Abstract
Background/Objectives: The rising use of antidepressants is linked to oral health risks, including xerostomia, caries, and periodontal disease. Recognizing these risks is essential for improving patient care. To systematically review oral manifestations in patients undergoing antidepressant treatment. Methods: This review follows the PRISMA guidelines and includes observational studies published in the last 21 years. A PICO-based question was developed to select relevant studies, which were assessed for quality using a modified STROBE checklist. Results: A total of 11 studies were analyzed, revealing a consistent association between antidepressant use and the increased risk of xerostomia, caries, and periodontal disease. Additional findings included taste dysfunction and oral bleeding complications. Among the antidepressants, selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants (TCAs) were most commonly associated with xerostomia and caries. However, no significant impact was observed on the chemical composition of saliva or on hemostasis in invasive dental procedures. Conclusions: Antidepressant use may lead to significant oral health issues, notably xerostomia and caries. Further studies are recommended to clarify the influence of specific antidepressants and confounding factors, such as treatment duration, dosage, and hygiene habits, on oral health outcomes.
1. Introduction
In a society where stress is on the rise and the search for a better quality of life is constant, psychic disorders such as depression are increasingly common, affecting 3.7% of the population [1,2]. This has led to an increase in the use of antidepressants, with one in six people consuming some type of psychodrug [3,4]. Depression, especially among young people, has increased considerably, being twice as prevalent in women [5].
Modern antidepressants may slightly reduce the symptoms of depression compared to placebos, although their effectiveness varies due to the heterogeneous nature of depression [6]. A gradual therapeutic approach is suggested, starting with social and psychological interventions, and then moving to more specific therapies and medicines [7]. Over time, several types of antidepressants have developed. Initially, in the 1950s, monoamine oxidase inhibitors (MAOIs) emerged, followed by tricyclic (TCAs) and tetracyclic antidepressants in the 1980s [8,9]. In 1987, selective serotonin reuptake inhibitors (SSRIs) appeared, such as citalopram and fluoxetine, which are the most commonly used today for their efficacy and safety [2,8].
Antidepressants not only treat depression, but also other disorders such as obsessive–compulsive disorder (OCD), anxiety, eating disorders, and irritable bowel syndrome, among others [2,9]. Although effective in 50–70% of patients, their latency time may lead to the discontinuation of treatment or increased risk of suicide [8]. Each class of antidepressants operates through distinct mechanisms of action, which lead to specific therapeutic effects as well as varied side effect profiles. This diversity in action and side effects underscores the importance of understanding the potential impacts of each type of antidepressant on oral health (Table 1). Antidepressants are mainly metabolized in the liver and may have temporary side effects such as anxiety, insomnia, and sexual dysfunctions, in addition to some that are more serious, but less common [2,9]; for example, SSRIs are associated with the risk of hyponatremia (low sodium level in the blood), which corresponds to the elderly but also the concomitant use of diuretics (Table 1). In summary, antidepressant use should be controlled and monitored due to its potential side effects and variability in patient response.

Table 1.
Antidepressant drugs, mechanisms of action, and their adverse effects [2,9].
Recent studies emphasize the impact of antidepressants on oral health, particularly on periodontium [10], caries, and xerostomia [11]. SSRIs, for example, are associated with increased periodontal disease risk due to their effects on bone density and inflammation [10]. Additionally, TCAs and other antidepressants with anticholinergic properties often lead to xerostomia, which reduces salivary flow and increases caries risk [11].
The increase in recent years of patients using antidepressants, and the knowledge that these drugs can cause alterations in the oral cavity evidence the need to perform a systematic review that synthesizes the oral manifestations that can occur in this type of patient. In this way, this article aims to make a qualitative synthesis of the scientific literature to identify the possible oral manifestations that patients treated with antidepressants may suffer.
The general objective of this systematic review is summarized in a qualitative synthesis of the available scientific information, by searching the literature in various databases, in order to identify the possible oral manifestations that could occur in people treated with antidepressants. The specific objectives are as follows: identify the most common oral condition in people treated with antidepressant drugs; indicate which type of antidepressants are most related to oral manifestations; and know which factors can influence the appearance of oral problems in people who use antidepressants.
2. Materials and Methods
This systematic review was conducted in accordance with the PRISMA 2020 guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) and was registered in the PROSPERO database (International Prospective Registry of Systematic Reviews) under the identification number CRD42023482042. The research question was formulated using the PICO framework as follows: What are the oral manifestations observed in patients undergoing treatment with antidepressant drugs? (P: human subjects; I: treatment with antidepressant drugs; C: individuals not receiving antidepressant treatment; and O: prevalence of oral manifestations in those treated with antidepressants). The search strategy, study selection, data extraction, and quality assessment (including the risk of bias evaluation) were carried out independently by two researchers (J.M.A.-H. and J.G.-G.). In cases where discrepancies arose, a third investigator (M.R.P.-L.) was consulted for resolution.
2.1. Search Strategy
The search strategy was conducted in December 2023 using five electronic databases: MEDLINE, Web of Science, Scopus, Cochrane Library, and SciELO. The search was restricted to the studies published between January 2003 and December 2023 across all the databases. The terms listed in Table 2 were used to build the strategy, combined with the Boolean operators “OR” and “AND”. Additionally, advanced search tools, such as the truncation symbol (*), were applied to refine the results.

Table 2.
Search strategy.
2.2. Eligibility Criteria
Regarding the eligibility criteria, which were defined according to the research question and the objectives of the study, we found the following: articles that study oral disorders and include patients treated with antidepressants; conducted on humans; written in English or Spanish; published in the last 20 years; and articles that are observational (cohorts, case–control, descriptive, and longitudinal) and experimental studies.
2.3. Study Selection
The references collected through the search strategy were imported to the EndNote appointment manager (Clarivate Analytics, London, UK) to remove duplicates. A selection process was then conducted by initially reviewing the titles and then summaries following the inclusion and exclusion criteria. Eligibility was then assessed and a qualitative synthesis of the articles that met these criteria was carried out through a thorough review of the full text.
