Comparison between Invasive Intervention and Conservative Treatment in Patients with In-Hospital Myocardial Infarctions: Results from the Regional Myocardial Infarction Registry of Saxony-Anhalt (RHESA) Study
Abstract
1. Introduction
2. Methods
2.1. Study Design, Dataset Description, and Data Collection
2.2. Variables and Outcomes
2.3. Statistical Analysis
2.4. Description of Directed Acyclic Graph (DAG)
2.5. Ethical Consideration
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMI | Acute myocardial infarction |
| BMI | Body mass index |
| CI | Confidence interval |
| RHESA | The Regional Myocardial Infarction Registry of Saxony-Anhalt |
| NSTEMI | Non-ST segment elevation myocardial infarction |
| SD | Standard deviation |
| STEMI | ST-segment elevation myocardial infarction |
| OHMI | Out-of-hospital myocardial infarction |
| OR | Odds ratio |
| ESC | European Society of Cardiology |
| CABG | Coronary artery bypass surgery |
| PCI | Percutaneous coronary intervention |
| ACE/ARB | Angiotensin converting enzyme inhibitors and/or Angiotensin II receptor blocker |
| DAG | Directed acyclic graph |
| CAD | Coronary artery disease |
References
- Assaf, M.; Costa, D.; Massag, J.; Weber, C.; Mikolajczyk, R.; Lückmann, S.L. Comparison between in-hospital and out-of-hospital acute myocardial infarctions: Results from the regional myocardial infarction registry of saxony-anhalt (RHESA) study. J. Clin. Med. 2023, 12, 6305. [Google Scholar] [CrossRef]
- Erne, P.; Bertel, O.; Urban, P.; Pedrazzini, G.; Lüscher, T.F.; Radovanovic, D.; Investigators, A.P. Inpatient versus outpatient onsets of acute myocardial infarction. Eur. J. Intern. Med. 2015, 26, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Maynard, C.; Lowy, E.; Rumsfeld, J.; Sales, A.E.; Sun, H.; Kopjar, B.; Fleming, B.; Jesse, R.L.; Rusch, R.; Fihn, S.D. The prevalence and outcomes of in-hospital acute myocardial infarction in the department of veterans affairs health system. Arch. Intern. Med. 2006, 166, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Bradley, S.M.; Borgerding, J.A.; Wood, G.B.; Maynard, C.; Fihn, S.D. Incidence, risk factors, and outcomes associated with in-hospital acute myocardial infarction. JAMA Netw. Open 2019, 2, e187348. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Bumgarner, J.; Spangler, A.; Meredith, D.; Smith, S.C.; Stouffer, G.A. Acute st-elevation myocardial infarction in patients hospitalized for noncardiac conditions. J. Am. Heart Assoc. 2013, 2, e000004. [Google Scholar] [CrossRef] [PubMed]
- Garberich, R.F.; Traverse, J.H.; Claussen, M.T.; Rodriguez, G.; Poulose, A.K.; Chavez, I.J.; Rutten-Ramos, S.; Hildebrandt, D.A.; Henry, T.D. St-elevation myocardial infarction diagnosed after hospital admission. Circulation 2014, 129, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Pande, A.N.; Jacobs, A.K. In-hospital st-segment-elevation myocardial infarction: An inside-out approach. Circulation 2014, 129, 1193–1195. [Google Scholar] [CrossRef] [PubMed]
- Stehli, J.; Dagan, M.; Dinh, D.T.; Lefkovits, J.; Dick, R.; Oxley, S.; Brennan, A.L.; Duffy, S.J.; Zaman, S. Differences in outcomes of patients with in-hospital versus out-of-hospital st-elevation myocardial infarction: A registry analysis. BMJ Open 2022, 12, e052000. [Google Scholar] [CrossRef] [PubMed]
- Zahn, R.; Schiele, R.; Seidl, K.; Kapp, T.; Glunz, H.G.; Jagodzinski, E.; Voigtländer, T.; Gottwik, M.; Berg, G.; Thomas, H. Acute myocardial infarction occurring in versus out of the hospital: Patient characteristics and clinical outcome. J. Am. Coll. Cardiol. 2000, 35, 1820–1826. [Google Scholar] [CrossRef] [PubMed]
