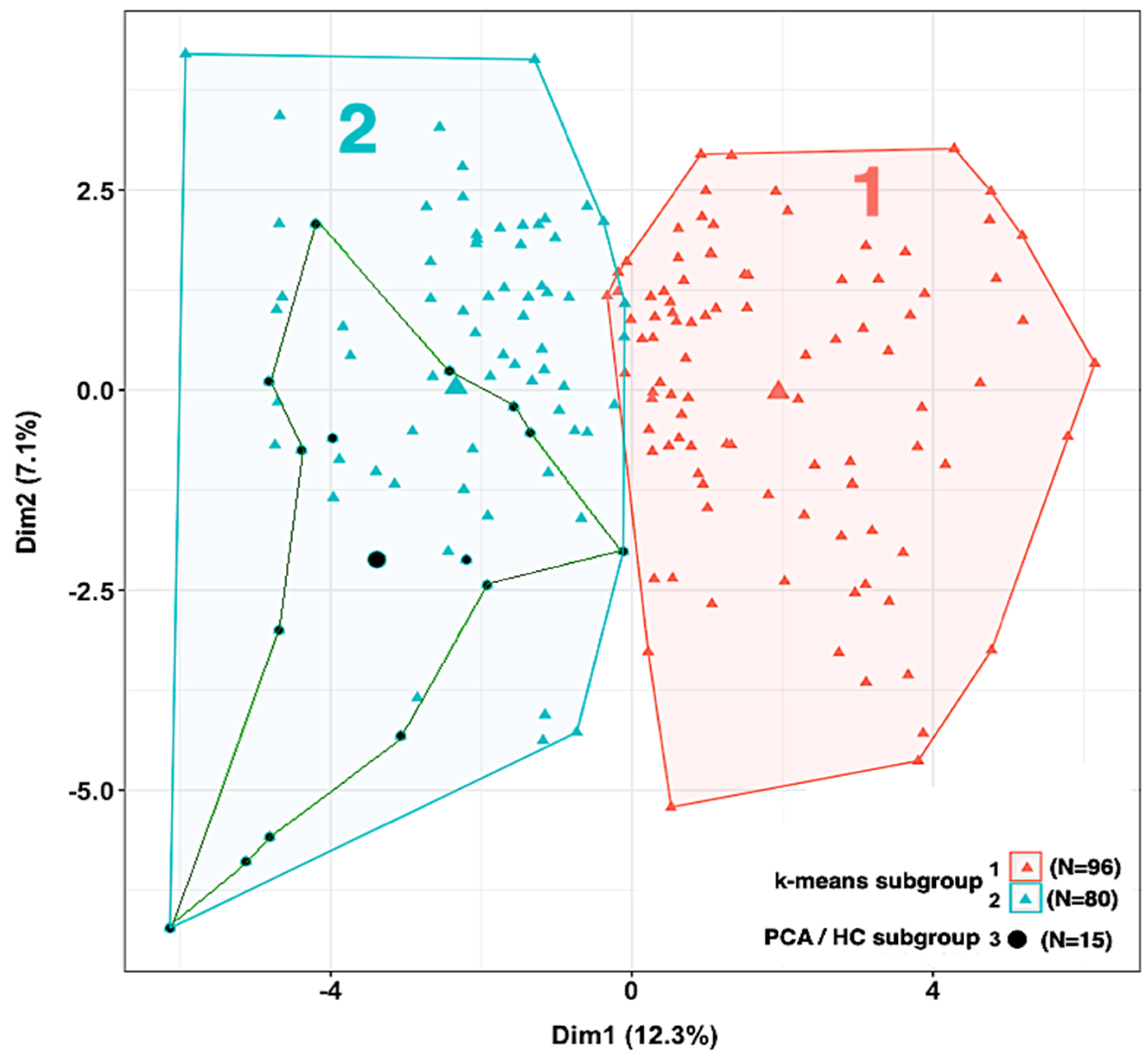
3.5.2. Phenotypic Subgrouping of the Septic Patients (N = 176) by k-Means Clustering
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, V.X.; Bhimarao, M.; Greene, J.D.M.; Manickam, R.N.; Martinez, A.; Schuler, A.; Barreda, F.M.; Escobar, G.J. The Presentation, Pace, and Profile of Infection and Sepsis Patients Hospitalized Through the Emergency Department: An Exploratory Analysis. Crit. Care Explor. 2021, 3, e0344. [Google Scholar] [CrossRef]
- Lengquist, M.; Varadarajan, A.; Alestam, S.; Friberg, H.; Frigyesi, A.; Mellhammar, L. Sepsis mimics among presumed sepsis patients at intensive care admission: A retrospective observational study. Infection 2024, 52, 1041–1053. [Google Scholar] [CrossRef]
- Seymour, C.W.; Kennedy, J.N.; Wang, S.; Chang, C.-C.H.; Elliott, C.F.; Xu, Z.; Berry, S.; Clermont, G.; Cooper, G.; Gomez, H.; et al. Derivation, Validation, and Potential Treatment Implications of Novel Clinical Phenotypes for Sepsis. JAMA 2019, 321, 2003–2017. [Google Scholar] [CrossRef]
- Fohner, A.E.; Greene, J.D.; Lawson, B.L.; Chen, J.H.; Kipnis, P.; Escobar, G.J.; Liu, V.X. Assessing clinical heterogeneity in sepsis through treatment patterns and machine learning. J. Am. Med. Inform. Assoc. 2019, 26, 1466–1477. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.; Sinha, P.; O’Kane, C.M.; Gordon, A.C.; Calfee, C.S.; McAuley, D.F. Subphenotypes in critical care: Translation into clinical practice. Lancet Respir. Med. 2020, 8, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Lopansri, B.K.; Miller, R.R., III; Burke, J.P.; Levy, M.; Opal, S.; Rothman, R.E.; D’alessio, F.R.; Sidhaye, V.K.; Balk, R.; Greenberg, J.A.; et al. Physician agreement on the diagnosis of sepsis in the intensive care unit: Estimation of concordance and analysis of underlying factors in a multicenter cohort. J. Intensiv. Care 2019, 7, 13. [Google Scholar] [CrossRef]
- Bhavani, S.V.; Semler, M.; Qian, E.T.; Verhoef, P.A.; Robichaux, C.; Churpek, M.M.; Coopersmith, C.M. Development and validation of novel sepsis subphenotypes using trajectories of vital signs. Intensiv. Care Med. 2022, 48, 1582–1592. [Google Scholar] [CrossRef]
- Sinha, P.; Kerchberger, V.E.; Willmore, A.; Chambers, J.; Zhuo, H.; Abbott, J.; Jones, C.; Wickersham, N.; Wu, N.; Neyton, L.; et al. Identifying molecular phenotypes in sepsis: An analysis of two prospective observational cohorts and secondary analysis of two randomised controlled trials. Lancet Respir. Med. 2023, 11, 965–974. [Google Scholar] [CrossRef]
