The Frontal Fibrosing Alopecia Treatment Dilemma
Abstract
:1. Aim and Methods
2. Introduction
3. Clinical Features and Diagnosis
4. Disease Severity
4.1. Five-Point Severity Scale
4.2. FFASI and FASS
4.3. Frontal Fibrosing Alopecia Global Staging Score and ALODEX-FFA
5. Clinical Course and Prognostic Factors
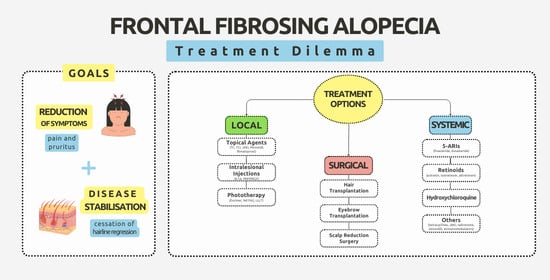
6. Treatment
6.1. Local Treatment
6.1.1. Anti-Inflammatory Agents
Corticosteroids/Calcineurin Inhibitors
JAK Inhibitors (JAKis)
Mechlorethamine
6.1.2. Hair Growth Modulators
Bimatoprost
Minoxidil
6.1.3. Phototherapy
Neodymium YAG Laser (Nd:YAG Laser)
Excimer Laser
Low-Level Light Therapy (LLLT)
6.1.4. Intralesional Treatment
Intralesional Triamcinolone Acetonide (ILTA) Injections
Platelet-Rich Plasma (PRP) and Plasma Rich in Growth Factors (PRGF)
6.2. Systemic Treatment
6.2.1. 5-ARIs
6.2.2. Retinoids
6.2.3. Hydroxychloroquine
6.2.4. Tetracyclines
6.2.5. JAKis
Tofacitinib
Baricitinib
6.2.6. Oral Minoxidil
6.2.7. Naltrexone
6.2.8. Pioglitazone
6.2.9. Other Anti-Inflammatory and Immunosuppressive Agents
Oral Corticosteroids
Calcineurin Inhibitors
Methotrexate
6.3. Surgical Treatment
6.3.1. Hair Transplantation (HT)
6.3.2. Scalp Reduction Surgery
6.3.3. Eyebrow Transplantation
7. Summary
8. Further Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kossard, S. Postmenopausal frontal fibrosing alopecia. Scarring alopecia in a pattern distribution. Arch. Dermatol. 1994, 130, 770–774. [Google Scholar] [CrossRef]
- To, D.; Beecker, J. Frontal Fibrosing Alopecia: Update and Review of Challenges and Successes. J. Cutan. Med. Surg. 2018, 22, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, L.; Rakowska, A. The increasing incidence of frontal fibrosing alopecia. In search of triggering factors. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1579–1580. [Google Scholar] [CrossRef] [PubMed]
- Vañó-Galván, S.; Molina-Ruiz, A.M.; Serrano-Falcón, C.; Arias-Santiago, S.; Rodrigues-Barata, A.R.; Garnacho-Saucedo, G.; Martorell-Calatayud, A.; Fernández-Crehuet, P.; Grimalt, R.; Aranegui, B.; et al. Frontal fibrosing alopecia: A multicenter review of 355 patients. J. Am. Acad. Dermatol. 2014, 70, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Kossard, S.; Lee, M.S.; Wilkinson, B. Postmenopausal frontal fibrosing alopecia: A frontal variant of lichen planopilaris. J. Am. Acad. Dermatol. 1997, 36, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Trager, M.H.; Lavian, J.; Lee, E.Y.; Wilkinson, B. Prevalence estimates for lichen planopilaris and frontal fibrosing alopecia in a New York City health care system. J. Am. Acad. Dermatol. 2021, 84, 1166–1169. [Google Scholar] [CrossRef]
- MacDonald, A.; Clark, C.; Holmes, S. Frontal fibrosing alopecia: A review of 60 cases. J. Am. Acad. Dermatol. 2012, 67, 955–961. [Google Scholar] [CrossRef]
- Porriño-Bustamante, M.L.; García-Lora, E.; Buendía-Eisman, A.; Arias-Santiago, S. Familial frontal fibrosing alopecia in two male families. Int. J. Dermatol. 2019, 58, e178–e180. [Google Scholar] [CrossRef] [PubMed]
- Alegre-Sánchez, A.; Saceda-Corralo, D.; Bernárdez, C.; Molina-Ruiz, A.; Arias-Santiago, S.; Vaño-Galvan, S. Frontal fibrosing alopecia in male patients: A report of 12 cases. J. Eur. Acad. Dermatol. Venereol. 2017, 31, e112–e114. [Google Scholar] [CrossRef] [PubMed]
- Tavakolpour, S.; Mahmoudi, H.; Abedini, R.; Hesari, K.K.; Kiani, A.; Daneshpazhooh, M. Frontal fibrosing alopecia: An update on the hypothesis of pathogenesis and treatment. Int. J. Womens Dermatol. 2019, 5, 116–123. [Google Scholar] [CrossRef]