2.4. Design of the Study
For the bibliometric analysis, the data collected from each article included the author, year of publication, the journal, and the country where the study was conducted. A summary table was also created to organize key details such as the author and publication year, study design, sample or study groups, participants’ ages, types of antidepressants administered, observed oral manifestations, significant findings, and conclusions.
2.5. Quality Analysis
An adapted version of the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) checklist was utilized to assess the risk of bias in the selected studies [12]. This adaptation focused on 11 specific criteria corresponding to items 5, 6, 7, 8, 10, 12, 14, and 15 from the original STROBE checklist. Compliance with each criterion was marked with a check (✓), while non-compliance was indicated with a cross (✗). Based on the total score, the studies were categorized as follows: a low risk of bias for scores between 8 and 11 points, a moderate risk for scores between 4 and 7 points, and a high risk for scores of 3 points or fewer.
3. Results
3.1. Selection of Studies and Flowchart
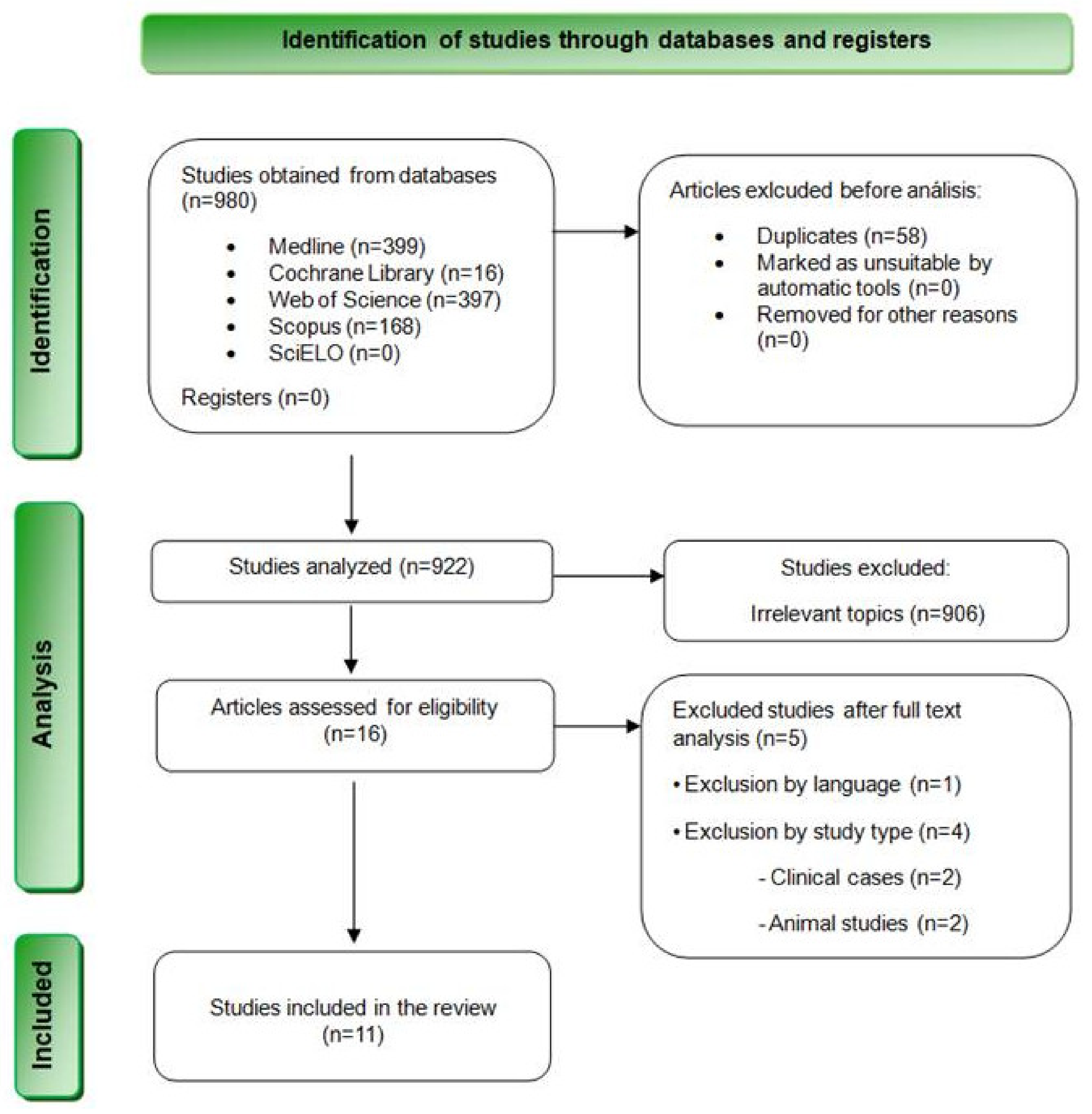
The bibliographic search resulted in a total of 980 results. Specifically, 399 articles were found in MEDLINE (PubMed), 397 in Web of Science, 168 in Scopus, 16 in Cochrane Library, and none in SciELO. Table 3 summarizes the results obtained from each database. Subsequently, 58 duplicate articles were removed, and 922 articles were selected for the revision of titles and summaries. At this stage, 853 articles were excluded after reviewing their titles, and 53 additional articles were discarded after reading their summaries and verifying that they did not meet the inclusion criteria. Finally, the remaining 16 articles were evaluated through a complete reading of their texts. Finally, 5 studies were eliminated after reading them in full text, and 11 articles were finally chosen for qualitative analysis (Figure 1).

Table 3.
Results obtained from each database.
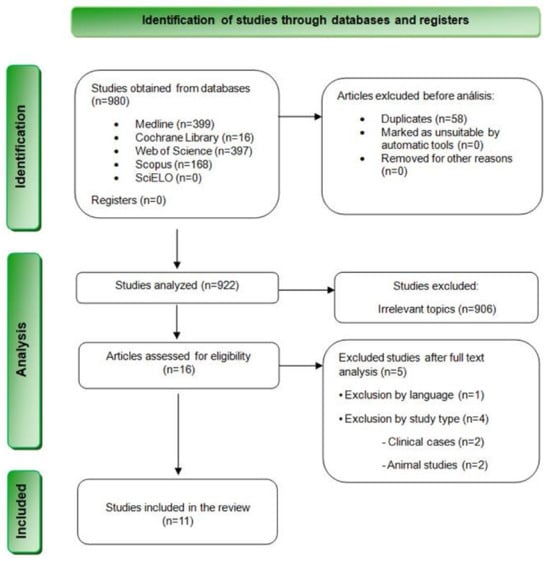
Figure 1.