- E Davenport, E.; Burnham, K.L.; Radhakrishnan, J.; Humburg, P.; Hutton, P.; Mills, T.C.; Rautanen, A.; Gordon, A.C.; Garrard, C.; Hill, A.V.S.; et al. Genomic landscape of the individual host response and outcomes in sepsis: A prospective cohort study. Lancet Respir. Med. 2016, 4, 259–271. [Google Scholar] [CrossRef]
- Scicluna, B.P.; van Vught, L.A.; Zwinderman, A.H.; Wiewel, M.A.; Davenport, E.E.; Burnham, K.L.; Nürnberg, P.; Schultz, M.J.; Horn, J.; Cremer, O.L.; et al. Classification of patients with sepsis according to blood genomic endotype: A prospective cohort study. Lancet Respir. Med. 2017, 5, 816–826. [Google Scholar] [CrossRef]
- Sweeney, T.E.; Azad, T.D.; Donato, M.; Haynes, W.A.; Perumal, T.M.; Henao, R.; Bermejo-Martin, J.F.; Almansa, R.; Tamayo, E.; Howrylak, J.A.; et al. Unsupervised Analysis of Transcriptomics in Bacterial Sepsis Across Multiple Datasets Reveals Three Robust Clusters. Crit. Care Med. 2018, 46, 915–925. [Google Scholar] [CrossRef]
- DeMerle, K.M.; Angus, D.C.; Baillie, J.K.; Brant, E.; Calfee, C.S.M.; Carcillo, J.; Chang, C.-C.H.; Dickson, R.; Evans, I.M.; Gordon, A.C.; et al. Sepsis Subclasses: A Framework for Development and Interpretation*. Crit. Care Med. 2021, 49, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Coburn, B.; Morris, A.M.; Tomlinson, G.; Detsky, A.S. Does This Adult Patient with Suspected Bacteremia Require Blood Cultures? JAMA 2012, 308, 502–511. [Google Scholar] [CrossRef]
- Vincent, J.-L.; Bogossian, E.; Menozzi, M. The Future of Biomarkers. Crit. Care Clin. 2020, 36, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.R., III; Lopansri, B.K.; Burke, J.P.; Levy, M.; Opal, S.; Rothman, R.E.; D’alessio, F.R.; Sidhaye, V.K.; Aggarwal, N.R.; Balk, R.; et al. Validation of a Host Response Assay, SeptiCyte LAB, for Discriminating Sepsis from Systemic Inflammatory Response Syndrome in the ICU. Am. J. Respir. Crit. Care Med. 2018, 198, 903–913. [Google Scholar] [CrossRef]
- Balk, R.; Esper, A.M.; Martin, G.S.; Miller, R.R., III; Lopansri, B.K.; Burke, J.P.; Levy, M.; Opal, S.; Rothman, R.E.; D’alessio, F.R.; et al. Validation of SeptiCyte RAPID to Discriminate Sepsis from Non-Infectious Systemic Inflammation. J. Clin. Med. 2024, 13, 1194. [Google Scholar] [CrossRef]
- McHugh, L.; Seldon, T.A.; Brandon, R.A.; Kirk, J.T.; Rapisarda, A.; Sutherland, A.J.; Presneill, J.J.; Venter, D.J.; Lipman, J.; Thomas, M.R.; et al. A Molecular Host Response Assay to Discriminate Between Sepsis and Infection-Negative Systemic Inflammation in Critically Ill Patients: Discovery and Validation in Independent Cohorts. PLoS Med. 2015, 12, e1001916. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; ISBN 3-900051-07-0. Available online: http://www.R-project.org/ (accessed on 1 September 2024).
