Unraveling the Connection: Pancreatic Cancer Cells and Schwann Cells
Abstract
1. Neural Invasion in Pancreatic Cancer
2. Schwann Cells’ Plasticity and Their Involvement in Cancer Neural Invasion
3. Enrollment and Activation of Schwann Cells in PDAC
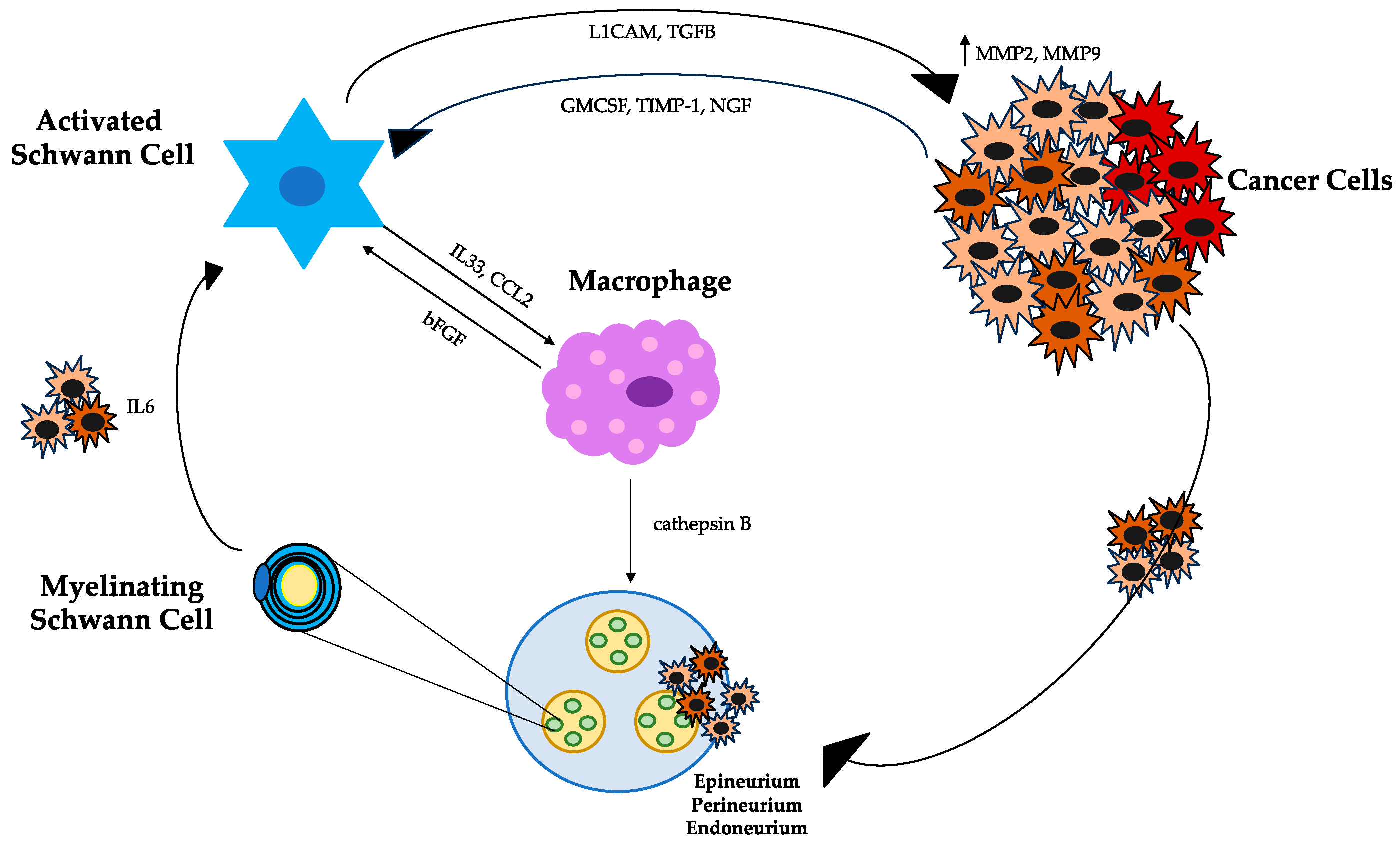
4. Schwann Cells, Tumor Cells, and Macrophages
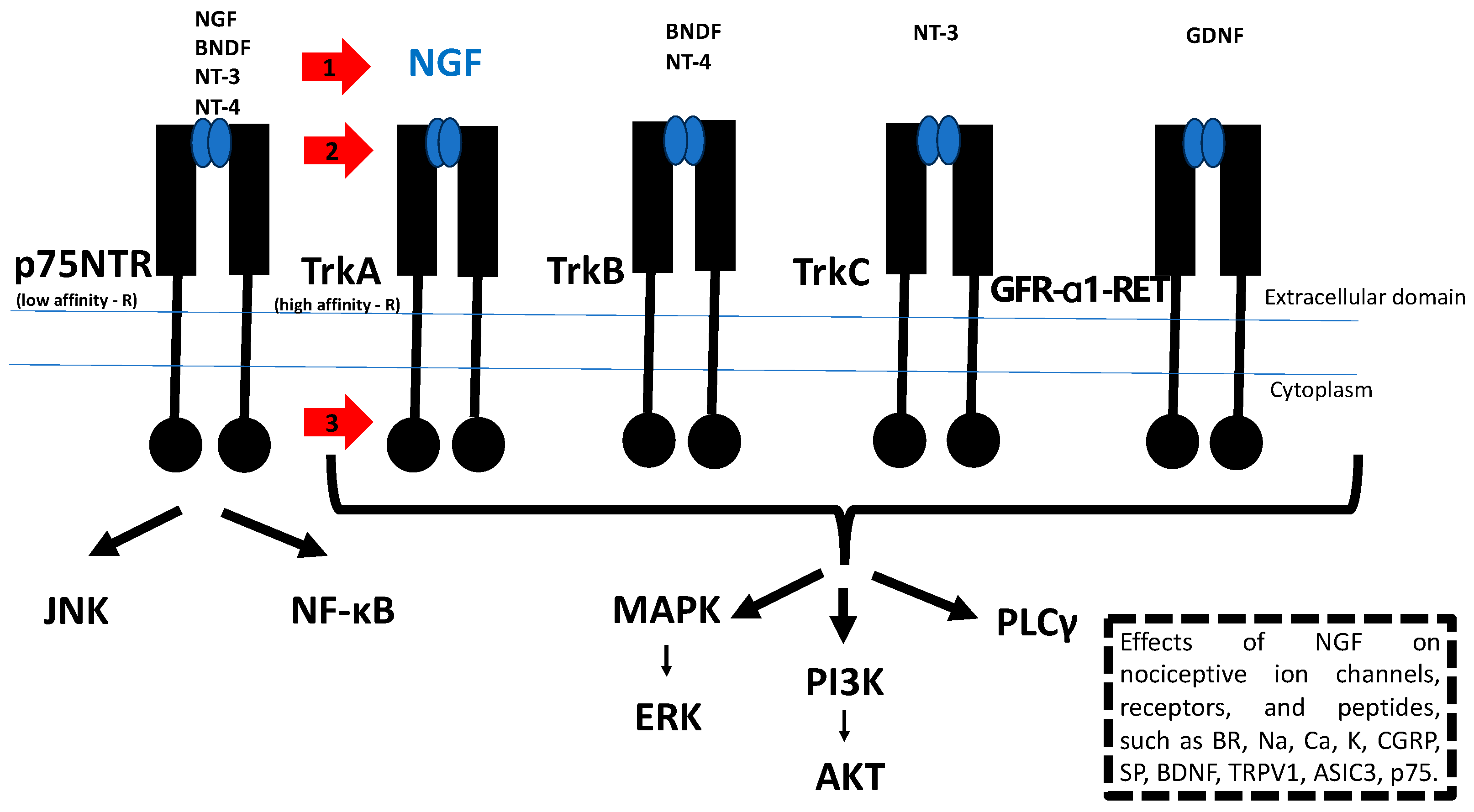
5. NGF/TrkA Signaling as a Therapeutic Target for the Treatment of Tumor Progression and Cancer Pain
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, G.; Pellegatta, M.; Crippa, S.; Lena, M.S.; Belfiori, G.; Doglioni, C.; Taveggia, C.; Falconi, M. Nerves and Pancreatic Cancer: New Insights into a Dangerous Relationship. Cancers 2019, 11, 893. [Google Scholar] [CrossRef] [PubMed]
- Bapat, A.A.; Hostetter, G.; Von Hoff, D.D.; Han, H. Perineural Invasion and Associated Pain in Pancreatic Cancer. Nat. Rev. Cancer 2011, 11, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Selvaggi, F.; Melchiorre, E.; Casari, I.; Cinalli, S.; Cinalli, M.; Aceto, G.M.; Cotellese, R.; Garajova, I.; Falasca, M. Perineural Invasion in Pancreatic Ductal Adenocarcinoma: From Molecules towards Drugs of Clinical Relevance. Cancers 2022, 14, 5793. [Google Scholar] [CrossRef]
- Crippa, S.; Pergolini, I.; Javed, A.A.; Honselmann, K.C.; Weiss, M.J.; Di Salvo, F.; Burkhart, R.; Zamboni, G.; Belfiori, G.; Ferrone, C.R.; et al. Implications of Perineural Invasion on Disease Recurrence and Survival After Pancreatectomy for Pancreatic Head Ductal Adenocarcinoma. Ann. Surg. 2020, 276, 378–385. [Google Scholar] [CrossRef]
- Garajová, I.; Balsano, R.; Donati, V.; Gnetti, L.; Capula, M.; Leonardi, F.; Valle, R.D.; Ravaioli, M.; Gelsomino, F.; Giovannetti, E. Comment on “Implications of Perineural Invasion on Disease Recurrence and Survival after Pancreatectomy for Pancreatic Head Ductal Adenocarcinoma by Crippa et Al”. Ann. Surg. 2022, 276, e136–e137. [Google Scholar] [CrossRef]
- Azam, S.H.; Pecot, C.V. Cancer’s Got Nerve: Schwann Cells Drive Perineural Invasion. J. Clin. Investig. 2016, 126, 1242–1244. [Google Scholar] [CrossRef]
