The sFlt-1/PlGF Ratio at 12, 24, and 32 Weeks Gestation in Twin Pregnancies as a Predictor of Placental Dysfunction
Abstract
1. Introduction
2. Materials and Methods
2.1. Statistics
2.2. Ethics
3. Results
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fox, N.S.; Rebarber, A.; Klauser, C.K.; Roman, A.S.; Saltzman, D.H. Intrauterine growth restriction in twin pregnancies: Incidence and associated risk factors. Am. J. Perinatol. 2011, 28, 267–272. [Google Scholar] [CrossRef]
- Gezer, A.; Rashidova, M.; Güralp, O.; Öçer, F. Perinatal mortality and morbidity in twin pregnancies: The relation between chorionicity and gestational age at birth. Arch. Gynecol. Obstet. 2012, 285, 353–360. [Google Scholar] [CrossRef]
- Romero, R.; Lockwood, C.; Oyarzun, E.; Hobbins, J.C. Toxemia: New concepts in an old disease. Semin. Perinatol. 1988, 12, 302–323. [Google Scholar]
- Magee, L.A.; Brown, M.A.; Hall, D.R.; Gupte, S.; Hennessy, A.; Karumanchi, S.A.; Kenny, L.C.; McCarthy, F.; Myers, J.; Poon, L.C.; et al. The 2021 International Society for the Study of Hypertension in Pregnancy classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2022, 27, 148–169. [Google Scholar] [CrossRef]
- Sibai, B.; Dekker, G.; Kupferminc, M. Pre-eclampsia. Lancet 2005, 365, 785–799. [Google Scholar] [CrossRef]
- Francisco, C.; Wright, D.; Benkő, Z.; Syngelaki, A.; Nicolaides, K.H. Hidden high rate of pre-eclampsia in twin compared with singleton pregnancy. Ultrasound Obstet. Gynecol. 2017, 50, 88–92. [Google Scholar] [CrossRef]
- Sibai, B.M.; Hauth, J.; Caritis, S.; Lindheimer, M.D.; MacPherson, C.; Klebanoff, M.; VanDorsten, J.; Landon, M.; Miodovnik, M.; Paul, R.; et al. Hypertensive disorders in twin versus singleton gestations. Am. J. Obstet. Gynecol. 2000, 182, 938–942. [Google Scholar] [CrossRef]
- Roberts, J.M.; Hubel, C.A. The two stage model of preeclampsia: Variations on the theme. Placenta 2009, 30, 32–37. [Google Scholar] [CrossRef]
- Ness, R.B.; Roberts, J.M. Heterogeneous causes constituting the single syndrome of preeclampsia: A hypothesis and its implications. Am. J. Obstet. Gynecol. 1996, 175, 1365–1370. [Google Scholar] [CrossRef]
- Bombrys, A.E.; Barton, J.R.; Nowacki, E.A.; Habli, M.; Pinder, L.; How, H.; Sibai, B.M. Expectant management of severe preeclampsia at less than 27 weeks’ gestation: Maternal and perinatal outcomes according to gestational age by weeks at onset of expectant management. Am. J. Obstet. Gynecol. 2008, 199, 247.e1–247.e6. [Google Scholar] [CrossRef]
- Von Dadelszen, P.; Magee, L.A.; Roberts, J.M. Subclassification of preeclampsia. Hypertens. Pregnancy 2003, 22, 143–148. [Google Scholar] [CrossRef]
- Townsend, R.; Khalil, A. Fetal growth restriction in twins. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 49, 79–88. [Google Scholar] [CrossRef]
- Chervenak, F.A.; Skupski, D.W.; Romero, R.; Myers, M.K.; Smith-Levitin, M.; Rosenwaks, Z.; Thaler, H.T. How accurate is fetal biometry in the assessment of fetal age? Am. J. Obstet. Gynecol. 1998, 178, 678–687. [Google Scholar] [CrossRef]
- Reforma, L.G.; Febres-Cordero, D.; Trochtenberg, A.; Modest, A.M.; Collier, A.-R.Y.; Spiel, M.H. Incidence of small-for-gestational-age infant birthweight following early intertwin fetal growth discordance in dichorionic and monochorionic twin pregnancies. Am. J. Obstet. Gynecol. 2022, 226, 726.e1–726.e9. [Google Scholar] [CrossRef]
- Hiersch, L.; Barrett, J.; Aviram, A.; Mei-Dan, E.; Yoon, E.W.; Zaltz, A.; Kingdom, J.; Melamed, N. Patterns of discordant growth and adverse neonatal outcomes in twins. Am. J. Obstet. Gynecol. 2021, 225, 187.e1–187.e14. [Google Scholar] [CrossRef]
- D’Antonio, F.; Odibo, A.O.; Prefumo, F.; Khalil, A.; Buca, D.; Flacco, M.E.; Liberati, M.; Manzoli, L.; Acharya, G. Weight discordance and perinatal mortality in twin pregnancy: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2018, 52, 11–23. [Google Scholar] [CrossRef]
- Dekalo, A.; Kogan, Z.; Herman, H.G.; Marelly, C.; Yaka, C.; Schreiber, L.; Weiner, E.; Miremberg, H. Fetal growth restriction, neonatal morbidity and placental pathology in dichorionic twins-a comparison of twin-specific versus singleton growth charts. Placenta 2023, 140, 6–10. [Google Scholar] [CrossRef]
- Herraiz, I.; Llurba, E.; Verlohren, S.; Galindo, A.; Bartha, J.L.; De La Calle, M.; Delgado, J.L.; De Paco, C.; Escudero, A.I.; Moreno, F.; et al. Update on the Diagnosis and Prognosis of Preeclampsia with the Aid of the sFlt-1/PlGF Ratio in Singleton Pregnancies. Fetal Diagn. Ther. 2018, 43, 81–89. [Google Scholar] [CrossRef]
- Alahakoon, T.I.; Zhang, W.; Trudinger, B.J.; Lee, V.W. Discordant clinical presentations of preeclampsia and intrauterine fetal growth restriction with similar pro- and anti-angiogenic profiles. J. Matern. Fetal Neonatal Med. 2014, 27, 1854–1859. [Google Scholar] [CrossRef]