- Amann, U.; Kirchberger, I.; Heier, M.; Thilo, C.; Kuch, B.; Peters, A.; Meisinger, C. Predictors of non-invasive therapy and 28-day-case fatality in elderly compared to younger patients with acute myocardial infarction: An observational study from the monica/kora myocardial infarction registry. BMC Cardiovasc. Disord. 2016, 16, 151. [Google Scholar] [CrossRef]
- Câlmâc, L.; Bătăilă, V.; Ricci, B.; Vasiljevic, Z.; Kedev, S.; Gustiene, O.; Trininic, D.; Knežević, B.; Miličić, D.; Dilic, M. Factors associated with use of percutaneous coronary intervention among elderly patients presenting with st segment elevation acute myocardial infarction (stemi): Results from the isacs-tc registry. Int. J. Cardiol. 2016, 217, S21–S26. [Google Scholar] [CrossRef] [PubMed]
- Kytö, V.; Prami, T.; Khanfir, H.; Hasvold, P.; Reissell, E.; Airaksinen, J. Usage of pci and long-term cardiovascular risk in post-myocardial infarction patients: A nationwide registry cohort study from finland. BMC Cardiovasc. Disord. 2019, 19, 123. [Google Scholar] [CrossRef] [PubMed]
- Negers, A.; Boddaert, J.; Mora, L.; Golmard, J.-L.; Moïsi, L.; Cohen, A.; Collet, J.-P.; Breining, A. Determinants of invasive strategy in elderly patients with non-st elevation myocardial infarction. J. Geriatr. Cardiol. 2017, 14, 465. [Google Scholar] [PubMed]
- Puymirat, E.; Taldir, G.; Aissaoui, N.; Lemesle, G.; Lorgis, L.; Cuisset, T.; Bourlard, P.; Maillier, B.; Ducrocq, G.; Ferrieres, J. Use of invasive strategy in non–st-segment elevation myocardial infarction is a major determinant of improved long-term survival: Fast-mi (french registry of acute coronary syndrome). JACC Cardiovasc. Interv. 2012, 5, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Hvelplund, A.; Galatius, S.; Madsen, M.; Rasmussen, J.N.; Rasmussen, S.; Madsen, J.K.; Sand, N.P.; Tilsted, H.-H.; Thayssen, P.; Sindby, E. Women with acute coronary syndrome are less invasively examined and subsequently less treated than men. Eur. Heart J. 2010, 31, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Prütz, F.; Rommel, A.; Kroll, L.E.; Lampert, T. 25 Years after the Fall of the Berlin Wall: Regional Differences in Health. 2014. Available online: https://edoc.rki.de/handle/176904/3133/ (accessed on 1 July 2023).
- Bohley, S.; Trocchi, P.; Robra, B.-P.; Mau, W.; Stang, A. The regional myocardial infarction registry of saxony-anhalt (RHESA) in germany–rational and study protocol. BMC Cardiovasc. Disord. 2015, 15, 45. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, K.; Bohley, S.; Mau, W.; Schmidt-Pokrzywniak, A. The RHESA-care study: An extended baseline survey of the regional myocardial infarction registry of saxony-anhalt (RHESA) design and objectives. BMC Cardiovasc. Disord. 2016, 16, 159. [Google Scholar] [CrossRef][Green Version]
- Bax, J.J.; Baumgartner, H.; Ceconi, C.; Dean, V.; UK, C.D.; Fagard, R.; Funck-Brentano, C.; Hasdai, D.; Hoes, A.; Kirchhof, P. Third universal definition of myocardial infarction. J. Am. Coll. Cardiol. 2012, 126, 2020–2035. [Google Scholar]
- Hippel, P.v. How to impute interactions, squares, and other transformed variables. Sociol. Methodol. 2009, 39, 265–291. [Google Scholar] [CrossRef]
- Textor, J.; Hardt, J.; Knüppel, S. Dagitty: A graphical tool for analyzing causal diagrams. Epidemiology 2011, 22, 745. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 1 July 2023).