- Vañó-Galván, S.; Saceda-Corralo, D.; Blume-Peytavi, U.; Cucchía, J.; Dlova, N.C.; Dias, M.F.R.G.; Grimalt, R.; Guzmán-Sánchez, D.; Harries, M.; Ho, A.; et al. Frequency of the Types of Alopecia at Twenty-Two Specialist Hair Clinics: A Multicenter Study. Skin Appendage Disord. 2019, 5, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.T.; Messenger, A.G. Frontal fibrosing alopecia: Clinical presentations and prognosis. Br. J. Dermatol. 2009, 160, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Arrones, O.M.; Saceda-Corralo, D.; Rodrigues-Barata, A.R.; Castellanos-Gonzalez, M.; Fernández-Pugnaire, M.A.; Grimalt, R.; Hermosa-Gelbard, A.; Bernardez, C.; Molina-Ruiz, A.M.; Ormaechea-Perez, N.; et al. Risk factors associated with frontal fibrosing alopecia: A multicentre case-control study. Clin. Exp. Dermatol. 2019, 44, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Donati, A.; Lindgren, B.R.; Abreu, G.; Hordinsky, M. Prevalence of frontal fibrosing alopecia among Brazilian dermatologists: A cross-sectional survey. JAAD Int. 2020, 1, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.M.; Anzai, A.; Duque-Estrada, B.; Farias, D.C.; Melo, D.F.; Mulinari-Brenner, F.; Pinto, G.M.; Abraham, L.S.; Santos, L.D.N.; Pirmez, R.; et al. Risk factors for frontal fibrosing alopecia: A case-control study in a multiracial population. J. Am. Acad. Dermatol. 2021, 84, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Aldoori, N.; Dobson, K.; Holden, C.R.; McDonagh, A.J.; Harries, M.; Messenger, A.G. Frontal fibrosing alopecia: Possible association with leave-on facial skin care products and sunscreens; a questionnaire study. Br. J. Dermatol. 2016, 175, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Porriño-Bustamante, M.L.; Fernández-Pugnaire, M.A.; Arias-Santiago, S. Frontal Fibrosing Alopecia: A Review. J. Clin. Med. 2021, 10, 1805. [Google Scholar] [CrossRef] [PubMed]
- Banka, N.; Mubki, T.; Bunagan, M.J.; McElwee, K.; Shapiro, J. Frontal fibrosing alopecia: A retrospective clinical review of 62 patients with treatment outcome and long-term follow-up. Int. J. Dermatol. 2014, 53, 1324–1330. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, L.; Kaczorowska, A.; Waśkiel-Burnat, A.; Rakowska, A.; Olszewska, M. Treatment of diseases associated with cicatricial alopecia. Dermatol. Rev./Przegląd Dermatol. 2022, 109, 32–42. [Google Scholar] [CrossRef]
- Ross, E.K.; Tan, E.; Shapiro, J. Update on primary cicatricial alopecias. J. Am. Acad. Dermatol. 2005, 53, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Elloudi, S.; Gallouj, S.; Meziane, M.; Mernissi, F.Z.; Rimani, M. Alopécie frontale fibrosante: Étude prospective de 20 cas [Frontal fibrosing alopecia: A prospective study of 20 cases]. Ann. Dermatol. Venereol. 2017, 144, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, L.; Olszewska, M.; Rakowska, A.; Slowinska, M. Trichoscopy update 2011. J. Dermatol. Case Rep. 2011, 5, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Melo, D.F.; de Mattos Barreto, T.; de Souza Albernaz, E.; Haddad, N.C.; Tortelly, V.D. Ten clinical clues for the diagnosis of frontal fibrosing alopecia. Indian J. Dermatol. Venereol. Leprol. 2019, 85, 559–564. [Google Scholar] [PubMed]
- Imhof, R.L.; Villalpando, B.; Proffer, S.; Cantwell, H.; Tolkachjov, S.; Torgerson, R. Eyebrow hair loss: Review of causes and treatment options. Clin. Cosmet. Investig. Dermatol. 2020, 13, 935–940. [Google Scholar]
- Katoulis, A.C.; Damaskou, V.; Diamanti, K.; Pouliakis, A.; Mortaki, D.; Zacharatou, A.; Bozi, E.; Sgouros, D.; Panayiotides, I.G. Eyebrow involvement in frontal fibrosing alopecia: A clinicopathologic cohort study for the reversibility of hair loss. J. Am. Acad. Dermatol. 2020, 82, 755–757. [Google Scholar] [CrossRef] [PubMed]