- Agrawal, S.; Shinar, S.; Cerdeira, A.S.; Redman, C.; Vatish, M. Predictive Performance of PlGF (Placental Growth Factor) for Screening Preeclampsia in Asymptomatic Women: A Systematic Review and Meta-Analysis. Hypertension 2019, 74, 1124–1135. [Google Scholar] [CrossRef]
- Herraiz, I.; Quezada, M.S.; Rodriguez-Calvo, J.; Gómez-Montes, E.; Villalaín, C.; Galindo, A. Longitudinal change of sFlt-1/PlGF ratio in singleton pregnancy with early-onset fetal growth restriction. Ultrasound Obstet. Gynecol. 2018, 52, 631–638. [Google Scholar] [CrossRef]
- Herraiz, I.; Dröge, L.A.; Gómez-Montes, E.; Henrich, W.; Galindo, A.; Verlohren, S. Characterization of the soluble fms-like tyrosine kinase-1 to placental growth factor ratio in pregnancies complicated by fetal growth restriction. Obstet. Gynecol. 2014, 124, 265–273. [Google Scholar] [CrossRef]
- Allen, R.E.; Rogozinska, E.; Cleverly, K.; Aquilina, J.; Thangaratinam, S. Abnormal blood biomarkers in early pregnancy are associated with preeclampsia: A meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 182, 194–201. [Google Scholar] [CrossRef]
- Barton, J.R.; Woelkers, D.A.; Newman, R.B.; Combs, C.A.; How, H.Y.; Boggess, K.A.; Martin, J.N., Jr.; Kupfer, K.; Sibai, B.M. Placental growth factor predicts time to delivery in women with signs or symptoms of early preterm preeclampsia: A prospective multicenter study. Am. J. Obstet. Gynecol. 2020, 222, 259.e1–259.e11. [Google Scholar] [CrossRef]
- Sovio, U.; Gaccioli, F.; Cook, E.; Charnock-Jones, D.S.; Smith, G.C.S. Slowing of fetal growth and elevated maternal serum sFLT1:PlGF are associated with early term spontaneous labor. Am. J. Obstet. Gynecol. 2021, 225, 520.e1–520.e10. [Google Scholar] [CrossRef]
- Satorres, E.; Martínez-Varea, A.; Diago-Almela, V. sFlt-1/PlGF ratio as a predictor of pregnancy outcomes in twin pregnancies: A systematic review. J. Matern. Fetal Neonatal Med. 2023, 36, 2230514. [Google Scholar] [CrossRef]
- Stepan, H.; Galindo, A.; Hund, M.; Schlembach, D.; Sillman, J.; Surbek, D.; Vatish, M. Clinical utility of sFlt-1 and PlGF in screening, prediction, diagnosis and monitoring of pre-eclampsia and fetal growth restriction. Ultrasound Obstet. Gynecol. 2023, 61, 168–180. [Google Scholar] [CrossRef]
- Hadlock, F.P.; Harrist, R.B.; Sharman, R.S.; Deter, R.L.; Park, S.K. Estimation of fetal weight with the use of head, body, and femur measurements—A prospective study. Am. J. Obstet. Gynecol. 1985, 151, 333–337. [Google Scholar] [CrossRef]
- Ferrero, A.; Maggi, E.; Giancotti, A.; Torcia, F.; Pachi, A. Regression formula for estimation of fetal weight with use of abdominal circumference and femur length: A prospective study. J. Ultrasound Med. 1994, 13, 823–833. [Google Scholar] [CrossRef]
- Schild, R.L.; Fell, K.; Fimmers, R.; Gembruch, U.; Hansmann, M. A new formula for calculating weight in the fetus of ≤1600 g. Ultrasound Obstet. Gynecol. 2004, 24, 775–780. [Google Scholar] [CrossRef]
- Woo, J.S.K.; Wan, C.W.; Cho, K.M. Computer-assisted evaluation of ultrasonic fetal weight prediction using multiple regression equations with and without the fetal femur length. J. Ultrasound Med. 1985, 4, 65–67. [Google Scholar] [CrossRef]
- Milner, J.; Arezina, J. The accuracy of ultrasound estimation of fetal weight in comparison to birth weight: A systematic review. Ultrasound 2018, 26, 32–41. [Google Scholar] [CrossRef]
- Geerts, L.; Widmer, T. Which is the most accurate formula to estimate fetal weight in women with severe preterm preeclampsia? J. Matern. Fetal Neonatal Med. 2011, 24, 271–279. [Google Scholar] [CrossRef]
- Bdolah, Y.; Lam, C.; Rajakumar, A.; Shivalingappa, V.; Mutter, W.; Sachs, B.P.; Lim, K.H.; Bdolah-Abram, T.; Epstein, F.H.; Karumanchi, S.A. Twin pregnancy and the risk of preeclampsia: Bigger placenta or relative ischemia? Am. J. Obstet. Gynecol. 2008, 198, 428.e1–428.e6. [Google Scholar] [CrossRef]
- Hytten, F. Blood volume changes in normal pregnancy. Clin. Haematol. 1985, 14, 601–612. [Google Scholar] [CrossRef]
- Shinohara, S.; Sunami, R.; Kasai, M.; Yasuda, G.; Uchida, Y. Predictive value of the sFlt-1/PlGF ratio for preeclampsia in twin pregnancies: A retrospective study. Hypertens. Pregnancy 2021, 40, 330–335. [Google Scholar] [CrossRef]
- Dröge, L.; Herraiz, I.; Zeisler, H.; Schlembach, D.; Stepan, H.; Küssel, L.; Henrich, W.; Galindo, A.; Verlohren, S. Maternal serum sFlt-1/PlGF ratio in twin pregnancies with and without pre-eclampsia in comparison with singleton pregnancies. Ultrasound Obstet. Gynecol. 2015, 45, 286–293. [Google Scholar] [CrossRef]
- Binder, J.; Palmrich, P.; Pateisky, P.; Kalafat, E.; Kuessel, L.; Zeisler, H.; Munkhbaatar, M.; Windsperger, K.; Thilaganathan, B.; Khalil, A. The Prognostic Value of Angiogenic Markers in Twin Pregnancies to Predict Delivery due to Maternal Complications of Preeclampsia. Hypertension 2020, 76, 176–183. [Google Scholar] [CrossRef]