Flowchart depicting the inclusion of studies in this systematic review based on the PRISMA 2020 Statement.
3.2. Quality Analysis
The quality assessment method used in this systematic review is based on an adapted version of the STROBE guide [12] for observational studies as shown in Table 4 The results of the analysis indicate that eight [13,14,15,16,17,18,19,20] of the works were considered low risk of bias, representing 72.72% of the total. Two studies were rated as moderate risk [21,22], which constitutes 18.18%, and another of the studies was identified with a high risk of bias [23], 9.09%, and was discarded in the extraction of data from the results and discussion of this work (Table 4).

Table 4.
Results of the quality analysis of the selected studies.
3.3. Characteristics of the Studies
3.3.1. Bibliometric Analysis
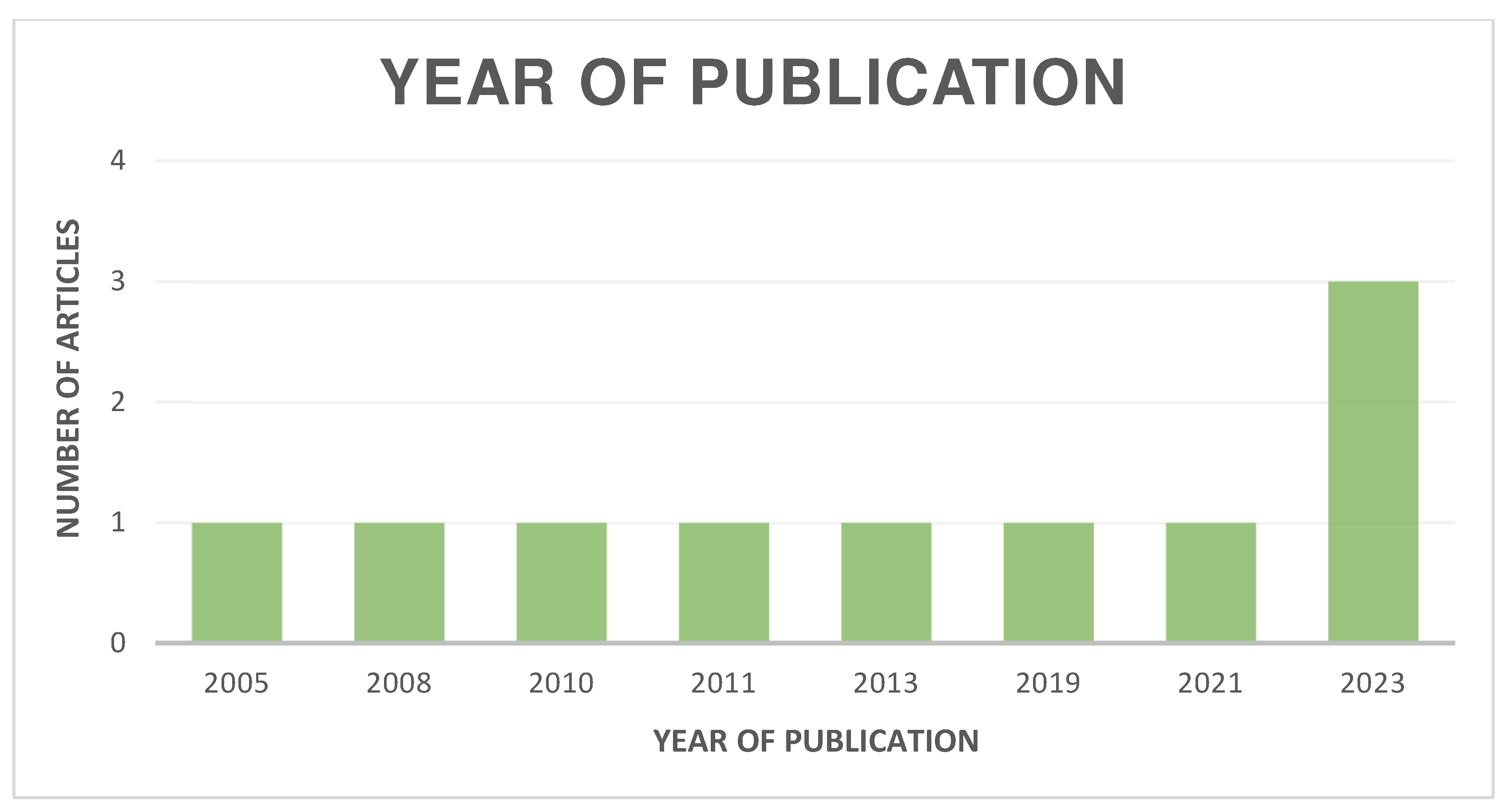
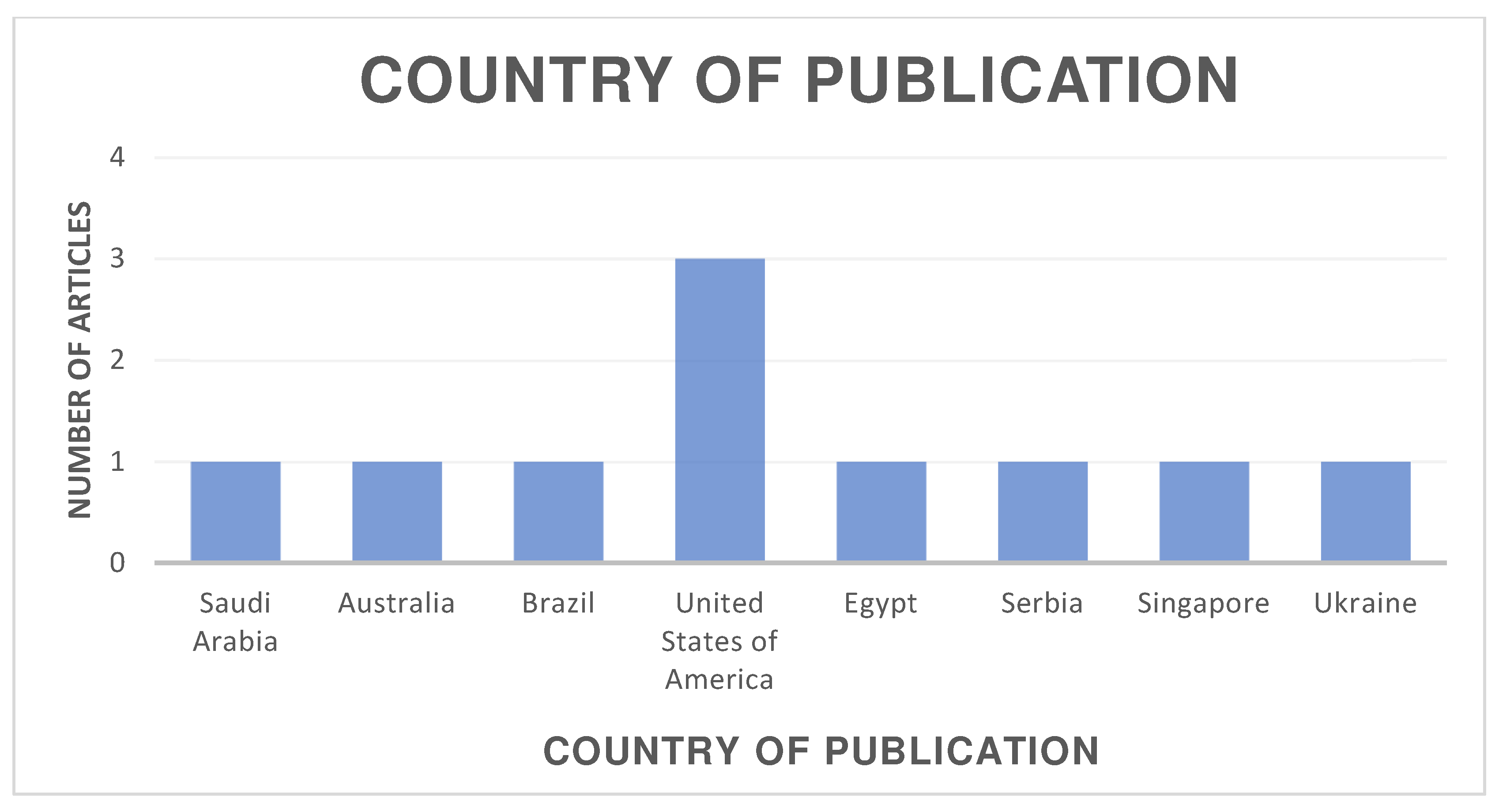
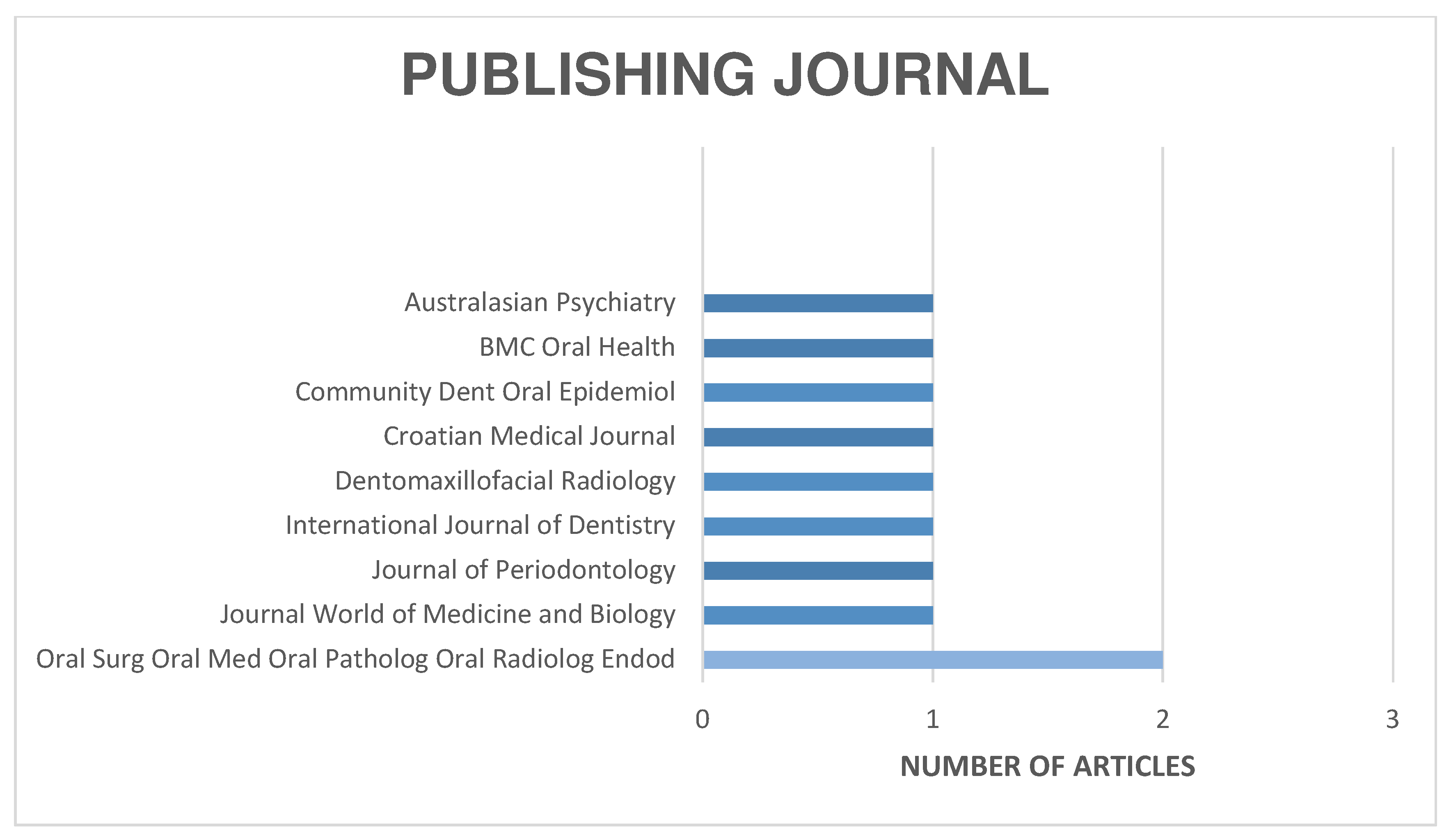
The distribution of the selected articles by publication year is shown in Figure 2, by country in Figure 3, and by journal in Figure 4.