- Strazzulla, L.C.; Avila, L.; Li, X.; Sicco, K.L.; Shapiro, J. Prognosis, treatment, and disease outcomes in frontal fibrosing alopecia: A retrospective review of 92 cases. J. Am. Acad. Dermatol. 2018, 78, 203–205. [Google Scholar] [CrossRef] [PubMed]
- López-Pestaña, A.; Tuneu, A.; Lobo, C.; Ormaechea, N.; Zubizarreta, J.; Vildosola, S.; Del Alcazar, E. Facial lesions in frontal fibrosing alopecia (FFA): Clinicopathological features in a series of 12 cases. J. Am. Acad. Dermatol. 2015, 73, e1–e987. [Google Scholar] [CrossRef]
- Ormaechea-Pérez, N.; López-Pestaña, A.; Zubizarreta-Salvador, J.; Jaka-Moreno, A.; Panes-Rodrigues, A.; Tuneu-Valls, A. Frontal Fibrosing Alopecia in Men: Presentations in 12 Cases and a Review of the Literature. Alopecia frontal fibrosante en el varón: Presentación de 12 casos y revisión de la literatura. Actas Dermosifiliogr. 2016, 107, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Pirmez, R.; Donati, A.; Valente, N.S.; Sodré, C.; Tosti, A. Glabellar red dots in frontal fibrosing alopecia: A further clinical sign of vellus follicle involvement. Br. J. Dermatol. 2014, 170, 745–746. [Google Scholar] [CrossRef] [PubMed]
- Inui, S.; Nakajima, T.; Shono, F.; Itami, S. Dermoscopic findings in frontal fibrosing alopecia: Report of four cases. Int. J. Dermatol. 2008, 47, 796–799. [Google Scholar] [CrossRef] [PubMed]
- Saceda-Corralo, D.; Moreno-Arrones, Ó.M.; Fonda-Pascual, P.; Pindado-Ortega, C.; Hermosa-Gelbard, A.; Rodrigues-Barata, A.R.; Vañó-Galván, S. Steroid-induced changes noted on trichoscopy of patients with frontal fibrosing alopecia. J. Am. Acad. Dermatol. 2018, 79, 956–957. [Google Scholar] [CrossRef] [PubMed]
- Waśkiel-Burnat, A.; Rakowska, A.; Kurzeja, M.; Czuwara, J.; Sikora, M.; Olszewska, M.; Rudnicka, L. The value of dermoscopy in diagnosing eyebrow loss in patients with alopecia areata and frontal fibrosing alopecia. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Stefanato, C.M. Histopathology of alopecia: A clinicopathological approach to diagnosis. Histopathology 2010, 56, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Hurt, M.A.; Weedon, D. Weedon D. Weedon’s Skin Pathology. Dermatol. Pract. Concept 2012, 2, 79–82. [Google Scholar] [CrossRef]
- Tolkachjov, S.N.; Chaudhry, H.M.; Imhof, R.L.; Camilleri, M.J.; Torgerson, R.R. Reply to: “Updated diagnostic criteria for frontal fibrosing alopecia”. J. Am. Acad. Dermatol. 2018, 78, e23–e24. [Google Scholar] [CrossRef] [PubMed]
- Vañó-Galván, S.; Saceda-Corralo, D.; Moreno-Arrones, Ó.M.; Camacho-Martinez, F.M. Updated diagnostic criteria for frontal fibrosing alopecia. J. Am. Acad. Dermatol. 2018, 78, e21–e22. [Google Scholar] [CrossRef] [PubMed]
- Olsen, E.A.; Harries, M.; Tosti, A.; Bergfeld, W.; Blume-Peytavi, U.; Callender, V.; Chasapi, V.; Correia, O.; Cotsarelis, G.; Dhurat, R.; et al. Guidelines for clinical trials of frontal fibrosing alopecia: Consensus recommendations from the International FFA Cooperative Group (IFFACG). Br. J. Dermatol. 2021, 185, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Holmes, S.; Ryan, T.; Young, D.; Harries, M. British Hair and Nail Society. Frontal Fibrosing Alopecia Severity Index (FFASI): A validated scoring system for assessing frontal fibrosing alopecia. Br. J. Dermatol. 2016, 175, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Saceda-Corralo, D.; Moreno-Arrones, Ó.M.; Fonda-Pascual, P.; Pindado-Ortega, C.; Buendía-Castaño, D.; Alegre-Sánchez, A.; Segurado-Miravalles, G.; Rodrigues-Barata, A.R.; Jaén-Olasolo, P.; Vaño-Galván, S. Development and validation of the Frontal Fibrosing Alopecia Severity Score. J. Am. Acad. Dermatol. 