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef]
- Delong, E.R.; Delong, D.M.; Clarke-Pearson, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Skalsk’a, H.; Freylich, V. Web-Bootstrap Estimate of Area Under ROC Curve. Austrian J. Stat. 2006, 35, 325–330. [Google Scholar] [CrossRef]
- Mason, S.J.; Graham, N.E. Areas beneath the relative operating characteristics (ROC) and relative operating levels (ROL) curves: Statistical significance and interpretation. Q. J. R. Meteorol. Soc. 2002, 128, 2145–2166. [Google Scholar] [CrossRef]
- Xu, W.; Dai, J.; Hung, Y.S.; Wang, Q. Estimating the area under a receiver operating characteristic (ROC) curve: Parametric and nonparametric ways. Signal Process. 2013, 93, 3111–3123. [Google Scholar] [CrossRef]
- Ghasemi, A.; Zahediasl, S. Normality Tests for Statistical Analysis: A Guide for Non-Statisticians. Int. J. Endocrinol. Metab. 2012, 10, 486–489. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Yang, C.; Yang, K.; Zhou, B. A hierarchical Clustering Method Based on PCA-Clusters Merging for Quasi-linear SVM. In Proceedings of the 2015 International Conference on Automation, Mechanical Control and Computational Engineering, Changsha, China, 24–25 October 2015. [Google Scholar]
- Maugeri, A.; Barchitta, M.; Basile, G.; Agodi, A. Applying a hierarchical clustering on principal components approach to identify different patterns of the SARS-CoV-2 epidemic across Italian regions. Sci. Rep. 2021, 11, 7082. [Google Scholar] [CrossRef]
- Koh, K.-Y.; Ahmad, S.; Lee, J.-I.; Suh, G.-H.; Lee, C.-M. Hierarchical Clustering on Principal Components Analysis to Detect Clusters of Highly Pathogenic Avian Influenza Subtype H5N6 Epidemic across South Korean Poultry Farms. Symmetry 2022, 14, 598. [Google Scholar] [CrossRef]
- Ikotun, A.M.; Ezugwu, A.E.; Abualigah, L.; Abuhaija, B.; Heming, J. K-means clustering algorithms: A comprehensive review, variants analysis, and advances in the era of big data. Inf. Sci. 2023, 622, 178–210. [Google Scholar] [CrossRef]
- Rousseeuw, P.J. Silhouettes: A graphical aid to the interpretation and validation of cluster analysis. J. Comput. Appl. Math. 1987, 20, 53–65. [Google Scholar] [CrossRef]
- Hu, J.; Gan, Q.; Zhou, D.; Xia, X.; Xiang, W.; Xiao, R.; Tang, J.; Li, J. Evaluating the risk of sepsis attributing to obesity: A two-sample Mendelian randomization study. Postgrad. Med. J. 2023, 99, 1266–1271. [Google Scholar] [CrossRef]
- Wang, J.; Hu, Y.; Zeng, J.; Li, Q.; He, L.; Hao, W.; Song, X.; Yan, S.; Lv, C. Exploring the Causality Between Body Mass Index and Sepsis: A Two-Sample Mendelian Randomization Study. Int. J. Public Health 2023, 68, 1605548. [Google Scholar] [CrossRef]
- Peri, A.M.; Rafiei, N.; O’callaghan, K.; Brischetto, A.; Graves, B.; Sinclair, H.; Eustace, M.; Lim, K.; Parkes-Smith, J.; Stewart, A.; et al. Host response signature trends in persistent bacteraemia and metastatic infection due to Staphylococcus aureus and Gram-negative bacilli: A prospective multicentre observational study. Infect. Dis. 2024, 56, 268–276. [Google Scholar] [CrossRef]
- Papathanakos, G.; Andrianopoulos, I.; Xenikakis, M.; Papathanasiou, A.; Koulenti, D.; Blot, S.; Koulouras, V. Clinical Sepsis Phenotypes in Critically Ill Patients. Microorganisms 2023, 11, 2165. [Google Scholar] [CrossRef]