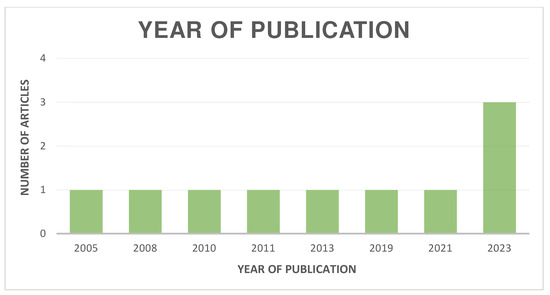
Figure 2.
Organization of articles according to their year of publication.
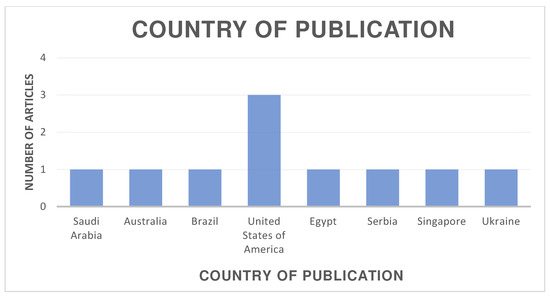
Figure 3.
Organization of articles according to their country of publication.
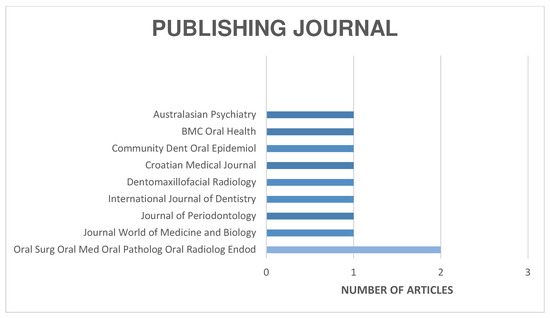
Figure 4.
Organization of articles according to their publication journal.
3.3.2. Design of the Study
Within the studies selected for review, the following study designs were identified: five case–control studies [13,14,15,17,19], 50% of the 10 articles selected; three cohort studies [18,19,21], representing 30% of the total; and two cross-sectional studies [16,22] representing 20% of the total (Table 5).

Table 5.
Results of Table 4: results of the articles included in the systematic review.
3.3.3. Groups or Sample
The sample size was very variable among all the articles, with five studies that exceeded 100 people sample [13,15,17,19,22], and five articles [14,16,18,20,21,24] that did not exceed that sample amount (Table 5).
3.3.4. Type of Antidepressant
As for the type of antidepressant, we can observe that two studies [15,16] do not specify the specific type of antidepressant patients receive from the study, representing 20% of the articles. As part of the remaining 80%, we found eight articles in which serotonin reuptake inhibitors (SSRIs) are studied, [13,14,17,18,19,20,21,22]. In the same way, we can see that in three articles [13,18,22] of these eight, tricyclic antidepressants (TCAs) are studied together with SSRIs, accounting for 30% of the total. Finally, in just one article [13], which represents 10% of the total, were also included patients treated with tetracyclic antidepressants or monoamine oxidase inhibitors (MAOIs) (Table 5).
3.3.5. Age of Participants
As for the age of the participants, in four articles [17,19,20,21], the average age of the studied groups was indicated, accounting for 40% of the total articles, in contrast to two articles [18,22] where it was indicated by an age range, assuming 20% of the total, and finally in the remaining 4 articles [13,14,15,16] it was indicated by an age range and the average age of the participants, accounting for 40% of the total articles included in this review (Table 5).
3.3.6. Oral Manifestations
As we see in Table 5, we found four articles that study the presence of restorations or cavities [13,15,16,22]. Also noteworthy are two articles that study xerostomia in patients treated with antidepressants [14,22]. On the other hand, we found three articles that study periodontal disease (EPO) [15,16,22]. On the other hand, we found two studies that study other oral manifestations such as periodontal and peri-implant status [19,20]; two other articles dealing with the composition of saliva and its restorative capacity [14,20]; an article on oral bleeding complications after invasive dental procedures [21]; another on mandibular bone mineral density [17]; another on other oral syndromes such as burning mouth, sialorrea, or geographic tongue; and finally, an article on gustatory dysfunction [18].
4. Discussion
The relationship between mental disorders and poor oral health is a frequent problem in clinical practice and medical research. This review explores how antidepressants of different types affect oral health, particularly in xerostomia, which is the perception of dry mouth and may be associated with a reduction in salivary flow, called hyposalivation [24]. Between 1% and 29% of the world’s population suffer from xerostomia, which affects the ability to speak and swallow, and can lead to caries [25,26].
Several studies analyzed in this review focus on xerostomia. On the other hand, De Almeida’s et al. study (2008) examined 33 patients divided into groups with and without psychotropic medication, finding a significant reduction in salivation in those treated with psychotropic drugs in this study [14]. However, no decrease in saliva was observed in patients treated with SSRIs at recommended doses compared with the control group. In groups treated with antidepressants, 15 cases of xerostomia were detected. This study claims that these drugs do not have as harmful an effect on salivation compared to the data in the literature, but [27] asserts that these medications cause xerostomia by reducing salivary flow. Similarly, other investigators [28] explain, through the neurotransmitter system that regulates salivary flow, how antidepressants decrease it due to their anticholinergic effect.