2018, 78, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Iorizzo, M.; Tosti, A. Frontal Fibrosing Alopecia: An Update on Pathogenesis, Diagnosis, and Treatment. Am. J. Clin. Dermatol. 2019, 20, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Arrones, O.M.; Saceda-Corralo, D.; Fonda-Pascual, P.; Rodrigues-Barata, A.; Buendia-Castaño, D.; Alegre-Sanchez, A.; Pindado-Ortega, C.; Molins, M.; Perosanz, D.; Segurado-Miravalles, G.; et al. Frontal fibrosing alopecia: Clinical and prognostic classification. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Batra, P.; Sukhdeo, K.; Shapiro, J. Hair Loss in Lichen Planopilaris and Frontal Fibrosing Alopecia: Not Always Irreversible. Skin Appendage Disord. 2020, 6, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Porriño-Bustamante, M.L.; Fernández-Pugnaire, M.A.; Arias-Santiago, S. A Cross-sectional Study of Rosacea and Risk Factors in Women with Frontal Fibrosing Alopecia. Acta Derm. Venereol. 2019, 99, 12. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Barata, A.R.; Moreno-Arrones, O.M.; Corralo, D.S.; Galvan, S.V. The “Starry Night Sky Sign” Using Ultraviolet-Light-Enhanced Trichoscopy: A New Sign That May Predict Efficacy of Treatment in Frontal Fibrosing Alopecia. Int. J. Trichol. 2018, 10, 241–243. [Google Scholar] [CrossRef] [PubMed]
- Kępińska, K.; Jałowska, M.; Bowszyc-Dmochowska, M. Frontal Fibrosing Alopecia—A review and a practical guide for clinicians. Ann. Agric. Environ. Med. 2022, 29, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Kerkemeyer, K.L.S.; Eisman, S.; Bhoyrul, B.; Pinczewski, J.; Sinclair, R.D. Frontal fibrosing alopecia. Clin. Dermatol. 2021, 39, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Kelati, A.; Mernissi, F.Z. Frontal Hairline Recession: A Diagnostic Pitfall. Skin Appendage Disord. 2017, 3, 18–19. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.; Shapiro, J. Medical therapy for frontal fibrosing alopecia: A review and clinical approach. J. Am. Acad. Dermatol. 2019, 81, 568–580. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Ramírez, D.; Ferrándiz, L.; Camacho, F.M. Alopecia frontal fibrosante. Valoración diagnóstica y terapéutica [Diagnostic and therapeutic assessment of frontal fibrosing alopecia]. Actas Dermosifiliogr. 2007, 98, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Plante, J.; Eason, C.; Snyder, A.; Elston, D. Tofacitinib in the treatment of lichen planopilaris: A retrospective review. J. Am. Acad. Dermatol. 2020, 83, 1487–1489. [Google Scholar] [CrossRef] [PubMed]
- Dunn, C.; Griffith, V.; Coican, A.; Dane, A.; Chow, W.; Aneja, S.; Nathoo, R.; Leavitt, A.; Hawkins, S.D. Janus kinase inhibition for the treatment of refractory frontal fibrosing alopecia: A case series and review of the literature. JAAD Case Rep. 2023, 40, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Pincelli, T.P.; Heckman, M.G.; Cochuyt, J.J.; Sluzevich, J.C. Valchlor® in the Treatment of Lichen Planopilaris and Frontal Fibrosing Alopecia: A Single Arm, Open-label, Exploratory Study. Int. J. Trichol. 2020, 12, 220–226. [Google Scholar]
- Woodward, D.F.; Liang, Y.; Krauss, A.H. Prostamides (prostaglandin-ethanolamides) and their pharmacology. Br. J. Pharmacol. 2008, 153, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Murad, A.; Bergfeld, W.F. Prostaglandin analogue for eyebrow loss in frontal fibrosing alopecia: A case report. J. Eur. Acad. Dermatol. Venereol. 2019, 33, e403–e405. [Google Scholar] [CrossRef] [PubMed]