- Wakiya, T.; Ishido, K.; Yoshizawa, T.; Kanda, T.; Hakamada, K. Roles of the Nervous System in Pancreatic Cancer. Ann. Gastroenterol. Surg. 2021, 5, 623–633. [Google Scholar] [CrossRef]
- Li, J.; Kang, R.; Tang, D. Cellular and Molecular Mechanisms of Perineural Invasion of Pancreatic Ductal Adenocarcinoma. Cancer Commun. 2021, 41, 642–660. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, Z.; Cao, Q.; Zhao, H.; Guo, Y.; Liu, T.; Li, J. Perineural Invasion-Associated Biomarkers for Tumor Development. Biomed. Pharmacother. 2022, 155, 113691. [Google Scholar] [CrossRef]
- Ayala, G. Neuroepithelial Interactions in Cancer. Annu. Rev. Pathol. Mech. Dis. 2023, 18, 493–514. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chen, S.; Chen, M. Schwann Cells in the Tumor Microenvironment: Need More Attention. J. Oncol. 2022, 2022, 1058667. [Google Scholar] [CrossRef]
- Wei, Z.; Fei, Y.; Su, W.; Chen, G. Emerging Role of Schwann Cells in Neuropathic Pain: Receptors, Glial Mediators and Myelination. Front. Cell Neurosci. 2019, 13, 116. [Google Scholar] [CrossRef]
- Deborde, S.; Wong, R.J. How Schwann Cells Facilitate Cancer Progression in Nerves. Cell. Mol. Life Sci. 2017, 74, 4405–4420. [Google Scholar] [CrossRef]
- Zhang, W.J.; Wu, C.L.; Liu, J. Schwann Cells as a Target Cell for the Treatment of Cancer Pain. Glia 2023, 71, 2309–2322. [Google Scholar] [CrossRef]
- Demir, I.E.; Friess, H.; Ceyhan, G.O. Neural Plasticity in Pancreatitis and Pancreatic Cancer. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 649–659. [Google Scholar] [CrossRef]
- Demir, I.E.; Tieftrunk, E.; Schorn, S.; Saricaoglu, Ö.C.; Pfitzinger, P.L.; Teller, S.; Wang, K.; Waldbaur, C.; Kurkowski, M.U.; Wörmann, S.M.; et al. Activated Schwann Cells in Pancreatic Cancer Are Linked to Analgesia via Suppression of Spinal Astroglia and Microglia. Gut 2016, 65, 1001–1014. [Google Scholar] [CrossRef]
- Ribeiro-Resende, V.T.; Koenig, B.; Nichterwitz, S.; Oberhoffner, S.; Schlosshauer, B. Strategies for Inducing the Formation of Bands of Büngner in Peripheral Nerve Regeneration. Biomaterials 2009, 30, 5251–5259. [Google Scholar] [CrossRef]