- Hoffmann, J.; Ossada, V.; Weber, M.; Stepan, H. An intermediate sFlt-1/PlGF ratio indicates an increased risk for adverse pregnancy outcome. Pregnancy Hypertens. 2017, 10, 165–170. [Google Scholar] [CrossRef]
- Martínez-Varea, A.; Martínez-Sáez, C.; Domenech, J.; Desco-Blay, J.; Monfort-Pitarch, S.; Hueso, M.; Diago-Almela, V. sFlt-1/PlGF Ratio at 24 Weeks Gestation in Twin Pregnancies as a Predictor of Preeclampsia or Fetal Growth Restriction. Fetal Diagn. Ther. 2022, 49, 206–214. [Google Scholar] [CrossRef]
- Karge, A.; Seiler, A.; Flechsenhar, S.; Haller, B.; Ortiz, J.U.; Lobmaier, S.M.; Axt-Fliedner, R.; Enzensberger, C.; Abel, K.; Kuschel, B.; et al. Prediction of adverse perinatal outcome and the mean time until delivery in twin pregnancies with suspected pre-eclampsia using sFlt-1/PIGF ratio. Pregnancy Hypertens. 2021, 24, 37–43. [Google Scholar] [CrossRef]
- Rana, S.; Hacker, M.R.; Modest, A.M.; Salahuddin, S.; Lim, K.H.; Verlohren, S.; Perschel, F.H.; Karumanchi, S.A. Circulating angiogenic factors and risk of adverse maternal and perinatal outcomes in twin pregnancies with suspected preeclampsia. Hypertension 2012, 60, 451–458. [Google Scholar] [CrossRef]
- Ong, C.Y.T.; Liao, A.W.; Spencer, K.; Munim, S.; Nicolaides, K.H. First trimester maternal serum free beta human chorionic gonadotrophin and pregnancy associated plasma protein A as predictors of pregnancy complications. BJOG 2000, 107, 1265–1270. [Google Scholar] [CrossRef]
- Smith, G.C.S.; Stenhouse, E.J.; Crossley, J.A.; Aitken, D.A.; Cameron, A.D.; Michael Connor, J. Early pregnancy levels of pregnancy-associated plasma protein a and the risk of intrauterine growth restriction, premature birth, preeclampsia, and stillbirth. J. Clin. Endocrinol. Metab. 2002, 87, 1762–1767. [Google Scholar] [CrossRef]
- Dugoff, L.; Hobbins, J.C.; Malone, F.D.; Porter, T.F.; Luthy, D.; Comstock, C.H.; Hankins, G.; Berkowitz, R.L.; Merkatz, I.; Craigo, S.D.; et al. First-trimester maternal serum PAPP-A and free-beta subunit human chorionic gonadotropin concentrations and nuchal translucency are associated with obstetric complications: A population-based screening study (The FASTER Trial). Am. J. Obstet. Gynecol. 2004, 191, 1446–1451. [Google Scholar] [CrossRef]
- Melchiorre, K.; Wormald, B.; Leslie, K.; Bhide, A.; Thilaganathan, B. First-trimester uterine artery Doppler indices in term and preterm pre-eclampsia. Ultrasound Obstet. Gynecol. 2008, 32, 133–137. [Google Scholar] [CrossRef]
- Martin, A.M.; Bindra, R.; Curcio, P.; Cicero, S.; Nicolaides, K.H. Screening for pre-eclampsia and fetal growth restriction by uterine artery Doppler at 11–14 weeks of gestation. Ultrasound Obstet. Gynecol. 2001, 18, 583–586. [Google Scholar] [CrossRef]
- Ananth, C.V.; Peltier, M.R.; Chavez, M.R.; Kirby, R.S.; Getahun, D.; Vintzileos, A.M. Recurrence of ischemic placental disease. Obstet. Gynecol. 2007, 110, 128–133. [Google Scholar] [CrossRef]
- Ananth, C.V.; Vintzileos, A.M. Ischemic placental disease: Epidemiology and risk factors. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 159, 77–82. [Google Scholar] [CrossRef]
- Ananth, C.V.; Peltier, M.R.; Kinzler, W.L.; Smulian, J.C.; Vintzileos, A.M. Chronic hypertension and risk of placental abruption: Is the association modified by ischemic placental disease? Am. J. Obstet. Gynecol. 2007, 197, 273.e1–273.e7. [Google Scholar] [CrossRef] [PubMed]
- Zeisler, H.; Llurba, E.; Chantraine, F.; Vatish, M.; Staff, A.C.; Sennström, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H.; Allegranza, D.; et al. Predictive Value of the sFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. N. Engl. J. Med. 2016, 374, 13–22. [Google Scholar] [CrossRef]
- Webster, K.; Fishburn, S.; Maresh, M.; Findlay, S.C.; Chappell, L.C. Diagnosis and management of hypertension in pregnancy: Summary of updated NICE guidance. BMJ 2019, 366, l5119. [Google Scholar] [CrossRef]
- Chaiworapongsa, T.; Chaemsaithong, P.; Yeo, L.; Romero, R. Pre-eclampsia part 1: Current understanding of its pathophysiology. Nat. Rev. Nephrol. 2014, 10, 466–480. [Google Scholar] [CrossRef]
- Figueras, F.; Gratacos, E. An integrated approach to fetal growth restriction. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 38, 48–58. [Google Scholar] [CrossRef]
- Khalil, A.; Rodgers, M.; Baschat, A.; Bhide, A.; Gratacos, E.; Hecher, K.; Kilby, M.D.; Lewi, L.; Nicolaides, K.H.; Oepkes, D.; et al. ISUOG Practice Guidelines: Role of ultrasound in twin pregnancy. Ultrasound Obstet. Gynecol. 2016, 47, 247–263. [Google Scholar] [CrossRef]
- Gómez, O.; Figueras, F.; Fernández, S.; Bennasar, M.; Martínez, J.M.; Puerto, B.; Gratacós, E. Reference ranges for uterine artery mean pulsatility index at 11–41 weeks of gestation. Ultrasound Obstet. Gynecol. 2008, 32, 128–132. [Google Scholar] [CrossRef]
- DeMers, D.; Wachs, D. Physiology, Mean Arterial Pressure. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538226/ (accessed on 5 December 2023).