- Murad, A.; Bergfeld, W. Prostaglandin analogue for treatment of eyebrow loss in frontal fibrosing alopecia: Three cases with different outcomes. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e138–e140. [Google Scholar] [CrossRef] [PubMed]
- Beyzaee, A.M.; Goldust, M.; Patil, A.; Ghoreishi, B.; Ghahremanloo, T.; Rokni, G.R. Treatment of Frontal Fibrosing Alopecia. Dermatol. Ther. 2023, 3, 1–22. [Google Scholar] [CrossRef]
- Subash, J.; Eginli, A.; Bomar, L.; McMichael, A. Frontal fibrosing alopecia treatment with Nd:YAG (1064 nm) nonablative laser. Int. J. Womens Dermatol. 2020, 7, 355–356. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.M.; Hadi, S.M. The excimer lasers. J. Drugs Dermatol. 2004, 3, 522–525. [Google Scholar] [PubMed]
- Grema, H.; Raulin, C. Der Excimer-Laser in der Dermatologie und ästhetischen Medizin [The excimer laser in dermatology and esthetic medicine]. Hautarzt 2004, 55, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Mehraban, S.; Feily, A. 308nm Excimer Laser in Dermatology. J. Lasers Med. Sci. 2014, 5, 8–12. [Google Scholar] [PubMed]
- Navarini, A.A.; Kolios, A.G.; Prinz-Vavricka, B.M.; Haug, S.; Trüeb, R.M. Low-dose excimer 308-nm laser for treatment of lichen planopilaris. Arch. Dermatol. 2011, 147, 1325–1326. [Google Scholar] [CrossRef] [PubMed]
- Anders, J.J.; Lanzafame, R.J.; Arany, P.R. Low-level light/laser therapy versus photobiomodulation therapy. Photomed. Laser Surg. 2015, 33, 183–184. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, L.F.; Hamblin, M.R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 7000417. [Google Scholar] [CrossRef] [PubMed]
- Avci, P.; Gupta, G.K.; Clark, J.; Wikonkal, N.; Hamblin, M.R. Low-level laser (light) therapy (LLLT) for treatment of hair loss. Lasers Surg. Med. 2014, 46, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Sorbellini, E.; Rucco, M.; Rinaldi, F. Photodynamic and photobiological effects of light-emitting diode (LED) therapy in dermatological disease: An update. Lasers Med. Sci. 2018, 33, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Bartosińska, J.; Gerkowicz, A.; Niewiedzioł, M.; Szczepanik-Kułak, P.; Kwaśny, M.; Krasowska, D. Application of photodynamic therapy with the use of superluminescent light-emitting diode (sLED) lamp in actinic keratosis. Dermatol. Rev./Przegl. Dermatol. 2019, 106, 372–383. [Google Scholar] [CrossRef]
- Gerkowicz, A.; Bartosińska, J.; Wolska-Gawron, K.; Michalska-Jakubus, M.; Kwaśny, M.; Krasowska, D. Application of superluminescent diodes (sLED) in the treatment of scarring alopecia—A pilot study. Photodiagnosis Photodyn. Ther. 2019, 28, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S.; Romeo, M.; Lankinen, P. Platelet-rich plasma for androgenetic alopecia: Does it work? Evidence from meta analysis. J. Cosmet. Dermatol. 2017, 16, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Dina, Y.; Aguh, C. Use of platelet-rich plasma in cicatricial alopecia. Dermatol. Surg. 2019, 45, 979–981. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Navarro, M.R.; Ramirez, A.; Pino, A.; Navarro, A.; Moles, I.; Gallego, E.; Anitua, E. Plasma Rich in Growth Factors as an Adjuvant Treatment for the Management of Frontal Fibrosing Alopecia: A Retrospective Observational Clinical Study. J. Cutan. Med. Surg. 2023, 27, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Özcan, D.; Tunçer Vural, A.; Özen, Ö. Platelet-rich plasma for treatment resistant frontal fibrosing alopecia: A case report. Dermatol. Ther. 2019, 32, e13072. [Google Scholar] [CrossRef] [PubMed]