- DiMeglio, M.; Dubensky, J.; Schadt, S.; Potdar, R.; Laudanski, K. Factors Underlying Racial Disparities in Sepsis Management. Healthcare 2018, 6, 133. [Google Scholar] [CrossRef]
- Guillamet, M.C.V.; Dodda, S.; Liu, L.; Kollef, M.H.; Micek, S.T. Race Does Not Impact Sepsis Outcomes When Considering Socioeconomic Factors in Multilevel Modeling. Crit. Care Med. 2022, 50, 410–417. [Google Scholar] [CrossRef]
- Frydrych, L.M.; Fattahi, F.; He, K.; Ward, P.A.; Delano, M.J. Diabetes and Sepsis: Risk, Recurrence, and Ruination. Front. Endocrinol. 2017, 8, 271. [Google Scholar] [CrossRef]
- Carey, I.M.; Critchley, J.A.; DeWilde, S.; Harris, T.; Hosking, F.J.; Cook, D.G. Risk of Infection in Type 1 and Type 2 Diabetes Compared with the General Population: A Matched Cohort Study. Diabetes Care 2018, 41, 513–521. [Google Scholar] [CrossRef]
- Ahlberg, C.D.; Wallam, S.; Tirba, L.A.; Itumba, S.N.; Gorman, L.; Galiatsatos, P. Linking Sepsis with chronic arterial hypertension, diabetes mellitus, and socioeconomic factors in the United States: A scoping review. J. Crit. Care 2023, 77, 154324. [Google Scholar] [CrossRef]
- Doi, K.; Leelahavanichkul, A.; Hu, X.; Sidransky, K.L.; Zhou, H.; Qin, Y.; Eisner, C.; Schnermann, J.; Yuen, P.S.; Star, R.A. Pre-existing renal disease promotes sepsis-induced acute kidney injury and worsens outcome. Kidney Int. 2008, 74, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.-M.; Lee, K.-H.; Tsai, M.-T.; Tseng, W.-C.; Chu, Y.-C.; Tarng, D.-C. Sepsis and the Risks of Long-Term Renal Adverse Outcomes in Patients with Chronic Kidney Disease. Front. Med. 2022, 9, 809292. [Google Scholar] [CrossRef]
- Gudiol, C.; Albasanz-Puig, A.; Cuervo, G.; Carratalà, J. Understanding and Managing Sepsis in Patients with Cancer in the Era of Antimicrobial Resistance. Front. Med. 2021, 8, 636547. [Google Scholar] [CrossRef] [PubMed]
- Nakada, T.-A.; Takahashi, W.; Nakada, E.; Shimada, T.; Russell, J.A.; Walley, K.R. Genetic Polymorphisms in Sepsis and Cardiovascular Disease: Do Similar Risk Genes Suggest Similar Drug Targets? Chest 2019, 155, 1260–1271. [Google Scholar] [CrossRef]
- Cavaillon, J.-M.; Chousterman, B.G.; Skirecki, T. Compartmentalization of the inflammatory response during bacterial sepsis and severe COVID-19. J. Intensiv. Med. 2024, 4, 326–340. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.-T.; Nguyen, T.-H.T.; Ju, S.-A.; Lee, Y.-S.; Han, S.H.; Lee, S.-C.; Kwon, B.S.; Yu, R.; Kim, G.Y.; Lee, B.J.; et al. CD137 Expressed on Neutrophils Plays Dual Roles in Antibacterial Responses against Gram-Positive and Gram-Negative Bacterial Infections. Infect. Immun. 2013, 81, 2168–2177. [Google Scholar] [CrossRef]
- Roger, P.-M.; Keïta-Perse, O.; Mainardi, J.-L. Diagnostic uncertainty in infectious diseases: Advocacy for a nosological framework. Infect. Dis. Now 2023, 53, 104751. [Google Scholar] [CrossRef]
- Takiguchi, H.; Chen, V.; Obeidat, M.; Hollander, Z.; FitzGerald, J.M.; McManus, B.M.; Ng, R.T.; Sin, D.D. Effect of short-term oral prednisone therapy on blood gene expression: A randomised controlled clinical trial. Respir. Res. 2019, 20, 176–210. [Google Scholar] [CrossRef]