- Deborde, S.; Omelchenko, T.; Lyubchik, A.; Zhou, Y.; He, S.; McNamara, W.F.; Chernichenko, N.; Lee, S.Y.; Barajas, F.; Chen, C.H.; et al. Schwann Cells Induce Cancer Cell Dispersion and Invasion. J. Clin. Investig. 2016, 126, 538–1554. [Google Scholar] [CrossRef]
- Nocera, G.; Jacob, C. Mechanisms of Schwann Cell Plasticity Involved in Peripheral Nerve Repair after Injury. Cell. Mol. Life Sci. 2020, 77, 3977–3989. [Google Scholar] [CrossRef]
- Deborde, S.; Gusain, L.; Powers, A.; Marcadis, A.; Yu, Y.; Chen, C.H.; Frants, A.; Kao, E.; Tang, L.H.; Vakiani, E.; et al. Reprogrammed Schwann Cells Organize into Dynamic Tracks That Promote Pancreatic Cancer Invasion. Cancer Discov. 2022, 12, 2454–2473. [Google Scholar] [CrossRef]
- Arthur-Farraj, P. Trick or Treat? Does Cancer Fool Schwann Cells by Mimicking Axons to Promote Metastasis into Nerves? Neural Regen. Res. 2023, 18, 1727. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The Repair Schwann Cell and Its Function in Regenerating Nerves. J. Physiol. 2016, 594, 3521–3531. [Google Scholar] [CrossRef]
- Swanson, B.J.; McDermott, K.M.; Singh, P.K.; Eggers, J.P.; Crocker, P.R.; Hollingsworth, M.A. MUC1 Is a Counter-Receptor for Myelin-Associated Glycoprotein (Siglec-4a) and Their Interaction Contributes to Adhesion in Pancreatic Cancer Perineural Invasion. Cancer Res. 2007, 67, 10222–10229. [Google Scholar] [CrossRef]
- Na’ara, S.; Amit, M.; Gil, Z. L1CAM Induces Perineural Invasion of Pancreas Cancer Cells by Upregulation of Metalloproteinase Expression. Oncogene 2019, 38, 596–608. [Google Scholar] [CrossRef]
- Roger, E.; Martel, S.; Bertrand-Chapel, A.; Depollier, A.; Chuvin, N.; Pommier, R.M.; Yacoub, K.; Caligaris, C.; Cardot-Ruffino, V.; Chauvet, V.; et al. Schwann Cells Support Oncogenic Potential of Pancreatic Cancer Cells through TGFβ Signaling. Cell Death Dis. 2019, 10, 886. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; He, R.; Yang, W.; Zhang, Y.; Yuan, Q.; Wang, J.; Liu, Y.; Chen, S.; Zhang, S.; Zhang, W.; et al. Autophagic Schwann Cells Promote Perineural Invasion Mediated by the NGF/ATG7 Paracrine Pathway in Pancreatic Cancer. J. Exp. Clin. Cancer Res. 2022, 41, 48. [Google Scholar] [CrossRef]