- The Fetal Medicine Foundation. Available online: https://fetalmedicine.org/research/assess/preeclampsia/background (accessed on 5 December 2023).
- Tan, M.Y.; Wright, D.; Syngelaki, A.; Akolekar, R.; Cicero, S.; Janga, D.; Singh, M.; Greco, E.; Wright, A.; Maclagan, K.; et al. Comparison of diagnostic accuracy of early screening for pre-eclampsia by NICE guidelines and a method combining maternal factors and biomarkers: Results of SPREE. Ultrasound Obstet. Gynecol. 2018, 51, 743–750. [Google Scholar] [CrossRef]
- Wright, D.; Syngelaki, A.; Akolekar, R.; Poon, L.C.; Nicolaides, K.H. Competing risks model in screening for preeclampsia by maternal characteristics and medical history. Am. J. Obstet. Gynecol. 2015, 213, 62.e1–62.e10. [Google Scholar] [CrossRef]
- Stepan, H.; Hund, M.; Andraczek, T. Combining Biomarkers to Predict Pregnancy Complications and Redefine Preeclampsia: The Angiogenic-Placental Syndrome. Hypertension 2020, 75, 918. [Google Scholar] [CrossRef]
- Benkő, Z.; Wright, A.; Rehal, A.; Cimpoca, B.; Syngelaki, A.; Delgado, J.L.; Tsokaki, T.; De Alvarado, M.; Vojtassakova, D.; Ntalianis, K.M.; et al. Prediction of pre-eclampsia in twin pregnancy by maternal factors and biomarkers at 11–13 weeks’ gestation: Data from EVENTS trial. Ultrasound Obstet. Gynecol. 2021, 57, 257–265. [Google Scholar] [CrossRef]
- Benkő, Z.; Chaveeva, P.; de Paco Matallana, C.; Zingler, E.; Wright, D.; Nicolaides, K.H. Revised competing-risks model in screening for pre-eclampsia in twin pregnancy by maternal characteristics and medical history. Ultrasound Obstet. Gynecol. 2019, 54, 617–624. [Google Scholar] [CrossRef]
- Herraiz, I.; Simón, E.; Gómez-Arriaga, P.I.; Quezada, M.S.; García-Burguillo, A.; López-Jiménez, E.A.; Galindo, A. Clinical implementation of the sFlt-1/PlGF ratio to identify preeclampsia and fetal growth restriction: A prospective cohort study. Pregnancy Hypertens. 2018, 13, 279–285. [Google Scholar] [CrossRef]