Another study, [22], surveyed doctors, dentists, pharmacists, and patients treated with antidepressants. A total of 83.9% of the patients reported xerostomia or caries, and a greater decrease in salivary flow was observed in patients treated with CTA (58%) compared with those treated with SSRIs (32%). More than 80% of the patients taking antidepressants had significant oral pathologies, recommending future research that correlates this disease with other variables such as changes in eating behavior, drug doses, and different antidepressant groups.
Periodontal disease, associated with bacteria such as Porphyromonas gingivalis [29], varies from gingivitis to periodontitis. Jovanovic and collaborators found that psychiatric patients taking antidepressants showed a higher CPOD index (decayed, missing, and blocked teeth) and plaque (IP) in their article [15]. Lalloo and collaborators observed an average CPOD of 17.7 in medicated psychiatric patients, higher than the Australian state average, with a high incidence of gingival inflammation and untreated caries, as they can demonstrate in their study [16]. Rindal and collaborators [13] concluded that patients taking antidepressants or non-xerogenic medication were more likely to need dental restorations compared to non-medicated ones, suggesting a relationship between antidepressant medication and poor oral care habits. However, these conclusions are confronted with those of Janket et al. [30], which do not find significant adverse effects of xerogenic medication on the caries index or on CPITN.
Taste dysfunctions, such as the loss of taste, are associated with hyposalivation and can affect the quality of life. Antidepressants with anticholinergic effects may reduce salivary flow, decreasing essential ions and enzymes for taste perception [31]. Additionally, alterations in serotonin pathways by some antidepressants may directly impact taste modulation [32]. Mikhail and collaborators [18] studied these dysfunctions in patients treated with antidepressants, finding that 70% of those treated with TCAs, 20% of those treated with SSRI, and those not medicated had taste problems, especially with sweet taste. Other studies, such as Arbisi and collaborators [33], also found a decreased ability to detect sweet taste in people with seasonal affective disorder.
The studies by Kotsailidi and collaborators [19] and Alharthi and collaborators [20] analyze the effects of antidepressants in patients with dental implants. Kotsailidi [19] found a significant association between SSRI use and marginal bone loss in 105 patients, suggesting an increased risk of peri-implantitis and implant failure in SSRI users. Alharthi and collaborators, however, found no significant differences in bone loss or other parameters in their study of 103 implants, provided adequate oral health is maintained [20]. In the same way, other works like that of Wu et al. [34] report implant failure rates more than double (4.6% non-SRIS users and 10.6% SSRI users), highlighting the need for more accurate and scrupulous implant treatment planning in SSRI users. Other cofactors may also contribute to implant failure in patients undergoing antidepressant therapy. Factors such as poor oral hygiene, smoking, systemic conditions like diabetes, and prolonged treatment duration can interact with the effects of antidepressants, potentially increasing the risk of peri-implantitis and bone loss. These cofactors may amplify inflammation and compromise bone density, further impacting the stability and success of dental implants [35].
In the context of mandibular bone mineral density, Gupta and collaborators [17] observed that patients treated with SSRIs had a higher probability of condylar pathology and lower mandibular bone quality, supporting the idea that SRIs negatively affect bone mineralization, as in other studies such as the one by Coşgunarslan et al. [36] which evidence that jaw bone areas with abundant bony trabeculae are harmed by the use of SSRIs. In addition, the use of SSRIs may lead to calcium and vitamin D depletion, increasing fracture risk in older adults, especially those with severe periodontitis. Calcium and vitamin D supplementation is recommended in these cases to help maintain bone health [37]. The effects of SSRIs on bone mineral density (BMD) may be influenced by treatment duration, especially in elderly patients. Additionally, fluoxetine’s anti-inflammatory and immunomodulatory properties could impact periodontal health and increase caries risk, underscoring the need for further research on its effects on oral and bone health [38].
Napeñas and collaborators [21] investigated oral hemorrhagic complications in patients treated with SSRIs, finding a minimal risk of hemorrhagic complications in invasive dental procedures, and concluding that peri- and postoperative precautions are sufficient to avoid problems. However, other studies such as the one by Weinrieb and collaborators [39] suggest that SSRIs can significantly increase the risk of gastrointestinal bleeding, especially when combined with NSAIDs.
Given the findings of this review, future research should focus on identifying preventive strategies to manage oral health risks in patients undergoing antidepressant therapy. Approaches such as the use of salivary substitutes to alleviate xerostomia and remineralizing agents to counteract enamel demineralization could be beneficial. Additionally, long-term studies with larger sample sizes are needed to investigate the cumulative effects of different classes of antidepressants on oral health, especially among individuals with additional risk factors, such as smoking, diabetes, or poor oral hygiene. Such research would provide valuable insights into tailored oral health protocols for this population, ultimately aiming to improve the quality of life and reduce complications associated with antidepressant treatment.
The systematic review has several significant limitations. One of them is the exclusion of many results that did not meet the inclusion criteria. This significantly reduced the number of studies analyzed. In addition, the lack of sufficient studies with comparable data made objective evaluation and the possibility of meta-analysis difficult. Another notable limitation was the restriction to articles written only in Spanish or English and the selection of studies published in the last 20 years. Although we initially considered shorter time ranges, the search was extended until January 2003 due to the lack of studies that met all the established criteria. Another limitation of this study is the difficulty in attributing symptoms like xerostomia solely to antidepressant use, particularly in patients with chronic comorbid conditions such as cancer, diabetes, or respiratory failure. These conditions often involve polypharmacy and can independently cause or exacerbate oral manifestations, making it difficult to isolate the specific contribution of antidepressants versus the underlying illness or the combined effects of both. This overlap underscores the complexity of assessing the impact of antidepressants on oral health in patients with coexisting health issues.
