Descemet Membrane Endothelial Keratoplasty Graft Preparation Using the Liquid Bubble Technique with Subtrabecular Hydrodissection: A Retrospective Real-Life Study
Abstract
1. Introduction
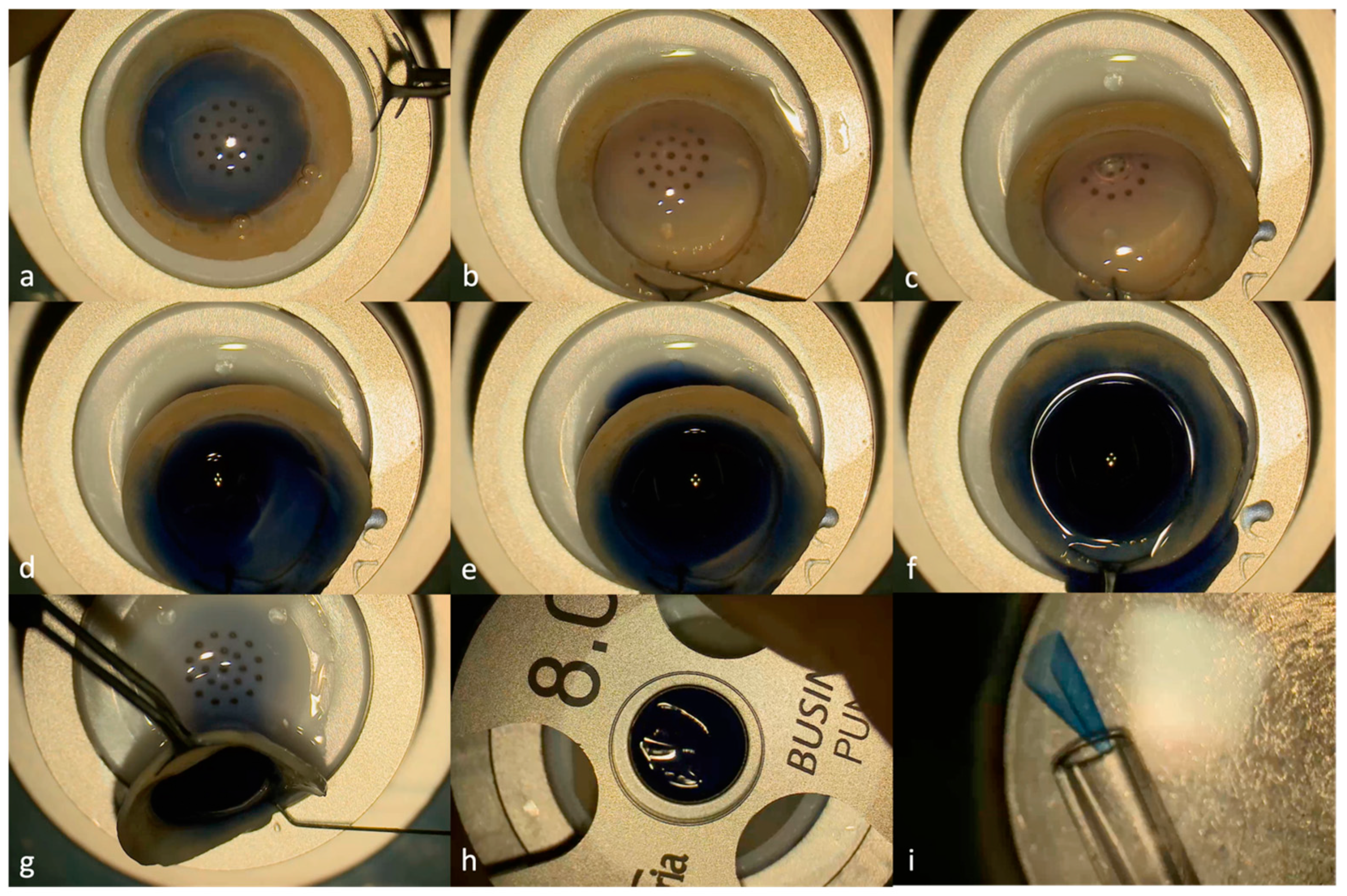
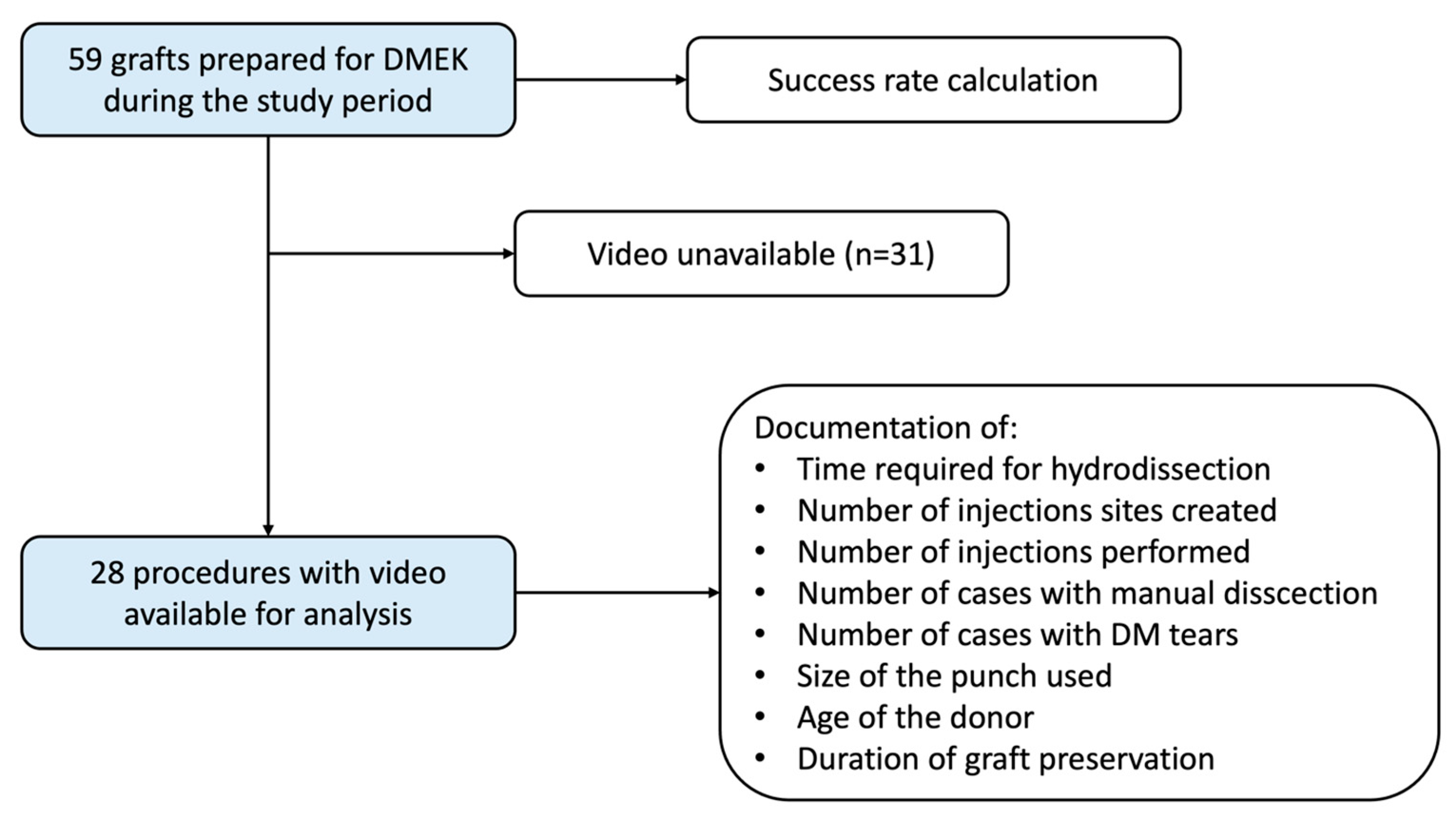
2. Materials and Methods
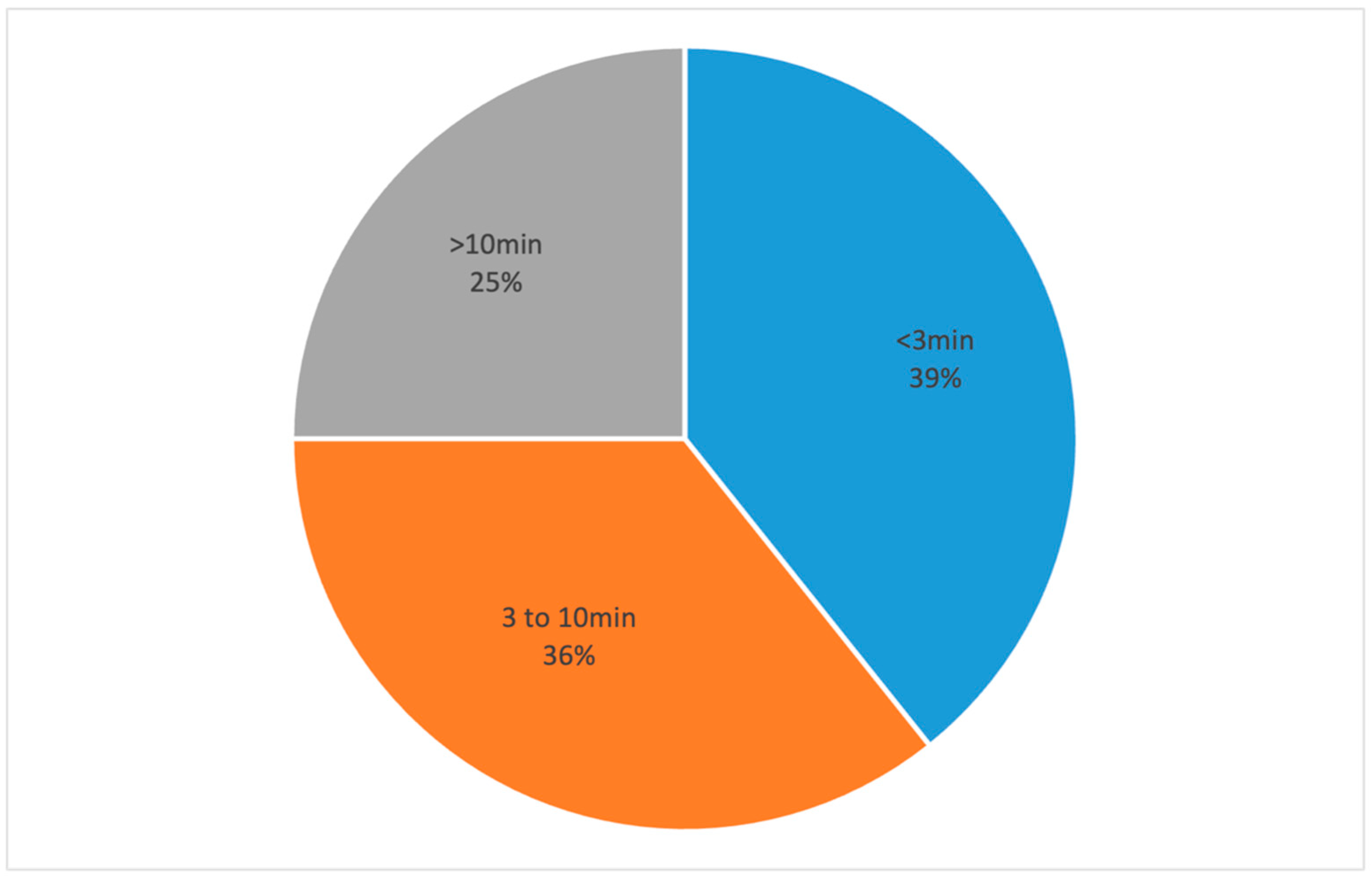
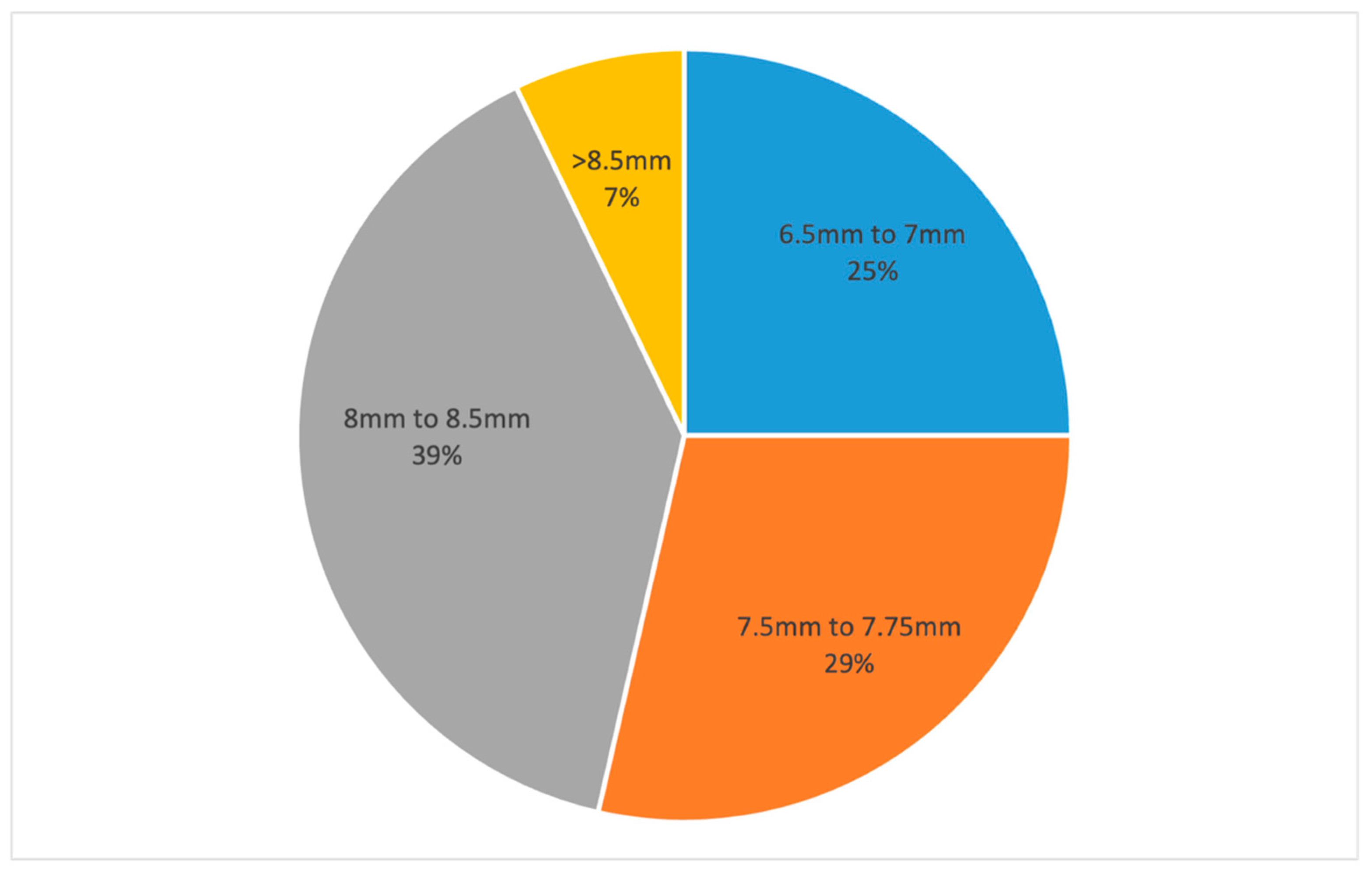
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, P.; Li, L.; Mukaida, N.; Zhang, X. Descemet membrane endothelial keratoplasty (DMEK). Cornea 2006, 25, 199–206. [Google Scholar] [CrossRef]
- Singh, P.; Sinha, A.; Nagpal, R.; Chaurasia, S. Descemet membrane endothelial keratoplasty: Update on preoperative considerations, surgical techniques, and outcomes. Indian J. Ophthalmol. 2022, 70, 3222–3238. [Google Scholar] [CrossRef]
- Birbal, R.S.; Sikder, S.; Lie, J.T.; Beek, E.A.G.-V.; Oellerich, S.; Melles, G.R.J. Donor Tissue Preparation for Descemet Membrane Endothelial Keratoplasty: An Updated Review. Cornea 2018, 37, 128–135. [Google Scholar] [CrossRef]
- Lie, J.T.; Birbal, R.; Ham, L.; van der Wees, J.; Melles, G.R.J. Donor tissue preparation for Descemet membrane endothelial keratoplasty. J. Cataract. Refract. Surg. 2008, 34, 1578–1583. [Google Scholar] [CrossRef]
- Kruse, F.E.; Laaser, K.; Cursiefen, C.; Heindl, L.M.; Schlötzer-Schrehardt, U.; Riss, S.; Bachmann, B.O. A stepwise approach to donor preparation and insertion increases safety and outcome of Descemet membrane endothelial keratoplasty. Cornea 2011, 30, 580–587. [Google Scholar] [CrossRef]