- RStudio Team. RStudio: Integrated Development Environment for R.; RStudio, P.: Boston, MA, USA, 2022; Available online: http://www.rstudio.com/ (accessed on 1 July 2023).
- Hamm, C.W.; Bassand, J.P.; Agewall, S.; Bax, J.; Boersma, E.; Bueno, H.; Caso, P.; Dudek, D.; Gielen, S.; Huber, K.; et al. ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent st-segment elevation: The task force for the management of acute coronary syndromes (ACS) in patients presenting without persistent st-segment elevation of the european society of cardiology (ESC). Eur. Heart J. 2011, 32, 2999–3054. [Google Scholar] [PubMed]
- Vij, A.; Kassab, K.; Chawla, H.; Kaur, A.; Kodumuri, V.; Jolly, N.; Doukky, R. Invasive therapy versus conservative therapy for patients with stable coronary artery disease: An updated meta-analysis. Clin. Cardiol. 2021, 44, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Levine, G.N.; Dai, X.; Henry, T.D.; Press, M.C.; Denktas, A.E.; Garberich, R.F.; Jacobs, A.K.; Jaski, B.E.; Kaul, P.; Kontos, M.C. In-hospital st-segment elevation myocardial infarction: Improving diagnosis, triage, and treatment. JAMA Cardiol. 2018, 3, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with st-segment elevation: The task force for the management of acute myocardial infarction in patients presenting with st-segment elevation of the european society of cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar]
- Newcastle-upon-Tyne Hospitals NHS Trust. The British Heart Foundation SENIOR-RITA Trial. Available online: https://trialbulletin.com/lib/entry/ct-03052036/ (accessed on 1 July 2023).
- Buiatti, E.; Barchielli, A.; Marchionni, N.; Balzi, D.; Carrabba, N.; Valente, S.; Olivotto, I.; Landini, C.; Filice, M.; Torri, M.; et al. Determinants of treatment strategies and survival in acute myocardial infarction: A population-based study in the florence district, italy: Results of the acute myocardial infarction florence registry (ami-florence). Eur. Heart J. 2003, 24, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Jaehn, P.; Andresen-Bundus, H.; Bergholz, A.; Pagonas, N.; Hauptmann, M.; Neugebauer, E.A.; Holmberg, C.; Ritter, O.; Sasko, B. Contextualising the association of socioeconomic deprivation with hospitalisation rates of myocardial infarction in a rural area in eastern germany. Rural Remote Health 2022, 22, 6658. [Google Scholar] [CrossRef]
- Loccoh, E.C.; Joynt Maddox, K.E.; Wang, Y.; Kazi, D.S.; Yeh, R.W.; Wadhera, R.K. Rural-urban disparities in outcomes of myocardial infarction, heart failure, and stroke in the united states. J. Am. Coll. Cardiol. 2022, 79, 267–279. [Google Scholar] [CrossRef]
- Abbasi, S.H.; Sundin, Ö.; Jalali, A.; Soares, J.; Macassa, G. Mortality from acute coronary syndrome: Does place of residence matter? J. Tehran Heart Cent. 2022, 17, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Nicolau, J.C.; Owen, R.; Furtado, R.H.M.; Goodman, S.G.; Granger, C.B.; Cohen, M.G.; Westermann, D.; Yasuda, S.; Simon, T.; Hedman, K.; et al. Long-term outcomes among stable post-acute myocardial infarction patients living in rural versus urban areas: Insights from the prospective, observational tigris registry. Open Heart 2023, 10, e002326. [Google Scholar] [CrossRef] [PubMed]
- Rittger, H.; Schnupp, S.; Sinha, A.M.; Breithardt, O.A.; Schmidt, M.; Zimmermann, S.; Mahnkopf, C.; Brachmann, J.; Rieber, J. Predictors of treatment in acute coronary syndromes in the elderly: Impact on decision making and clinical outcome after interventional versus conservative treatment. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2012, 80, 735–743. [Google Scholar] [CrossRef]
- Birkemeyer, R.; Schneider, H.; Rillig, A.; Ebeling, J.; Akin, I.; Kische, S.; Paranskaya, L.; Jung, W.; Ince, H.; Nienaber, C.A. Do gender differences in primary pci mortality represent a different adherence to guideline recommended therapy? A multicenter observation. BMC Cardiovasc. Disord. 2014, 14, 71. [Google Scholar] [CrossRef] [PubMed]
- L’Abbate, A.; Carpeggiani, C.; Testa, R.; Michelassi, C.; Biagini, A.; Severi, S. In-hospital myocardial infarction. Pre-infarction features and their correlation with short-term prognosis. Eur. Heart J. 1986, 7, 53–61. [Google Scholar] [PubMed]