5. Conclusions
Antidepressants can cause xerostomia, cavities, taste dysfunctions, the failure of dental implants, and the deterioration of jawbone quality. These effects are linked to the drugs’ mechanisms of action on neurotransmitter pathways, which can influence oral health outcomes. Antidepressants do not affect the composition and pH of saliva or hemostatic capacity in oral invasive treatments. Xerostomia is the most common oral manifestation, with no significant association found between specific antidepressant types and oral manifestations. Factors such as dosage, eating habits, the duration of treatment, a lack of hygiene, and smoking also influence these outcomes. Proactive measures, including regular dental check-ups and the use of salivary substitutes or remineralization agents, may help mitigate these risks. More studies with consistent methodologies are needed to reach firm conclusions.
Author Contributions
Conceptualization, J.M.A.-H. and J.G.-G.; methodology, J.M.A.-H.; software, M.R.P.-L.; validation, J.G.-G. and J.M.A.-H.; formal analysis, M.R.P.-L.; investigation, J.M.A.-H. and J.G.-G.; resources, J.G.-G.; data curation, J.M.A.-H.; writing—original draft preparation, J.M.A.-H.; writing—review and editing, J.G.-G. and M.R.P.-L.; supervision, J.G.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gavrila-Ardelean, M.; Moldovan, O.D. The Occupational Stress to The Young Workers Came From Institutionalized Environment. Procedia Soc. Behav. Sci. 2014, 159, 589–592. [Google Scholar] [CrossRef][Green Version]
- Chávez León, E.; Ontiveros Uribe, M.P.; Serrano Gómez, C. Los antidepresivos inhibidores selectivos de recaptura de serotonina (ISRS, ISR-5HT). Salud Mental. 2008, 31, 307–319. [Google Scholar]
- Braslow, J.T.; Marder, S.R. History of Psychopharmacology. Annu. Rev. Clin. Psychol. 2019, 15, 25–50. [Google Scholar] [CrossRef]
- Forns, J.; Pottegård, A.; Reinders, T.; Poblador-Plou, B.; Morros, R.; Brandt, L.; Cainzos-Achirica, M.; Hekkfritzsch, M.; Schink, T.; Prados-Torres, A.; et al. Antidepressant use in Denmark, Germany, Spain, and Sweden between 2009 and 2014: Incidence and comorbidities of antidepressant initiators. J. Affect. Disord. 2019, 249, 242–252. [Google Scholar] [CrossRef]
- Morishita, S.; Kinoshita, T.; Arita, S. Gender Differences in Response to Antidepressants. Antidepressants: Types, Efficiency and Possible Side Effects. In Enciclopedia of Pharmacology Research; Nova Science Publishing, Inc.: New York, NY, USA, 2010; pp. 199–219. [Google Scholar]
- Hetrick, S.E.; McKenzie, J.E.; Bailey, A.P.; Sharma, V.; Moller, C.I.; Badcock, P.B.; Cox, G.R.; Merry, S.N.; Meader, N. New generation antidepressants for depression in children and adolescents: A network meta-analysis. Cochrane Database Syst. Rev. 2021, 5, Cd013674. [Google Scholar]
- Thapar, A.; Eyre, O.; Patel, V.; Brent, D. Depression in young people. Lancet 2022, 400, 617–631. [Google Scholar] [CrossRef]
- Gourion, D. Antidepressants and their onset of action: A major clinical, methodological and pronostical issue. Enceph. Rev. Psychiatr. Clin. Biol. Ther. 2008, 34, 73–81. [Google Scholar]
- Khawam, E.A.; Laurencic, G.; Malone, D.A., Jr. Side effects of antidepressants: An overview. Cleve Clin. J. Med. 2006, 73, 351–353. [Google Scholar] [CrossRef]
- Taccardi, D.; Chiesa, A.; Maiorani, C.; Pardo, A.; Lombardo, G.; Scribante, A.; Sabatini, S.; Butera, A. Periodontitis and Depressive Disorders: The Effects of Antidepressant Drugs on the Periodontium in Clinical and Preclinical Models: A Narrative Review. J. Clin. Med. 2024, 13, 4524. [Google Scholar] [CrossRef]
- Thomson, W.M.; Ferguson, C.A.; Janssens, B.E.; Kerse, N.M.; Ting, G.S.; Smith, M.B. Xerostomia and polypharmacy among dependent older New Zealanders: A national survey. Age Ageing 2021, 50, 248–251. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Rindal, D.B.; Rush, W.A.; Peters, D.; Maupomé, G. Antidepressant xerogenic medications and restoration rates. Community Dent. Oral Epidemiol. 2005, 33, 74–80. [Google Scholar] [CrossRef]
- de Almeida, P.D.; Grégio, A.M.T.; Brancher, J.A.; Ignácio, S.A.; Machado, M.A.N.; de Lima, A.A.S.; Azevedo, L.R. Effects of antidepressants and benzodiazepines on stimulated salivary flow rate and biochemistry composition of the saliva. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2008, 106, 58–65. [Google Scholar] [CrossRef]
- Jovanovic, S.; Milovanovic, S.D.; Gajic, I.; Mandic, J.; Latas, M.; Jankovic, L. Oral Health Status of Psychiatric In-patients in Serbia and Implications for Their Dental Care. Croat. Med. J. 2010, 51, 443–450. [Google Scholar] [CrossRef]
- Lalloo, R.; Kisely, S.; Amarasinghe, H.; Perera, R.; Johnson, N. Oral health of patients on psychotropic medications: A study of outpatients in Queensland. Australas. Psychiatry 2013, 21, 338–342. [Google Scholar] [CrossRef]
- Gupta, B.; Acharya, A.; Singh, S.; Brazzoli, S.; Ghorab, M.; Malik, S.; Pelekos, G.; Rossouw, E. Evaluation of jawbone morphology and bone density indices in panoramic radiographs of selective serotonin reuptake inhibitor users: A preliminary study. Dentomaxillofac. Radiol. 2019, 48, 20170360. [Google Scholar] [CrossRef]