- Mu, W.; Wang, Z.; Zöller, M. Ping-Pong—Tumor and Host in Pancreatic Cancer Progression. Front. Oncol. 2019, 9, 1359. [Google Scholar] [CrossRef]
- Stratton, J.A.; Holmes, A.; Rosin, N.L.; Sinha, S.; Vohra, M.; Burma, N.E.; Trang, T.; Midha, R.; Biernaskie, J. Macrophages Regulate Schwann Cell Maturation after Nerve Injury. Cell Rep. 2018, 24, 2561–2572.e6. [Google Scholar] [CrossRef] [PubMed]
- Hirose, M.; Kuroda, Y.; Murata, E. NGF/TrkA Signaling as a Therapeutic Target for Pain. Pain Pract. 2016, 16, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Halvorson, K.G.; Kubota, K.; Sevcik, M.A.; Lindsay, T.H.; Sotillo, J.E.; Ghilardi, J.R.; Rosol, T.J.; Boustany, L.; Shelton, D.L.; Mantyh, P.W. A Blocking Antibody to Nerve Growth Factor Attenuates Skeletal Pain Induced by Prostate Tumor Cells Growing in Bone. Cancer Res. 2005, 65, 9426–9435. [Google Scholar] [CrossRef]
- Sevcik, M.A.; Ghilardi, J.R.; Peters, C.M.; Lindsay, T.H.; Halvorson, K.G.; Jonas, B.M.; Kubota, K.; Kuskowski, M.A.; Boustany, L.; Shelton, D.L.; et al. Anti-NGF Therapy Profoundly Reduces Bone Cancer Pain and the Accompanying Increase in Markers of Peripheral and Central Sensitization. Pain 2005, 115, 128–141. [Google Scholar] [CrossRef]
- Wang, W.; Chen, J.; Guo, X. The Role of Nerve Growth Factor and Its Receptors in Tumorigenesis and Cancer Pain. Biosci. Trends 2014, 8, 68–74. [Google Scholar] [CrossRef]
- Arthur-Farraj, P.J.; Latouche, M.; Wilton, D.K.; Quintes, S.; Chabrol, E.; Banerjee, A.; Woodhoo, A.; Jenkins, B.; Rahman, M.; Turmaine, M.; et al. C-Jun Reprograms Schwann Cells of Injured Nerves to Generate a Repair Cell Essential for Regeneration. Neuron 2012, 75, 633–647. [Google Scholar] [CrossRef]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-ΚB Pathway for the Therapy of Diseases: Mechanism and Clinical Study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef]