- Sandoval, Y.; Jaffe, A.S. Type 2 myocardial infarction: Jacc review topic of the week. J. Am. Coll. Cardiol. 2019, 73, 1846–1860. [Google Scholar] [CrossRef] [PubMed]
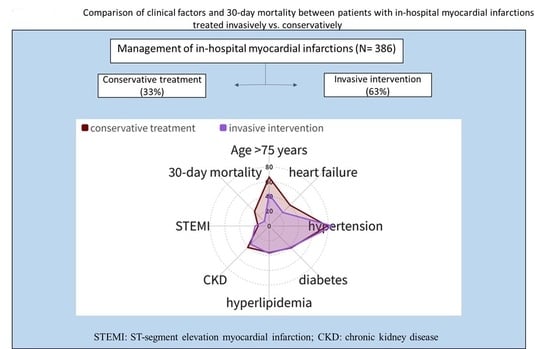
| Total Number of In-Hospital AMIs N = 386 | Conservative Treatment: N = 127 | Invasive Intervention: N = 259 | ||
|---|---|---|---|---|
| N (%) or Mean (SD) | 95% CI | N (%) or Mean (SD) | 95% CI | |
| Characteristics | ||||
| Age (years) | 75.4 (12.7) | 73.2–77.6 | 69.9 (12.4) | 68.4–71.4 |
| Age > 75 years | 84 (66.1) | 57.6–73.9 | 111 (42.9) | 36.9–48.9 |
| Female | 65 (51.1) | 42.1–60 | 95 (36.6) | 30.6–42.3 |
| Region: | ||||
| Halle (urban) | 52 (40.9) | 32.7–54.3 | 197 (76.1) | 70.6–80.9 |
| Altmark (rural) | 75 (59.1) | 50.4–67.3 | 62 (23.9) | 19.1–29.4 |
| Body mass index group (kg/m2) | ||||
| <25 | 21 (16.5) | 10.9–23.7 | 46 (17.8) | 13.5–22.8 |
| 25–<30 | 63 (49.6) | 41.0–58.2 | 135 (52.1) | 46.0–58.2 |
| 30–35 | 30 (23.6) | 16.9–31.5 | 61 (23.6) | 18.7–29.0 |
| >35 | 12 (9.4) | 5.3–15.4 | 17 (6.6) | 4.0–10.1 |
| Smoking Status | ||||
| Never smoker | 85 (66.9) | 58.4–74.7 | 133 (51.4) | 45.3–57.4 |
| Smoker | 28 (22.0) | 15.5–29.8 | 89 (34.4) | 28.8–40.3 |
| Former smoker | 14 (11.0) | 6.5–17.3 | 37 (14.3) | 10.4–18.9 |
| Pre-existing comorbidities | ||||
| Diabetes | 53 (41.7) | 33.4–50.4 | 106 (40.9) | 35.1–47.0 |
| Hypertension | 100 (78.7) | 71.0–85.2 | 222 (85.7) | 81.1–89.6 |
| Hyperlipidemia | 46 (36.2) | 28.2–44.8 | 94 (36.6) | 30.6–42.3 |
| Stroke | 20 (15.7) | 10.2–22.8 | 26 (10.1) | 6.8–14.1 |
| Atrial fibrillation | 35 (27.6) | 20.4–35.8 | 66 (25.5) | 20.5–31.0 |
| Heart failure | 51 (40.2) | 31.9–48.8 | 67 (25.9) | 20.8–30.4 |
| Chronic kidney disease | 52 (40.9) | 32.7–49.6 | 91 (35.1) | 29.5–41.1 |
| Total Number of In-Hospital AMIs: N = 386 | Conservative Treatment: N = 127 | Invasive Intervention: N = 259 | ||
|---|---|---|---|---|
| N (%) or Mean (SD) | 95% CI | N (%) | 95% CI | |