- Mikhail, C.; Elgaaly, K.; Abd El Hamid, A.A.E.L.; Shaker, O.; Ali, S. Gustatory dysfunction among a sample of depressed Egyptian adults under antidepressants therapy: A retrospective cohort study. Int. J. Dent. 2021, 2021, C7-5543840. [Google Scholar] [CrossRef]
- Kotsailidi, E.A.; Gagnon, C.; Johnson, L.; Basir, A.B.; Tsigarida, A. Association of selective serotonin reuptake inhibitor use with marginal bone level changes around osseointegrated dental implants: A retrospective study. J. Periodontol. 2023, 94, 1008–1017. [Google Scholar] [CrossRef]
- Alharthi, S.S.; BinShabaib, M.S.; Alwahibi, A.; Gamal, S.; Elashiry, E., Jr.; Almershed, S.E.; Alkhamis, H.A.; Anweigi, L. Periodontal and peri-implant status and whole salivary interleukin 1-beta levels among individuals using selective serotonin reuptake inhibitors: An observational study. BMC Oral Health 2023, 23, 310. [Google Scholar] [CrossRef]
- Napeñas, J.J.; Hong, C.H.L.; Kempter, E.; Brennan, M.T.; Furney, S.L.; Lockhart, P.B. Selective serotonin reuptake inhibitors and oral bleeding complications after invasive dental treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2011, 112, 463–467. [Google Scholar] [CrossRef]
- Khaitovych, M.V.; Mazur, I.P.; Voitova, Y.V.; Yunakova, N.M.; Turchak, D.V.; Temirova, O.A.; Polyakova, D.S. Side Effects of Antidepressants on Oral Health According to Questionnaires of Patients and Healthcare Professionals. World Med. Biol. 2023, 84, 163–167. [Google Scholar] [CrossRef]
- Gandhi, P.; Saxena, A.; Pai, K.; Ahmed, J.; Ongole, R. Oral Manifestations of Psychotropic Drugs on the Oral Cavity: Observational Study. J. Contemp. Dent. Pract. 2022, 23, 443–446. [Google Scholar]
- Napeñas, J.J.; Brennan, M.T.; Fox, P.C. Diagnosis and treatment of xerostomia (dry mouth). Odontology 2009, 97, 76–83. [Google Scholar] [CrossRef]
- Tanasiewicz, M.; Hildebrandt, T.; Obersztyn, I. Xerostomia of Various Etiologies: A Review of the Literature. Adv. Clin. Exp. Med. 2016, 25, 199–206. [Google Scholar] [CrossRef]
- Llena-Puy, C. The role of saliva in maintaining oral health and as an aid to diagnosis. Med. Oral Patol. Oral Cir. Bucal. 2006, 11, E449–E455. [Google Scholar]
- Kisely, S.; Sawyer, E.; Siskind, D.; Lalloo, R. The oral health of people with anxiety and depressive disorders—A systematic review and meta-analysis. J. Affect. Disord. 2016, 200, 119–132. [Google Scholar] [CrossRef]
- Peeters, F.P.M.L.; deVries, M.W.; Vissink, A. Risks for Oral Health with the Use of Antidepressants. Gen. Hosp. Psychiatry 1998, 20, 150–154. [Google Scholar] [CrossRef]
- Wadhawan, A.; Reynolds, M.A.; Makkar, H.; Scott, A.J.; Potocki, E.; Hoisington, A.J.; Brenner, L.A.; Dagdag, A.; Lowry, C.A.; Dwivedi, Y.; et al. Periodontal Pathogens and Neuropsychiatric Health. Curr. Top. Med. Chem. 2020, 20, 1353–1397. [Google Scholar] [CrossRef]
- Janket, S.J.; Jones, J.A.; Rich, S.; Meurman, J.; Garcia, R.; Miller, D. Xerostomic medications and oral health: The Veterans Dental Study (part I). Gerodontology 2003, 20, 41–49. [Google Scholar] [CrossRef]
- Schiffman, S.S.; Zervakis, J.; Suggs, M.S.; Budd, K.C.; Iuga, L. Effect of tricyclic antidepressants on taste responses in humans and gerbils. Pharmacol. Biochem. Behav. 2000, 65, 599–609. [Google Scholar] [CrossRef]
- Huang, Y.A.; Dando, R.; Roper, S.D. Autocrine and paracrine roles for ATP and serotonin in mouse taste buds. J. Neurosci. 2009, 29, 13909–13918. [Google Scholar] [CrossRef]
- Arbisi, P.A.; Levine, A.S.; Nerenberg, J.; Wolf, J. Seasonal alteration in taste detection and recognition threshold in seasonal affective disorder: The proximate source of carbohydrate craving. Psychiatry Res. 1996, 59, 171–182. [Google Scholar] [CrossRef]
- Wu, X.; Al-Abedalla, K.; Rastikerdar, E.; Abi Nader, S.; Daniel, N.G.; Nicolau, B.; Tamimi, F. Selective serotonin reuptake inhibitors and the risk of osseointegrated implant failure: A cohort study. J. Dent. Res. 2014, 93, 1054–1061. [Google Scholar] [CrossRef]
- Do, T.A.; Le, H.S.; Shen, Y.W.; Huang, H.L.; Fuh, L.J. Risk Factors related to Late Failure of Dental Implant—A Systematic Review of Recent Studies. Int. J. Environ. Res. Public Health 2020, 17, 3931. [Google Scholar] [CrossRef]
- Coşgunarslan, A.; Aşantoğrol, F.; Soydan Çabuk, D.; Canger, E.M. The effect of selective serotonin reuptake inhibitors on the human mandible. Oral Radiol. 2021, 37, 20–28. [Google Scholar] [CrossRef]
- Goldman, A.L.; Donlon, C.M.; Cook, N.R.; Manson, J.E.; Buring, J.E.; Copeland, T.; Yu, C.Y.; LeBoff, M.S. VITamin D and OmegA-3 TriaL (VITAL) bone health ancillary study: Clinical factors associated with trabecular bone score in women and men. Osteoporos. Int. 2018, 29, 2505–2515. [Google Scholar] [CrossRef]
- Wernicke, J.F. Safety and side effect profile of fluoxetine. Expert Opin. Drug Saf. 2004, 3, 495–504. [Google Scholar] [CrossRef]
- Weinrieb, R.M.; Auriacombe, M.; Lynch, K.G.; Lewis, J.D. Selective serotonin re-uptake inhibitors and the risk of bleeding. Expert Opin. Drug Saf. 2005, 4, 337–344. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).