- Ignacio, T.S.; Nguyen, T.T.; Sarayba, M.A.; Sweet, P.M.; Piovanetti, O.; Chuck, R.S.; Behrens, A. A technique to harvest Descemet’s membrane with viable endothelial cells for selective transplantation. Am. J. Ophthalmol. 2005, 139, 325–330. [Google Scholar] [CrossRef]
- Szurman, P.; Januschowski, K.; Rickmann, A.; Damm, L.J.; Boden, K.T.; Opitz, N. Novel liquid bubble dissection technique for DMEK lenticule preparation. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 1819–1823. [Google Scholar] [CrossRef] [PubMed]
- Parekh, M.; Borroni, D.; Ruzza, A.; Levis, H.J.; Ferrari, S.; Ponzin, D.; Romano, V. A comparative study on different Descemet membrane endothelial keratoplasty graft preparation techniques. Acta Ophthalmol. 2018, 96, e718–e726. [Google Scholar] [CrossRef] [PubMed]
- Din, N.; Mimouni, M.; Slomovic, J.; Aldrees, S.; Trinh, T.; Cohen, E.; Gouvea, L.; Alshaker, S.; Chan, C.C.; Chew, H.F.; et al. Comparative evaluation of four Descemet membrane endothelial keratoplasty graft preparation techniques. Can. J. Ophthalmol. 2023, 58, 191–197. [Google Scholar] [CrossRef]
- Muraine, M.; Gueudry, J.; He, Z.; Piselli, S.; Lefevre, S.; Toubeau, D. Novel technique for the preparation of corneal grafts for descemet membrane endothelial keratoplasty. Am. J. Ophthalmol. 2013, 156, 851–859. [Google Scholar] [CrossRef]