- Danesh, M.; Murase, J.E. Increasing utility of finasteride for frontal fibrosing alopecia. J. Am. Acad. Dermatol. 2015, 72, e157. [Google Scholar] [CrossRef] [PubMed]
- Georgala, S.; Katoulis, A.C.; Befon, A.; Danopoulou, I.; Georgala, C. Treatment of postmenopausal frontal fibrosing alopecia with oral dutasteride. J. Am. Acad. Dermatol. 2009, 61, 157–158. [Google Scholar] [CrossRef] [PubMed]
- Rogers, N.E.; Avram, M.R. Medical treatments for male and female pattern hair loss. J. Am. Acad. Dermatol. 2008, 59, 547–568. [Google Scholar] [CrossRef] [PubMed]
- Kini, M.A.; Riaz, R.; Jolliffe, V. A Retrospective Analysis of Efficacy and Safety of Intralesional Triamcinolone Injections in the Treatment of Frontal Fibrosing Alopecia Either as Monotherapy or as a Concomitant Therapy. Int. J. Trichol. 2018, 10, 162–168. [Google Scholar]
- Pham, C.T.; Hosking, A.M.; Cox, S.; Mesinkovska, N.A. Therapeutic response of facial papules and inflammation in frontal fibrosing alopecia to low-dose oral isotretinoin. JAAD Case Rep. 2020, 6, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Hong, J.S.; Lee, S.H.; Lee, A.Y. Successful treatment of frontal fibrosing alopecia with alitretinoin. Dermatol. Ther. 2019, 32, e13037. [Google Scholar] [CrossRef] [PubMed]
- Pirmez, R.; Duque-Estrada, B.; Barreto, T.; Quintella, D.C.; Cuzzi, T. Successful Treatment of Facial Papules in Frontal Fibrosing Alopecia with Oral Isotretinoin. Skin Appendage Disord. 2017, 3, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Rakowska, A.; Gradzińska, A.; Olszewska, M.; Rudnicka, L. Efficacy of Isotretinoin and Acitretin in Treatment of Frontal Fibrosing Alopecia: Retrospective Analysis of 54 Cases. J. Drugs Dermatol. 2017, 16, 988–992. [Google Scholar] [PubMed]
- Terry-Flores, M.; Garcia-Arpa, M.; Franco-Muñóz, L.; González-Ruiz, L. Facial Response to Isotretinoin. Actas Dermo Sifiliogr. 2018, 109, 831–833. [Google Scholar]
- Zbiciak-Nylec, M.; Brzezińska-Wcisło, L.; Salwowska, N. The efficacy of antimalarial drugs in the therapy of selected forms of cicatricial alopecia. Adv. Dermatol. Allergol./Postępy Dermatol. I Alergol. 2021, 38, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Gamret, A.C.; Potluri, V.S.; Krishnamurthy, K.; Fertig, R.M. Frontal fibrosing alopecia: Efficacy of treatment modalities. Int. J. Womens Health 2019, 11, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Van Beek, M.J.; Piette, W.W. Antimalarials. Dermatol. Clin. 2001, 19, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Saber, M.; Farokhshahi, M.; Fatemi Naeini, F.; Mohaghegh, F.; Asilian, A. Clinical effectiveness of finasteride versus hydroxychloroquine in the treatment of frontal fibrosing alopecia: A randomized controlled trial. J. Cosmet. Dermatol. 2024, 23, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Khalil, S.; Bardawil, T.; Stephan, C.; Darwiche, N.; Abbas, O.; Kibbi, A.G.; Nemer, G.; Kurban, M. Retinoids: A journey from the molecular structures and mechanisms of action to clinical uses in dermatology and adverse effects. J. Dermatolog. Treat. 2017, 28, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Khanna, T.; Sallee, B.; Christiano, A.M.; Bordone, L.A. Tofacitinib for the treatment of lichen planopilaris: A case series. Dermatol. Ther. 2018, 31, e12656. [Google Scholar] [CrossRef] [PubMed]
- Damsky, W.; King, B.A. JAK inhibitors in dermatology: The promise of a new drug class. J. Am. Acad. Dermatol. 2017, 76, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Pirmez, R.; Spagnol Abraham, L. Eyebrow regrowth in patients with frontal fibrosing alopecia treated with low-dose oral minoxidil. Skin Appendage Disord. 2021, 7, 112–114. [Google Scholar] [CrossRef] [PubMed]