- Fazal, S.V.; Gomez-Sanchez, J.A.; Wagstaff, L.J.; Musner, N.; Otto, G.; Janz, M.; Mirsky, R.; Jessen, K.R. Graded Elevation of C-Jun in Schwann Cells in Vivo: Gene Dosage Determines Effects on Development, Remyelination, Tumorigenesis, and Hypomyelination. J. Neurosci. 2017, 37, 12297–12313. [Google Scholar] [CrossRef]
- Fallon, M.; Sopata, M.; Dragon, E.; Brown, M.T.; Viktrup, L.; West, C.R.; Bao, W.; Agyemang, A. A Randomized Placebo-Controlled Trial of the Anti-Nerve Growth Factor Antibody Tanezumab in Subjects with Cancer Pain Due to Bone Metastasis. Oncologist 2023, 28, e1268–e1278. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Tang, L.; Xie, Y.; Xianyu, Y.; Zhang, L.; Wang, P.; Hamada, Y.; Jiang, K.; Zheng, W.; Jiang, X. Gold Nanoclusters-Assisted Delivery of NGF SiRNA for Effective Treatment of Pancreatic Cancer. Nat. Commun. 2017, 8, 15130. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Peng, X.; Yang, S.; Wei, S.; Fan, Q.; Liu, J.; Yang, L.; Li, H. Targeting Tumor Innervation: Premises, Promises, and Challenges. Cell Death Discov. 2022, 8, 131. [Google Scholar] [CrossRef] [PubMed]
- Yurteri, Ü.; Çifcibaşı, K.; Friess, H.; Ceyhan, G.O.; Istvanffy, R.; Demir, I.E. Schwann Cells in Peripheral Cancers: Bystanders or Promoters? Adv. Biol. 2022, 6, 2200033. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garajová, I.; Trentini, F.; Leonardi, F.; Giovannetti, E. Unraveling the Connection: Pancreatic Cancer Cells and Schwann Cells. J. Clin. Med. 2024, 13, 1785. https://doi.org/10.3390/jcm13061785
Garajová I, Trentini F, Leonardi F, Giovannetti E. Unraveling the Connection: Pancreatic Cancer Cells and Schwann Cells. Journal of Clinical Medicine. 2024; 13(6):1785. https://doi.org/10.3390/jcm13061785
Chicago/Turabian StyleGarajová, Ingrid, Francesca Trentini, Francesco Leonardi, and Elisa Giovannetti. 2024. "Unraveling the Connection: Pancreatic Cancer Cells and Schwann Cells" Journal of Clinical Medicine 13, no. 6: 1785. https://doi.org/10.3390/jcm13061785
APA StyleGarajová, I., Trentini, F., Leonardi, F., & Giovannetti, E. (2024). Unraveling the Connection: Pancreatic Cancer Cells and Schwann Cells. Journal of Clinical Medicine, 13(6), 1785. https://doi.org/10.3390/jcm13061785