- Price, M.O.; Giebel, A.W.; Fairchild, K.M.; Price, F.W. Descemet’s membrane endothelial keratoplasty: Prospective multicenter study of visual and refractive outcomes and endothelial survival. Ophthalmology 2009, 116, 2361–2368. [Google Scholar] [CrossRef] [PubMed]
- Studeny, P.; Farkas, A.; Vokrojova, M.; Liskova, P.; Jirsova, K. Descemet membrane endothelial keratoplasty with a stromal rim (DMEK-S). Br. J. Ophthalmol. 2010, 94, 909–914. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sella, R.; Einan-Lifshitz, A.; Sorkin, N.; Chan, C.C.; Afshari, N.A.; Rootman, D.S. Learning curve of two common Descemet membrane endothelial keratoplasty graft preparation techniques. Can. J. Ophthalmol. 2019, 54, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Solley, K.D.; Berges, A.; Diaz, C.; Ostrander, B.T.; Ding, A.S.; Larson, S.A.; Frank, K.; Lee, D.; Guerrero, J.; deCarvalho, T.; et al. Evaluation of Efficacy, Efficiency, and Cell Viability of a Novel Descemet Membrane Endothelial Keratoplasty Graft Preparation Device, DescePrep, in Nondiabetic and Diabetic Human Donor Corneas. Cornea 2022, 41, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Yoeruek, E.; Schmidt, B. Novel surgical instruments facilitating Descemet membrane dissection. Cornea 2013, 32, 523–526. [Google Scholar] [CrossRef]
- Stuhlmacher, E.S.; Suffo, S.; Munteanu, C.; Seitz, B.; Daas, L. Assessing the Learning Curve for DMEK Using Post-Procedural Clinical Outcomes-Comparison of Four Different Surgeons during Two Different Periods. J. Clin. Med. 2023, 12, 811. [Google Scholar] [CrossRef]