- Cranwell, W.C.; Sinclair, R. Familial frontal fibrosing alopecia treated with dutasteride, minoxidil and artificial hair transplantation. Australas. J. Dermatol. 2017, 58, e94–e96. [Google Scholar] [CrossRef] [PubMed]
- Jaros, J.; Lio, P. Low dose naltrexone in dermatology. J. Drugs Dermatol. 2019, 18, 235–238. [Google Scholar] [PubMed]
- Lee, B.; Elston, D.M. The uses of naltrexone in dermatologic conditions. J. Am. Acad. Dermatol. 2019, 80, 1746–1752. [Google Scholar] [CrossRef] [PubMed]
- Ekelem, C.; Juhasz, M.; Khera, P.; Mesinkovska, N.A. Utility of naltrexone treatment for chronic inflammatory dermatologic conditions: A systematic review. JAMA Dermatol. 2019, 155, 229–236. [Google Scholar] [CrossRef]
- Sikora, M.; Rakowska, A.; Olszewska, M.; Rudnicka, L. The use of naltrexone in dermatology; current evidence and future directions. Curr. Drug Targets. 2019, 20, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Hamel, R.K.; Chen, L.; O’Connell, C.; Mann, C. Oral Low-Dose Naltrexone in the Treatment of Frontal Fibrosing Alopecia and Lichen Planopilaris: An Uncontrolled Open-Label Prospective Study. Cureus 2023, 15, 34169. [Google Scholar]
- Spring, P.; Spanou, Z.; de Viragh, P.A. Lichen planopilaris treated by the peroxisome proliferator activated receptor-γ agonist pioglitazone: Lack of lasting improvement or cure in the majority of patients. J. Am. Acad. Dermatol. 2013, 69, 830–832. [Google Scholar] [CrossRef] [PubMed]
- Mesinkovska, N.A.; Tellez, A.; Dawes, D.; Piliang, M.; Bergfeld, W. The use of oral pioglitazone in the treatment of lichen planopilaris. J. Am. Acad. Dermatol. 2015, 72, 355–356. [Google Scholar] [CrossRef]
- Ladizinski, B.; Bazakas, A.; Selim, M.A.; Olsen, E.A. Frontal fibrosing alopecia: A retrospective review of 19 patients seen at Duke University. J. Am. Acad. Dermatol. 2013, 68, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Ioannides, D.; Vakirlis, E.; Kemeny, L.; Marinovic, B.; Massone, C.; Murphy, R.; Nast, A.; Ronnevig, J.; Ruzicka, T.; Cooper, S.; et al. European S1 guidelines on the management of lichen planus: A cooperation of the European Dermatology Forum with the European Academy of Dermatology and Venereology. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, L.; Rosman, I.S.; Mann, C.M. Frontal fibrosing alopecia demographics: A survey of 29 patients. Cutis 2019, 103, E16–E22. [Google Scholar] [PubMed]
- Suchonwanit, P.; Pakornphadungsit, K.; Leerunyakul, K.; Khunkhet, S.; Sriphojanart, T.; Rojhirunsakool, S. Frontal fibrosing alopecia in Asians: A retrospective clinical study. Int. J. Dermatol. 2020, 59, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Morandi Stumpf, M.A.; do Rocio Valenga Baroni, E.; Schafranski, M.D. Frontal fibrosing alopecia: Successfully treated with methotrexate or just the natural disease progression? Acta Dermatovenerol. Croat. 2020, 28, 202–203. [Google Scholar]
- Lee, J.A.; Levy, D.A.; Patel, K.G.; Brennan, E.A.; Oyer, S.L. Hair Transplantation in Frontal Fibrosing Alopecia and Lichen Planopilaris: A Systematic Review. Laryngoscope 2020, 131, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Unger, W.; Unger, R.; Wesley, C. The surgical treatment of cicatricial alopecia. Dermatol. Ther. 2008, 21, 295–311. [Google Scholar] [CrossRef] [PubMed]
- Arasu, A.; Meah, N.; Marzola, M.; Sinclair, R. Scalp reduction surgery does not reactivate frontal fibrosing alopecia: A case report. Dermatol. Ther. 2022, 35, e15451. [Google Scholar] [CrossRef] [PubMed]
- Klingbeil, K.D.; Fertig, R. Eyebrow and eyelash hair transplantation: A systematic review. J. Clin. Aesthet. Dermatol. 2018, 1, 21–30. [Google Scholar]