- Chinenov, Y.; Coppo, M.; Gupte, R.; A Sacta, M.; Rogatsky, I. Glucocorticoid receptor coordinates transcription factor-dominated regulatory network in macrophages. BMC Genom. 2014, 15, 656. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Sakhuja, A.; Kumar, G.; McGrath, E.; Nanchal, R.S.; Kashani, K.B. Culture-Negative Severe Sepsis: Nationwide Trends and Outcomes. Chest 2016, 150, 1251–1259. [Google Scholar] [CrossRef]
- Hirosawa, T.; Sakamoto, T.; Hanai, S.; Harada, Y.; Shimizu, T. Effect of Prior Antibiotic Treatment on Blood Culture in an Outpatient Department of General Internal Medicine: A Retrospective Case–Control Analysis. Int. J. Gen. Med. 2023, 16, 2709–2717. [Google Scholar] [CrossRef]
- Rhee, C.; Kadri, S.S.; Danner, R.L.; Suffredini, A.F.; Massaro, A.F.; Kitch, B.T.; Lee, G.; Klompas, M. Diagnosing sepsis is subjective and highly variable: A survey of intensivists using case vignettes. Crit. Care 2016, 20, 1–89. [Google Scholar] [CrossRef]
- Opal, S.M.; Wittebole, X. Biomarkers of Infection and Sepsis. Crit. Care Clin. 2020, 36, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Pierrakos, C.; Velissaris, D.; Bisdorff, M.; Marshall, J.C.; Vincent, J.-L. Biomarkers of sepsis: Time for a reappraisal. Crit. Care 2020, 24, 287. [Google Scholar] [CrossRef]
- Liu, V.X.; Fielding-Singh, V.; Greene, J.D.; Baker, J.M.; Iwashyna, T.J.; Bhattacharya, J.; Escobar, G.J. The Timing of Early Antibiotics and Hospital Mortality in Sepsis. Am. J. Respir. Crit. Care Med. 2017, 196, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Umemura, Y.; Abe, T.; Ogura, H.; Fujishima, S.; Kushimoto, S.; Shiraishi, A.; Saitoh, D.; Mayumi, T.; Otomo, Y.; Hifumi, T.; et al. Hour-1 bundle adherence was associated with reduction of in-hospital mortality among patients with sepsis in Japan. PLoS ONE 2022, 17, e0263936. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensiv. Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Cohen, J. A Coefficient of Agreement for Nominal Scales. Educ. Psychol. Meas. 1960, 20, 213–220. [Google Scholar] [CrossRef]
- Fleiss, J.L.; Cohen, J.; Everitt, B.S. Large sample standard errors of kappa and weighted kappa. Psychol. Bull. 1969, 72, 323–327. [Google Scholar] [CrossRef]
- Goldstein-Greenwood, J.; (UVA Library StatLab). ROC Curves and AUC for Models Used for Binary Classification. 2022. Available online: https://library.virginia.edu/data/articles/roc-curves-and-auc-for-models-used-for-binary-classification (accessed on 19 August 2024).
- Kuye, I.; Anand, V.; Klompas, M.; Chan, C.; Kadri, S.S.; Rhee, C. Prevalence and Clinical Characteristics of Patients with Sepsis Discharge Diagnosis Codes and Short Lengths of Stay in U.S. Hospitals. Crit. Care Explor. 2021, 3, e0373. [Google Scholar] [CrossRef]
- Qin, C.; Zhang, S.; Zhao, Y.; Ding, X.; Yang, F.; Zhao, Y. Diagnostic value of metagenomic next-generation sequencing in sepsis and bloodstream infection. Front. Cell. Infect. Microbiol. 2023, 13, 1117987. [Google Scholar] [CrossRef]
- Shoonjans, F. MedCalc Manual: Easy-to-Use Statistical Software; MedCalc Software bvba: Ostend, Belgium, 2017; ISBN 978-1520321578. [Google Scholar]
- Yang, L.; Lin, Y.; Wang, J.; Song, J.; Wei, B.; Zhang, X.; Yang, J.; Liu, B. Comparison of Clinical Characteristics and Outcomes Between Positive and Negative Blood Culture Septic Patients: A Retrospective Cohort Study. Infect. Drug Resist. 2021, 14, 4191–4205. [Google Scholar] [CrossRef]