- Greiner, M.A.; Rixen, J.J.; Wagoner, M.D.; Schmidt, G.A.; Stoeger, C.G.; Straiko, M.D.; Zimmerman, M.; Kitzmann, A.S.; Goins, K.M. Diabetes mellitus increases risk of unsuccessful graft preparation in Descemet membrane endothelial keratoplasty: A multicenter study. Cornea 2014, 33, 1129–1133. [Google Scholar] [CrossRef]
- McKee, H.D.; Irion, L.C.D.; Carley, F.M.; Jhanji, V.; Brahma, A.K. Donor preparation using pneumatic dissection in endothelial keratoplasty: DMEK or DSEK? Cornea 2012, 31, 798–800. [Google Scholar] [CrossRef]
- Parekh, M.; Romano, D.; Wongvisavavit, R.; Coco, G.; Giannaccare, G.; Ferrari, S.; Rocha-De-Lossada, C.; Levis, H.J.; Semeraro, F.; Calvo-De-Mora, M.R.; et al. DMEK graft: One size does not fit all. Acta Ophthalmol. 2023, 101, e14–e25. [Google Scholar] [CrossRef]
- Parekh, M.; Ruzza, A.; Salvalaio, G.; Ferrari, S.; Camposampiero, D.; Busin, M.; Ponzin, D. Descemet membrane endothelial keratoplasty tissue preparation from donor corneas using a standardized submerged hydro-separation method. Am. J. Ophthalmol. 2014, 158, 277–285. [Google Scholar] [CrossRef]
- Salvalaio, G.; Parekh, M.; Ruzza, A.; Ferrari, S.; Camposampiero, D.; Ponzin, D. DMEK lenticule preparation from donor corneas using a novel ‘SubHyS’ technique followed by anterior corneal dissection. Br. J. Ophthalmol. 2014, 98, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Bhogal, M.; Balda, M.S.; Matter, K.; Allan, B.D. Global cell-by-cell evaluation of endothelial viability after two methods of graft preparation in Descemet membrane endothelial keratoplasty. Br. J. Ophthalmol. 