- Madar, H.; Goffinet, F.; Seco, A.; Rozenberg, P.; Dupont, C.; Deneux-Tharaux, C. Severe Acute Maternal Morbidity in Twin Compared with Singleton Pregnancies. Obstet. Gynecol. 2019, 133, 1141–1150. [Google Scholar] [CrossRef]
- De La Calle, M.; Delgado, J.L.; Verlohren, S.; Escudero, A.I.; Bartha, J.L.; Campillos, J.M.; De La Cruz, A.A.; Chantraine, F.; Hernández, J.G.; Herraiz, I.; et al. Gestational Age-Specific Reference Ranges for the sFlt-1/PlGF Immunoassay Ratio in Twin Pregnancies. Fetal Diagn. Ther. 2021, 48, 288–296. [Google Scholar] [CrossRef]
- Perales, A.; Delgado, J.L.; de la Calle, M.; García-Hernández, J.A.; Escudero, A.I.; Campillos, J.M.; Sarabia, M.D.; Laíz, B.; Duque, M.; Navarro, M.; et al. sFlt-1/PlGF for prediction of early-onset pre-eclampsia: STEPS (Study of Early Pre-eclampsia in Spain). Ultrasound Obstet. Gynecol. 2017, 50, 373–382. [Google Scholar] [CrossRef]
- Chappell, L.C.; Duckworth, S.; Seed, P.T.; Griffin, M.; Myers, J.; Mackillop, L.; Simpson, N.; Waugh, J.; Anumba, D.; Kenny, L.C.; et al. Diagnostic accuracy of placental growth factor in women with suspected preeclampsia: A prospective multicenter study. Circulation 2013, 128, 2121–2131. [Google Scholar] [CrossRef]
- Griffin, M.; Seed, P.T.; Duckworth, S.; North, R.; Myers, J.; Mackillop, L.; Simpson, N.; Waugh, J.; Anumba, D.; Kenny, L.C.; et al. Predicting delivery of a small-for-gestational-age infant and adverse perinatal outcome in women with suspected pre-eclampsia. Ultrasound Obstet. Gynecol. 2018, 51, 387–395. [Google Scholar] [CrossRef]
- Rana, S.; Schnettler, W.T.; Powe, C.; Wenger, J.; Salahuddin, S.; Cerdeira, A.S.; Verlohren, S.; Perschel, F.H.; Arany, Z.; Lim, K.-H.; et al. Clinical characterization and outcomes of preeclampsia with normal angiogenic profile. Hypertens. Pregnancy 2013, 32, 189–201. [Google Scholar] [CrossRef]