- Zhou, X.H.; Obuchowski, N.A.; McClish, D.K. Statistical Methods in Diagnostic Medicine, 2nd ed.; John Wiley and Sons: Hoboken, NJ, USA, 2011; ISBN 978-0-470-18314-4. [Google Scholar]
- Zweig, M.H.; Campbell, G. Receiver-operating characteristic (ROC) plots: A fundamental evaluation tool in clinical medicine. Clin. Chem. 1993, 39, 561–577, Erratum in Clin. Chem. 1993, 39, 1589. https://doi.org/10.1093/clinchem/39.8.1589. [Google Scholar] [CrossRef] [PubMed]

| Global Mean Imputation | ||||
|---|---|---|---|---|
| Cluster 1 | Cluster 2 | Cluster 3 | ||
| Group Mean Imputation | Cluster 1 | 251 | 0 | 1 |
| Cluster 2 | 0 | 29 | 0 | |
| Cluster 3 | 21 | 0 | 117 | |
| Condition | N Sepsis | N SIRS | AUC | AUC 95% CI | p-Value | DeLong’s p-Value |
|---|---|---|---|---|---|---|
| Male (M) | 95 | 137 | 0.81 | 0.75–0.87 | 1.4 × 10−15 | M vs. F: 0.52 |
| Female (F) | 81 | 106 | 0.84 | 0.78–0.90 | 1.6 × 10−15 | |
| Age <60 years | 77 | 138 | 0.83 | 0.77–0.89 | 3.9 × 10−14 | Age (<60) vs. (≥60): 0.82 |
| Age ≥60 years | 99 | 105 | 0.82 | 0.76–0.88 | 5.7 × 10−16 | |
| Black (B) * | 45 | 70 | 0.85 | 0.77–0.93 | 5.9 × 10−11 | B vs. W: 0.30 B vs. H: 0.21 B vs. A: 0.62 W vs. H: 0.03 W vs. A: 0.23 H vs. A: 0.63 |
| White (W) * | 108 | 146 | 0.80 | 0.74–0.86 | 7.2 × 10−16 | |
| Hispanic (H) * | 10 | 12 | 0.93 | 0.81–1.05 | 0.00014 | |
| Asian (A) * | 10 | 11 | 0.89 | 0.74–1.04 | 0.0013 |
| Stratification | N Sepsis | N SIRS | AUC | AUC 95% CI | p-Value | Delong’s p-Value |
|---|---|---|---|---|---|---|
| Hyperglycemia (HG) | 50 | 78 | 0.83 | 0.76–0.92 | 5.3 × 10−10 | HG vs. no HG noted: 0.76 HGd vs. no HG noted: 0.51 HGd vs. HG¬d: 0.37 |
| Diabetic hyperglycemia (HGd) | 10 | 16 | 0.75 | 0.51–0.93 | 0.035 (T) * 0.038 (U) * | |
| Diabetic hyperglycemia not noted (HG¬d) | 40 | 62 | 0.85 | 0.77–0.93 | 3.7 × 10−9 | |
| No HG noted | 126 | 165 | 0.82 | 0.76–0.86 | 5.8 × 10−21 | |
| Impaired immunity (II) | 27 | 32 | 0.83 | 0.72–0.94 | 6.8 × 10−6 | II vs. no II noted: 0.84 |
| No impaired immunity noted | 149 | 211 | 0.82 | 0.77–0.87 | 4.9 × 10−25 | |
| Hypertension (HT) | 24 | 26 | 0.79 | 0.66–0.92 | 1.8 × 10−4 | HT vs. no HT noted: 0.59 |
| No hypertension noted | 152 | 217 | 0.83 | 0.78–0.88 | 1.0 × 10−26 | |
| Cardiovascular disease (CVD) | 16 | 23 | 0.85 | 0.72–0.98 | 1.7 × 10−4 | CVD vs. no CVD noted: 0.63 |
| Cardiovascular disease (acute) | 7 | 6 | 1.0 | 0.75–1.0 | 0.0031 (T) 0.0034 (U) | |
| Cardiovascular disease (chronic) | 9 | 17 | 0.81 | 0.62–1.00 | 0.0046 (T) 0.0105 (U) * | |
| No cardiovascular disease noted | 160 | 220 | 0.82 | 0.78–0.86 | 1.9 × 10−26 | |
| Kidney disease (KD) | 33 | 34 | 0.83 | 0.73–0.93 | 5.6 × 10−7 | |
| Kidney disease (acute) | 13 | 13 | 0.86 | 0.70–1.00 | 0.0012 (T) 0.0023 (U) | KD vs. no KD noted: 0.89 |
| Kidney disease (chronic) | 20 | 21 | 0.83 | 0.70–0.96 | 0.0001 T) 0.0004 (U) | |
| No kidney disease noted | 143 | 209 | 0.82 | 0.77–0.87 | 7.4 × 10−24 | |
| Obesity (BMI ≥30) | 6 | 14 | 0.86 | 0.66–1.06 | 0.0141 (T) * 0.0135 (U) * | Obesity vs. no obesity noted: 0.63 |
| No obesity noted | 170 | 229 | 0.82 | 0.78–0.86 | 1.8 × 10−28 |