2016, 100, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, R.; Jun, A.S. Emerging alternatives to keratoplasty for corneal endothelial cell dysfunction. Curr. Opin. Ophthalmol. 2024, 35, 415–422. [Google Scholar] [CrossRef]
- Din, N.M.; Cohen, E.; Popovic, M.; Mimouni, M.; Trinh, T.M.; Gouvea, L.; Alshaker, S.M.; Tone, S.M.O.; Chan, C.C.M.; Slomovic, A.R.M. Surgical Management of Fuchs Endothelial Corneal Dystrophy: A Treatment Algorithm and Individual Patient Meta-Analysis of Descemet Stripping Only. Cornea 2022, 41, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Macsai, M.S.; Shiloach, M. Use of Topical Rho Kinase Inhibitors in the Treatment of Fuchs Dystrophy after Descemet Stripping Only. Cornea 2019, 38, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Toda, M.; Numa, K.; Tanaka, H.; Imai, K.; Bush, J.; Teramukai, S.; Okumura, N.; Koizumi, N.; Yamamoto, A.; et al. Superiority of Mature Differentiated Cultured Human Corneal Endothelial Cell Injection Therapy for Corneal Endothelial Failure. Am. J. Ophthalmol. 2022, 237, 267–277. [Google Scholar] [CrossRef]
- Lai, J.Y.; Chen, K.H.; Hsiue, G.H. Tissue-engineered human corneal endothelial cell sheet transplantation in a rabbit model using functional biomaterials. Transplantation 2007, 84, 1222–1232. [Google Scholar] [CrossRef]