- Rana, S.; Powe, C.E.; Salahuddin, S.; Verlohren, S.; Perschel, F.H.; Levine, R.J.; Lim, K.-H.; Wenger, J.B.; Thadhani, R.; Karumanchi, S.A. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation 2012, 125, 911–919. [Google Scholar] [CrossRef]
- Heimberger, S.; Mueller, A.; Ratnaparkhi, R.; Perdigao, J.L.; Rana, S. Angiogenic factor abnormalities and risk of peripartum complications and prematurity among urban predominantly obese parturients with chronic hypertension. Pregnancy Hypertens. 2020, 20, 124–130. [Google Scholar] [CrossRef]
- Faupel-Badger, J.M.; McElrath, T.F.; Lauria, M.; Houghton, L.C.; Lim, K.H.; Parry, S.; Cantonwine, D.; Lai, G.; Karumanchi, S.A.; Hoover, R.N.; et al. Maternal circulating angiogenic factors in twin and singleton pregnancies. Am. J. Obstet. Gynecol. 2015, 212, 636.e1–636.e8. [Google Scholar] [CrossRef]



| Twin Pregnancies without Placental Dysfunction (n = 49) | Twin Pregnancies with Placental Dysfunction (n = 21) | p Value | |
|---|---|---|---|
| BMI | 23.7 (4.0) | 24.1 (4.3) | 0.776 |
| Age | 35.0 (4.6) | 35.8 (4.5) | 0.512 |
| ART | 26 (53.06%) | 14 (66.67%) | 0.172 |
| Nulliparous | 35 (71.42%) | 17 (80.95%) | |
| Smoking | 6 (12.24%) | 1 (4.76%) | |
| Ethnicity: Caucasian | 46 (93.88%) | 20 (95.24%) | |
| Ethnicity: Black | 1 (2.04%) | ||
| Ethnicity: Asian | 2 (4.08%) | 1 (4.76%) | |
| Dichorionic | 44 (89.80%) | 16 (76.19%) | 0.154 |
| Monochorionic | 5 (10.20%) | 5 (23.81%) | |
| High-risk of early-onset PE in first trimester | 5 (10.20%) | 4 (19.05%) | |
| Low-risk of early-onset PE in first trimester | 43 (87.76%) | 16 (76.19%) | |
| PAPP-A levels, week 10 | 6.6 (9.2) | 3.4 (2.6) | 0.044 |
| Mean uterine artery pulsatility index | 1.2 (0.4) | 1.4 (0.5) | 0.134 |
| Blood pressure, week 12 | 85.7 (9.3) | 90.1 (11.2) | 0.069 |
| Blood pressure, week 24 | 85.7 (8.4) | 88.6 (10.9) | 0.238 |
| Blood pressure, week 32 | 87.0 (9.2) | 98.2 (10.9) | <0.001 |
| Twin Pregnancies without Placental Dysfunction (n = 49) | Twin Pregnancies with Placental Dysfunction (n = 21) | p Value | |