| Therapeutic Intervention | N Sepsis | N SIRS | AUC (95% CI) | Sepsis vs. SIRS p-Value | DeLong’s p-Value |
|---|---|---|---|---|---|
| Immunosuppressants (IS) | 29 | 31 | 0.80 (0.67–0.91) | 1.6 × 10−5 | IS vs. no IS: 0.75 |
| No immunosuppressants | 147 | 212 | 0.82 (0.77–0.87) | 5.8 × 10−25 | |
| Antibiotics (Abx) 1 day prior to SeptiScore | 110 | 80 | 0.79 (0.73–0.85) | 7.5 × 10−13 | Abx prior vs. on = 0.46 Abx on vs. after = 0.71 Abx after vs. prior = 0.79 |
| Antibiotics same day as SeptiScore | 34 | 42 | 0.84 (0.75–0.93) | 4.9 × 10−8 | |
| Antibiotics 1 day after SeptiScore | 29 | 119 | 0.81 (0.71–0.91) | 9.4 × 10−6 | |
| Vasopressors (V) or inotropes (I) | 81 | 76 | 0.83 (0.77–0.89) | 2.3 × 10−14 | V/I vs. no V/I: 0.60 |
| No vasopressors (V) or inotropes (I) | 95 | 167 | 0.81 (0.75–0.87) | 4.8 × 10−15 | |
| Mechanical ventilation (MV) | 62 | 91 | 0.80 (0.72–0.88) | 3.1 × 10−10 | MV vs. no MV: 0.36 |
| No mechanical ventilation | 114 | 152 | 0.84 (0.79–0.89) | 5.4 × 10−21 |
| Comparison | N Sepsis | N SIRS | AUC | Sepsis vs. SIRS p-Value | DeLong’s p-Value |
|---|---|---|---|---|---|
| Sepsis PCA subgroup 1 vs. SIRS | 110 | 243 | 0.81 | 6.9 × 10−19 | 1 vs. 2 p = 0.95 1 vs. 3 p = 0.015 2 vs. 3 p = 0.035 |
| Sepsis PCA subgroup 2 vs. SIRS | 50 | 243 | 0.81 | 4.5 × 10−9 | |
| Sepsis PCA subgroup 3 vs. SIRS | 15 | 243 | 0.93 | 2.2 × 10−6 |
| Characteristic | Missing Values (%) | Subgroup 1 (N = 110), Median (IQR) | Subgroup 2 (N = 50), Median (IQR) | Subgroup 3 (N = 15), Median (IQR) | p-Value |
|---|---|---|---|---|---|
| Vital Signs | |||||
| Temperature (Min) | 24 (14%) | 36.3 (35.5–36.7) | 36.0 (35.3–36.7) | 35.4 (33.5–35.7) | <0.001 |
| Heart rate (Max) | 0 (0%) | 113 (102–128) | 128 (114–138) | 138 (122–156) | <0.001 |
| Mean arterial pressure (Min) | 2 (1%) | 62 (55–74) | 59 (48–65) | 50 (45–57) | 0.006 |
| Respiratory rate (Max) | 53 (30%) | 27 (24–31) | 27 (23–31) | 37 (33–43) | <0.001 |
| Respiratory rate (Min) | 56 (32%) | 20 (14–24) | 17 (12–23) | 32 (15–36) | <0.001 |
| Clinical Parameters | |||||
| Glucose (Min) | 5 (3%) | 123 (98–160) | 129 (108–160) | 90 (65–115) | 0.009 |
| WBC (Max) | 7 (4%) | 12 (8–15) | 21 (18–29) | 23 (18–29) | <0.001 |
| WBC (Min) | 5 (3%) | 9 (6–12) | 18 (15–23) | 14 (8–23) | <0.001 |
| PCT | 27 (15%) | 3 (0–17) | 5 (2–13) | 36 (14–130) | <0.001 |
| Lactate | 47 (27%) | 2.0 (1.45–3.15) | 2.4 (1.60–3.40) | 9.3 (4.13–11.88) | <0.001 |
| Platelets (Min) | 52 (30%) | 145 (102–212) | 309 (238–367) | 80 (53–221) | <0.001 |
| SOFA | 19 (11%) | 6 (4–9) | 6 (4–8) | 11 (7–14) | <0.001 |
| Vasopressor (Y/N) | - | 42 Y (38%) | 20 Y (40%) | 12 Y (80%) | 0.008 |
| Infection Site * | 1 vs. 2, 1 vs. 3, 2 vs. 3 (proportions test) | ||||
| Pulmonary | 34 (30.9%) | 20 (40%) | 4 (26.7%) | 0.26, 0.73, 0.35 | |
| Abdominal | 15 (13.6%) | 10 (20%) | 5 (33.3%) | 0.30, 0.05, 0.29 | |
| Blood | 12 (10.9%) | 4 (8.0%) | 1 (6.7%) | 0.57, 0.23, 0.33 | |
| CNS | 6 (5.5%) | 0 (0%) | 0 (0%) | 0.08, 0.33, ND | |
| Urinary tract | 13 (11.8%) | 8 (16%) | 3 (20%) | 0.47, 0.37, 0.13 | |
| Other | 9 (8.2%) | 4 (8.0%) | 1 (6.7%) | 0.97, 0.84, 0.87 | |
| Site not identified ** | 21 (19.1%) | 4 (8.0%) | 1 (6.7%) | 0.07, 0.24, 0.87 | |
| Pathogens ** | 1 vs. 2, 1 vs. 3, 2 vs. 3 (proportions test) | ||||