- Audickaite, A.; Alam, M.; Jimenez, F. Eyebrow Transplantation in Frontal Fibrosing Alopecia: Pitfalls of Short- and Long-Term Results. Dermatol. Surg. 2020, 46, 922–925. [Google Scholar] [CrossRef]


| Major Criteria | Minor Criteria |
|---|---|
|
|
| Grade of Severity | Cicatricial Area Largest Measure [cm] |
|---|---|
| I | <1 |
| II | 1–2.99 |
| III | 3–4.99 |
| IV | 5–6.99 |
| V | ≥7 |
| Signs and Symptoms | Grade | Punctuation |
|---|---|---|
| Clinical symptoms | ||
| Hairline recession [cm] | ||
| Frontal |
| grade × 2 |
| Temporal left | grade × 1 | |
| Temporal right | grade × 1 | |
| Eyebrow loss | No | 0 |
| Partial | 0.5 | |
| Total | 1 | |
| Alopecia score | the sum of clinical symptoms punctuation max. 21 | |
| Inflammation | ||
| Severity | ||
| Perifollicular hyperkeratosis | No | 0 |
| Mild | 0.1 | |
| Severe | 0.2 | |
| Perifollicular erythema | No | 0 |
| Mild | 0.1 | |
| Severe | 0.2 | |
| Extent along the frontotemporal line [%] | ||
| Perifollicular hyperkeratosis | No | 0 |
| <25% | 0.1 | |
| >25% | 0.2 | |
| Perifollicular erythema | No | 0 |
| <75% | 0.1 | |
| >75% | 0.2 | |
| Associated symptoms | ||
| Pruritus | ||
| Severity | No | 0 |
| Mild | 0.3 | |
| Severe | 0.6 | |
| Frequency | No | 0 |
| Occasional | 0.3 | |
| Daily | 0.6 | |
| Pain | ||
| Severity | No | 0 |
| Mild | 0.3 | |
| Severe | 0.6 | |
| Frequency | No | 0 |
| Occasional | 0.3 | |
| Daily | 0.6 | |
| Inflammation score | the sum of inflammation and associated symptoms punctuation max. 4 | |
| FFASS | the sum of alopecia score and inflammation score max. 25 | |
| Symptom | Grade | Punctuation |
|---|---|---|
| Scalp hair loss | None | 0 |
| Minimal (<1 cm) | 1 | |
| Mild (1 to <3 cm) | 2 | |
| Moderate (3 to <5 cm) | 3 | |
| Severe (≥5 cm) | 4 | |
| Eyebrow loss | None | 0 |
| Partial | 1 | |
| Total loss in at least one eyebrow | 2 | |
| Facial papules | None | 0 |
| Some | 1 | |
| Prominent forehead veins | None | 0 |
| Some | 1 | |
| Facial hyperpigmentation | None | 0 |
| Some | 1 |
| Local Therapy | Systemic Therapy | Surgical Procedures |
|---|---|---|
| Topical agents | 5-alpha-reductase inhibitors Dutasteride Finasteride Antimalarial agents Hydroxychloroquine Retinoids Isotretinoin Acitretin Alitretinoin Tetracyclines Tetracycline Doxycycline JAK inhibitors Tofacitinib Baricitinib Minoxidil Pioglitazone Naltrexone Calcineurin inhibitors Cyclosporine Mycophenolate mofetil Azathioprine Oral corticosteroids | Hair transplantation Scalp reduction surgery Eyebrow transplantation |
| Corticosteroids Betamethasone Clobetasol Calcineurin Inhibitors Tacrolimus Pimecrolimus JAK inhibitors Tofacitinib Ruxolitinib Mechlorethamine Hair Growth Modulators Minoxidil Bimatoprost | ||
| Intralesional injections | ||
| ILTA * PRP/PRGF | ||
| Phototherapy | ||
| Low-Level Light Therapy Excimer Laser Nd:YAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzesłowska, W.J.; Woźniacka, A. The Frontal Fibrosing Alopecia Treatment Dilemma. J. Clin. Med. 2024, 13, 2137. https://doi.org/10.3390/jcm13072137
Krzesłowska WJ, Woźniacka A. The Frontal Fibrosing Alopecia Treatment Dilemma. Journal of Clinical Medicine. 2024; 13(7):2137. https://doi.org/10.3390/jcm13072137
Chicago/Turabian StyleKrzesłowska, Wiktoria Julia, and Anna Woźniacka. 2024. "The Frontal Fibrosing Alopecia Treatment Dilemma" Journal of Clinical Medicine 13, no. 7: 2137. https://doi.org/10.3390/jcm13072137
APA StyleKrzesłowska, W. J., & Woźniacka, A. (2024). The Frontal Fibrosing Alopecia Treatment Dilemma. Journal of Clinical Medicine, 13(7), 2137. https://doi.org/10.3390/jcm13072137