|---|---|---|---|
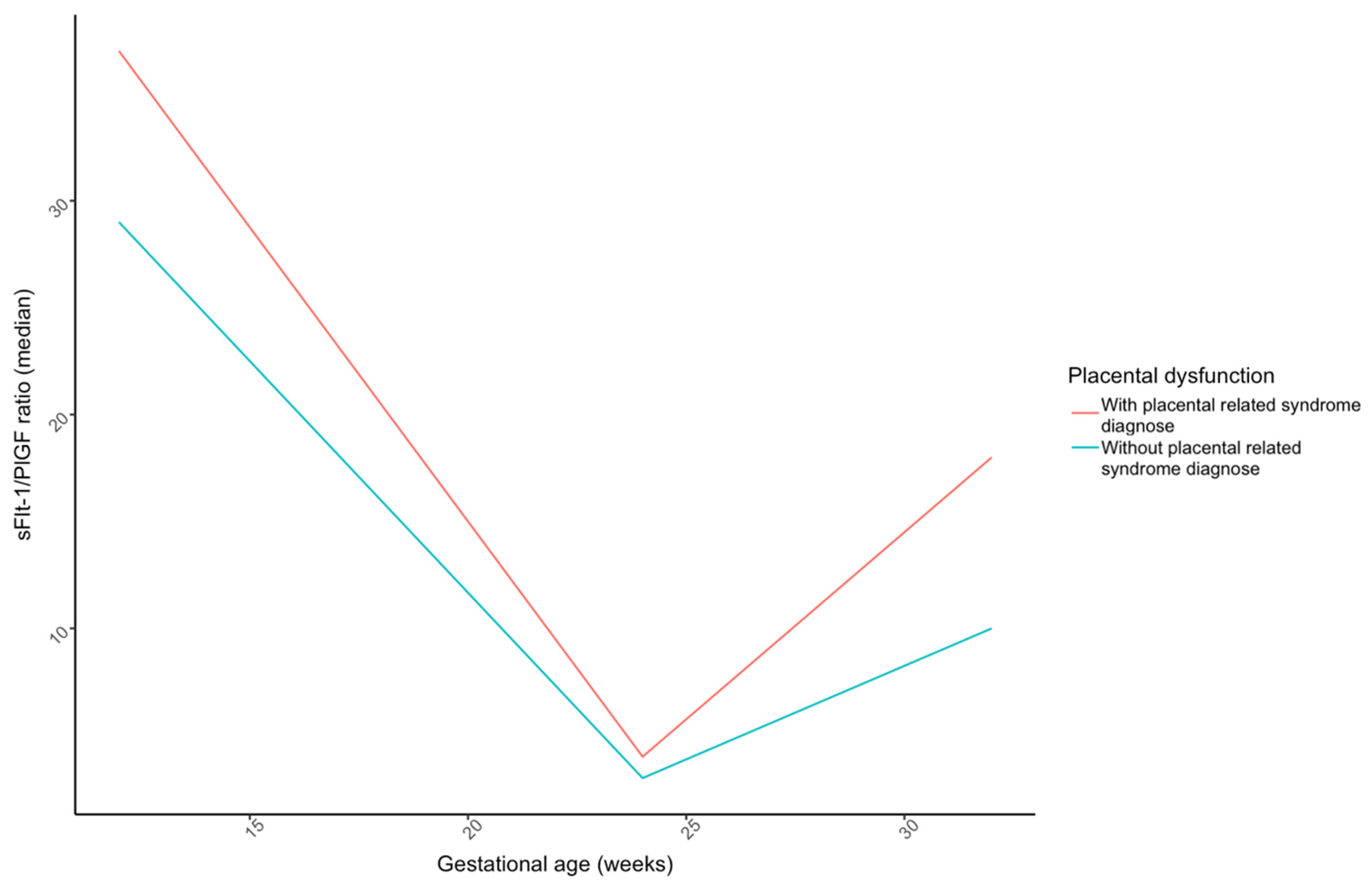
| sFlt-1/PlGF ratio—week 12 | 33.0 (19.2) | 46.0 (36.0) | 0.109 |
| sFlt-1/PlGF ratio—week 24 | 4.0 (2.9) | 6.1 (5.7) | 0.496 |
| sFlt-1/PlGF ratio—week 32 | 13.6 (12.0) | 31.6 (32.8) | 0.007 |
| sFlt-1—week 12 | 2541.4 (918) | 2350.3 (902.7) | 0.19 |
| sFlt-1—week 24 | 3258.5 (1585) | 3513.8 (1636.5) | 0.31 |
| sFlt-1—week 32 | 5917.4 (4318) | 6975.7 (4644.5) | 0.14 |
| PlGF—week 12 | 106.2 (73.3) | 69.4 (34.1) | 0.021 |
| PlGF—week 24 | 1184.7 (577) | 854.4 (702.7) | 0.11 |
| PlGF—week 32 | 852.6 (619) | 386.6 (343.7) | 0.02 |
| Twin Pregnancies without Placental Dysfunction (n = 49) | Twin Pregnancies with Placental Dysfunction (n = 21) | p Value | |
|---|---|---|---|
| Gestational age at birth (days) | 254.9 (8.4) | 245.2 (16.2) | 0.004 |
| Weight first newborn | 2451.7 (420) | 2072 (450.7) | 0.002 |
| Weight second newborn | 2373.7 (444.8) | 2015.2 (507.1) | 0.007 |
| Apgar 5′ for first newborn | 9.9 | 9.6 | 0.389 |
| Apgar 5′ for second newborn | 9.8 | 9.6 | 0.256 |
| Arterial pH first newborn | 7.3 (0.1) | 7.3 (0.1) | 0.317 |
| Arterial pH second newborn | 7.3 (0.1) | 7.3 (0.1) | 0.989 |
| Admission days | 2.5 (6.4) | 13.4 (21.3) | 0.001 |
| Prediction | Cutoff | AUC | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|---|---|
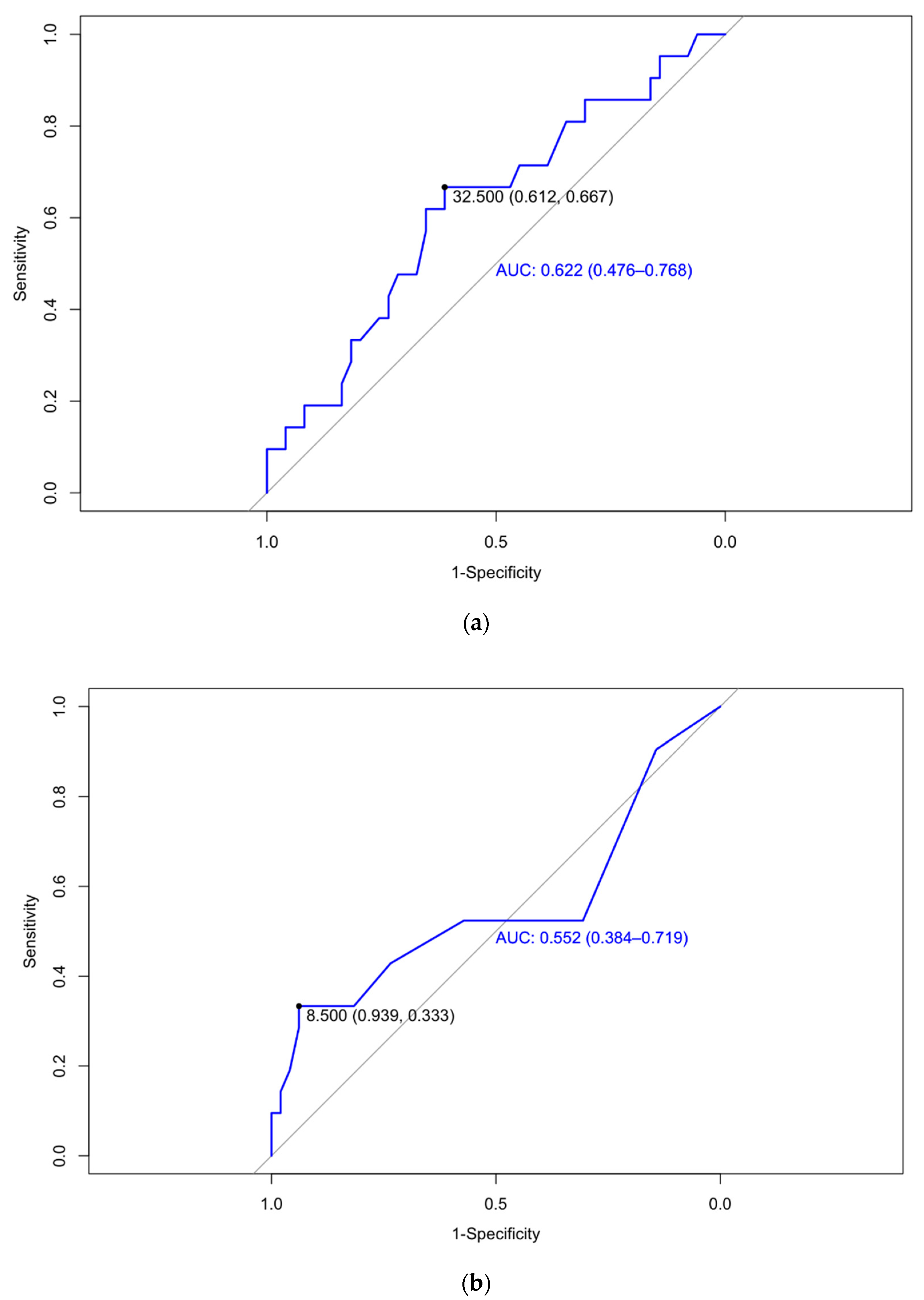
| Week 12 | Placental dysfunction | 32.5 | 0.622 (0.476–0.768) | 66.7 | 61.2 | 42.4 | 81.1 |
| Week 24 | Placental dysfunction | 8.5 | 0.552 (0.384–0.719) | 33.33 | 93.88 | 70.00 | 76.67 |
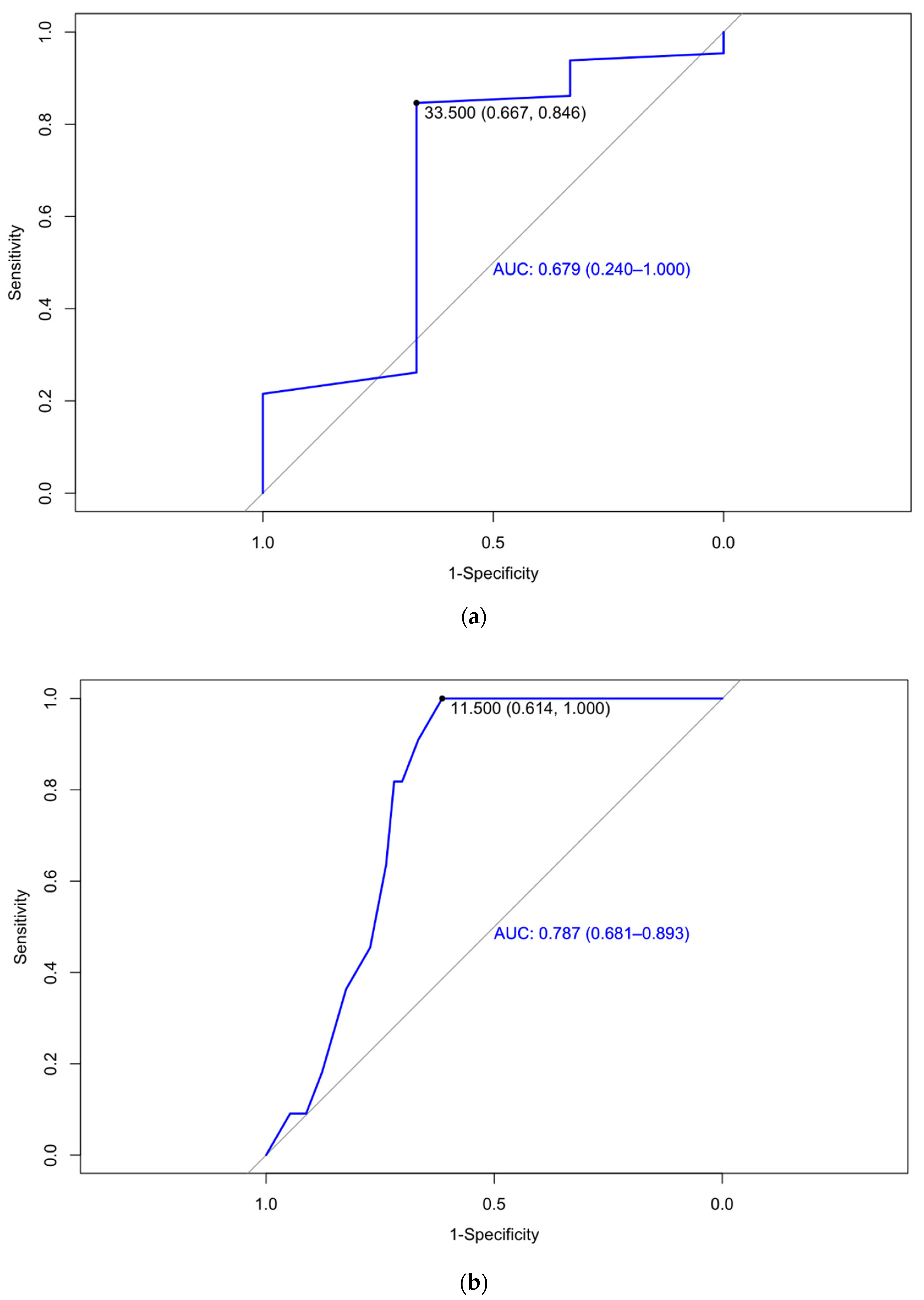
| Birth weight under 1500 g | 33.5 | 0.679 (0.240–1.00) | 66.7 | 84.6 | 98.21 | 16.67 | |
| Week 32 | Placental dysfunction | 30.5 | 0.709 (0.570–0.849) | 45 | 87.8 | 60 | 79.6 |
| Birth weight under 2500 g | 11.5 | 61.4 | 100 | 33.33 | 100 | 0.787 (0.681–0.893) |
| Measurement | Event | PCC | |