| Viral (including coinfections) | 23 (21%) | 6 (12%) | 0 (0%) | 0.17, 0.05, 0.16 | |
| Escherichia coli | 11 (10%) | 5 (10%) | 2 (13.3%) | 1.00, 0.70, 0.72 | |
| Staphylococcus aureus | 18 (16.4%) | 15 (30%) | 2 (13.3%) | 0.05, 0.76, 0.20 | |
| Streptococcus | 6 (5.5%) | 3 (6%) | 2 (13.3%) | 0.90, 0.25, 0.36 | |
| Enterococcus | 4 (3.6%) | 3 (6%) | 0 (0%) | 0.49, 0.46, 0.34 |
| Characteristic | Missing Values (%) | Subgroup 1 (N = 96), Median (IQR) | Subgroup 2 (N = 80), Median (IQR) | p-Value * |
|---|---|---|---|---|
| Vital Signs | ||||
| Temperature (Min) | 24 (14%) | 36.4 (36.0–36.8) | 35.3 (34.4–35.8) | <0.001 |
| Mean arterial pressure (Min) | 2 (1%) | 67 (57–78) | 55 (46–61) | <0.001 |
| Respiratory rate (Max) | 53 (30%) | 25 (23–31) | 29 (25–35) | 0.01 |
| Clinical Chemistry | ||||
| WBC (Max) | 7 (4%) | 12 (8–18) | 18 (14–25) | <0.001 |
| WBC (Min) | 5 (3%) | 10 (6–14) | 14 (9–19) | <0.001 |
| Lactate | 47 (27%) | 2.10 (1.45–3.00) | 3.00 (1.83–5.85) | <0.001 |
| GCS < 15 (qSOFA component) | 25 (14%) | 18 (18.8%) | 42 (52.5%) | <0.001 |
| qSOFA ≥ 2 | - | 22 (23%) | 68 (85%) | <0.001 |
| qSOFA < 2 | - | 62 (65%) | 8 (10%) | <0.001 |
| SOFA | 19 (11%) | 4 (2–6) | 9 (7–12) | <0.001 |
| PCT_binary (>0.5 ng/mL) | 27 (15%) | 52 (64%) | 65 (96%) | <0.001 |
| Interventions | ||||
| Vasopressors used | - | 15 (16%) | 60 (75%) | <0.001 |
| Mechanical ventilation | - | 18 (19%) | 44 (55%) | <0.001 |
| Infection Site * | ||||
| Pulmonary | - | 36 (37.5%) | 23 (28.8%) | 0.22 |
| Abdominal | - | 13 (13.5%) | 17 (21.2%) | 0.18 |
| Blood | - | 13 (13.5%) | 4 (5%) | 0.06 |
| Central nervous system (CNS) | - | 4 (4.2%) | 2 (2.5%) | 0.54 |
| Other | - | 5 (5.2%) | 9 (11.2%) | 0.14 |
| Urinary tract infection (UTI) | - | 9 (9.4%) | 15 (18.8%) | 0.07 |
| Site not confirmed initially | - | 16 (16.7%) | 10 (12.5%) | 0.44 |
| Pathogens * | ||||
| Viral (including coinfections) | - | 23 (24%) | 6 (7.5%) | 0.006 |
| Escherichia coli | - | 8 (8.3%) | 10 (12.5%) | 0.36 |
| Staphylococcus aureus | - | 20 (20.8%) | 15 (18.8%) | 0.74 |
| Streptococcus | - | 5 (5.2%) | 6 (7.5%) | 0.53 |
| Enterococcus | - | 4 (4.2%) | 3 (3.8%) | 0.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balk, R.; Esper, A.M.; Martin, G.S.; Miller, R.R., III; Lopansri, B.K.; Burke, J.P.; Levy, M.; Rothman, R.E.; D’Alessio, F.R.; Sidhaye, V.K.; et al. Rapid and Robust Identification of Sepsis Using SeptiCyte RAPID in a Heterogeneous Patient Population. J. Clin. Med. 2024, 13, 6044. https://doi.org/10.3390/jcm13206044
Balk R, Esper AM, Martin GS, Miller RR III, Lopansri BK, Burke JP, Levy M, Rothman RE, D’Alessio FR, Sidhaye VK, et al. Rapid and Robust Identification of Sepsis Using SeptiCyte RAPID in a Heterogeneous Patient Population. Journal of Clinical Medicine. 2024; 13(20):6044. https://doi.org/10.3390/jcm13206044
Chicago/Turabian StyleBalk, Robert, Annette M. Esper, Greg S. Martin, Russell R. Miller, III, Bert K. Lopansri, John P. Burke, Mitchell Levy, Richard E. Rothman, Franco R. D’Alessio, Venkataramana K. Sidhaye, and et al. 2024. "Rapid and Robust Identification of Sepsis Using SeptiCyte RAPID in a Heterogeneous Patient Population" Journal of Clinical Medicine 13, no. 20: 6044. https://doi.org/10.3390/jcm13206044