- Crouzet, E.; He, Z.; Ben Moussa, O.; Mentek, M.; Isard, P.; Peyret, B.; Forest, F.; Gain, P.; Koizumi, N.; Okumura, N.; et al. Tissue engineered endothelial keratoplasty in rabbit: Tips and tricks. Acta Ophthalmol. 2022, 100, 690–699. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blavakis, E.; Kecik, M.; Panos, G.D.; Thumann, G.; Massa, H. Descemet Membrane Endothelial Keratoplasty Graft Preparation Using the Liquid Bubble Technique with Subtrabecular Hydrodissection: A Retrospective Real-Life Study. J. Clin. Med. 2024, 13, 6048. https://doi.org/10.3390/jcm13206048
Blavakis E, Kecik M, Panos GD, Thumann G, Massa H. Descemet Membrane Endothelial Keratoplasty Graft Preparation Using the Liquid Bubble Technique with Subtrabecular Hydrodissection: A Retrospective Real-Life Study. Journal of Clinical Medicine. 2024; 13(20):6048. https://doi.org/10.3390/jcm13206048
Chicago/Turabian StyleBlavakis, Emmanouil, Mateusz Kecik, Georgios D. Panos, Gabriele Thumann, and Horace Massa. 2024. "Descemet Membrane Endothelial Keratoplasty Graft Preparation Using the Liquid Bubble Technique with Subtrabecular Hydrodissection: A Retrospective Real-Life Study" Journal of Clinical Medicine 13, no. 20: 6048. https://doi.org/10.3390/jcm13206048
APA StyleBlavakis, E., Kecik, M., Panos, G. D., Thumann, G., & Massa, H. (2024). Descemet Membrane Endothelial Keratoplasty Graft Preparation Using the Liquid Bubble Technique with Subtrabecular Hydrodissection: A Retrospective Real-Life Study. Journal of Clinical Medicine, 13(20), 6048. https://doi.org/10.3390/jcm13206048