|---|---|---|---|
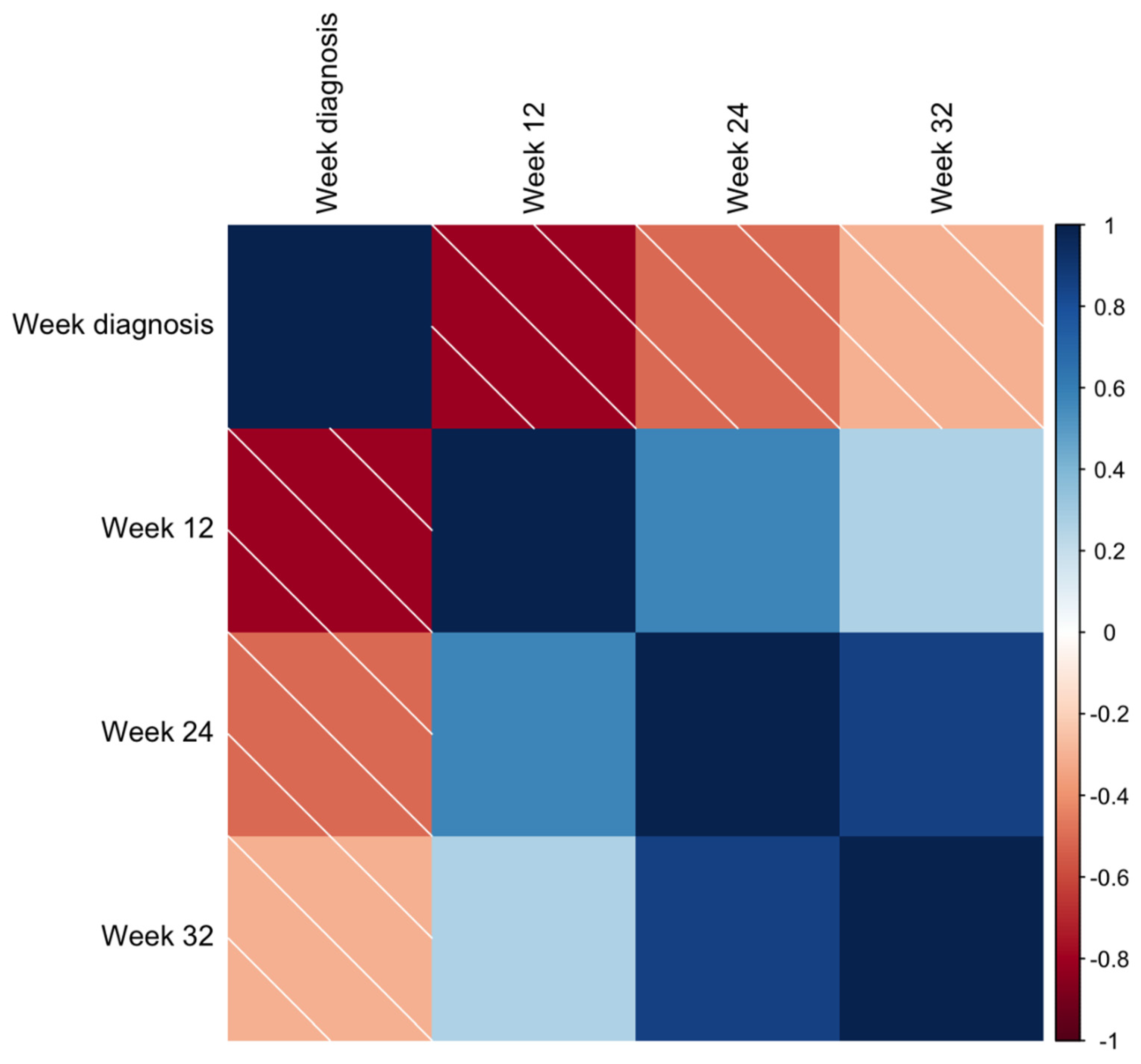
| Week 12 | sFlt-1/PlGF ratio | Week of pre-eclampsia diagnosis | −0.81; p < 0.05 |
| Days of neonatal admission | 0.36; p < 0.05 | ||
| PlGF | Week of pre-eclampsia diagnosis | 0.77; p < 0.05 | |
| sFlt-1 | Week of pre-eclampsia diagnosis | −0.38; p < 0.10 | |
| Week 24 | sFlt-1/PlGF ratio | Birth weight (both newborns) | −0.29 and −0.36; p < 0.05 |
| Days of neonatal admission | 0.50; p < 0.05 | ||
| PlGF | Week of FGR diagnosis | 0.43; p < 0.05 | |
| Week 32 | sFlt-1/PlGF ratio | Birth weight (both newborns) | 0.41 and −0.33; p < 0.05 |
| Days of neonatal admission | 0.62; p < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satorres-Pérez, E.; Martínez-Varea, A.; Novillo-Del Álamo, B.; Morales-Roselló, J.; Diago-Almela, V. The sFlt-1/PlGF Ratio at 12, 24, and 32 Weeks Gestation in Twin Pregnancies as a Predictor of Placental Dysfunction. J. Clin. Med. 2024, 13, 1784. https://doi.org/10.3390/jcm13061784
Satorres-Pérez E, Martínez-Varea A, Novillo-Del Álamo B, Morales-Roselló J, Diago-Almela V. The sFlt-1/PlGF Ratio at 12, 24, and 32 Weeks Gestation in Twin Pregnancies as a Predictor of Placental Dysfunction. Journal of Clinical Medicine. 2024; 13(6):1784. https://doi.org/10.3390/jcm13061784
Chicago/Turabian StyleSatorres-Pérez, Elena, Alicia Martínez-Varea, Blanca Novillo-Del Álamo, José Morales-Roselló, and Vicente Diago-Almela. 2024. "The sFlt-1/PlGF Ratio at 12, 24, and 32 Weeks Gestation in Twin Pregnancies as a Predictor of Placental Dysfunction" Journal of Clinical Medicine 13, no. 6: 1784. https://doi.org/10.3390/jcm13061784
APA StyleSatorres-Pérez, E., Martínez-Varea, A., Novillo-Del Álamo, B., Morales-Roselló, J., & Diago-Almela, V. (2024). The sFlt-1/PlGF Ratio at 12, 24, and 32 Weeks Gestation in Twin Pregnancies as a Predictor of Placental Dysfunction. Journal of Clinical Medicine, 13(6), 1784. https://doi.org/10.3390/jcm13061784