| Inpatient clinical metrics | ||||
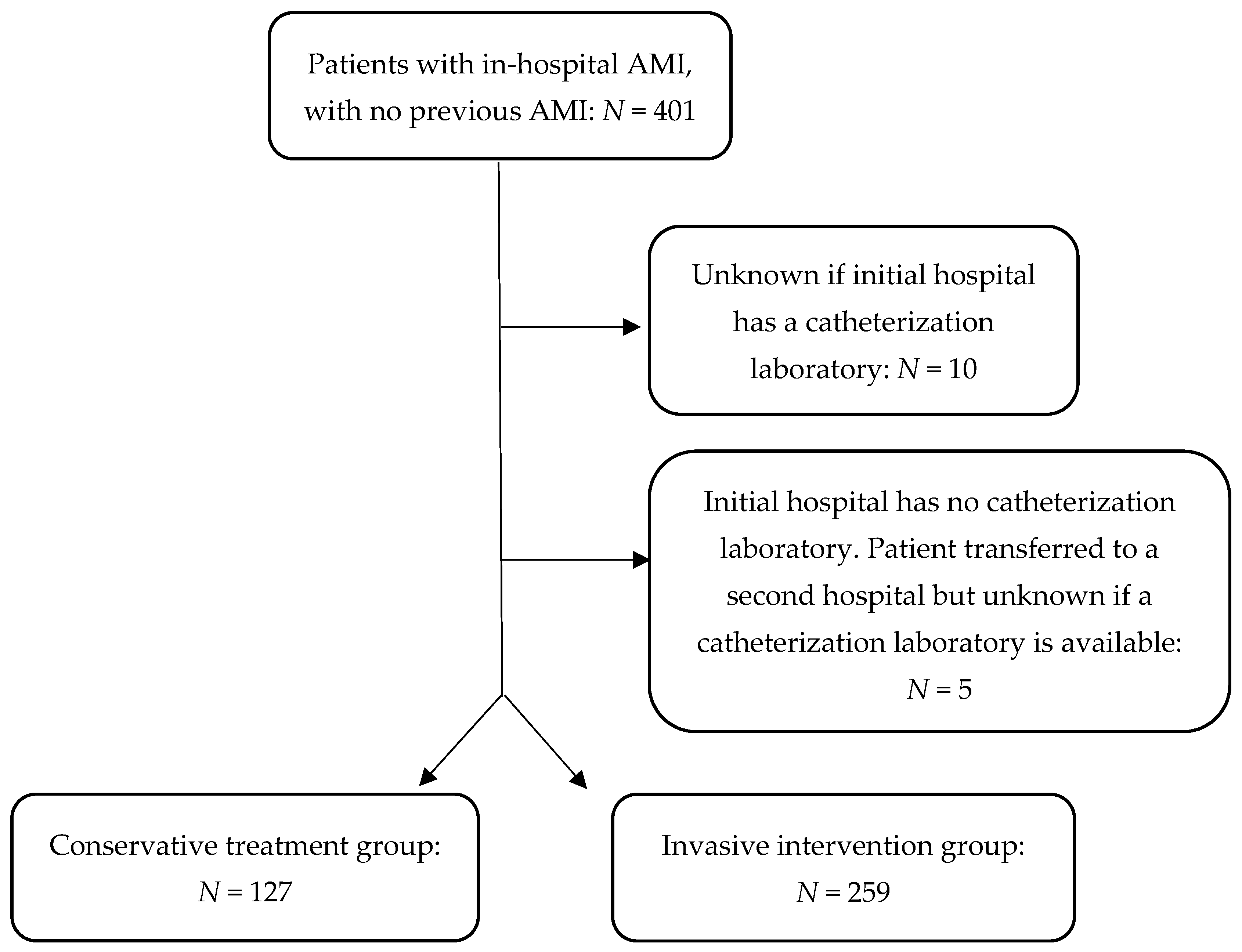
| Patient initially found in a hospital with no cardiac catheter laboratory | 80 (63) | 54.4–71 | 78 (30.1) | 24.8–35.9 |
| If yes, number of patients transferred to a hospital with cardiac catheterization laboratory | 11 died in original hospital (5 within 24 h) | 0 died in KH1 | ||
| 7 were discharged home | 0 were discharged home | |||
| 62 were transferred to a hospital with a catheterization laboratory but still received no intervention | 78 were transferred to a hospital with a catheterization laboratory where they received intervention | |||
| Heart rate on presentation (beats/min) | 88 (23) | 84–93 | 82 (23) | 79–86 |
| Systolic blood pressure on presentation (mmHg) | 140 (31) | 135–146 | 139 (30) | 136–143 |
| STEMI | 19 (15.0) | 9.6–21.2 | 72 (27.8) | 22.6–33.5 |
| Occurrence of shock upon presentation | 7 (5.5) | 2.5–10.5 | 25 (9.7) | 6.5–13.7 |
| Initial medical treatments | ||||
| ASS | 94 (74.0) | 65.9–81.0 | 164 (63.3) | 57.3–69.0 |
| P2Y12 inhibitor | 51 (40.2) | 31.9–48.8 | 101 (39.0) | 33.2–45.0 |
| Heparin | 80 (62.4) | 53.0–69.4 | 180 (69.8) | 63.7–75.2 |
| Thrombolytic agent | 1 (0.8) | 0.1–3.6 | 7 (2.7) | 1.2–5.2 |
| Outcomes | ||||
| In-hospital complications | 40 (31.5) | 23.9–39.9 | 73 (28.2) | 23.0–33.9 |
| 30-day mortality | 24 (18.9) | 13.8–26.4 | 22 (8.5) | 5.6–12.4 |
| Factors | Adjusted OR | 95% CI |
|---|---|---|
| Age ≤ 75 years | 0.99 | 0.95–1.03 |
| Age > 75 years | 0.85 | 0.76–0.94 |
| Sex (reference: male) | 1.16 | 0.63–2.12 |
| BMI group (reference: <25 kg/m2) | ||
| 25–<30 | 1.54 | 0.71–3.36 |
| 30–35 | 1.02 | 0.42–2.44 |
| >35 | 0.43 | 0.14–1.33 |
| Smoking status (never smoker) | ||
| Current smoker | 1.41 | 0.66–2.99 |
| Previous smoker | 0.97 | 0.42–2.29 |
| Diabetes | 1.34 | 0.71–2.53 |
| Hypertension | 2.86 | 1.45–5.62 |
| Hyperlipidemia | 0.59 | 0.32–1.12 |
| History of stroke | 0.71 | 0.30–1.69 |
| Atrial fibrillation | 1.19 | 0.63–2.36 |
| Chronic kidney disease | 1.95 | 0.97–3.91 |
| Heart failure | 0.52 | 0.30–0.90 |
| Heart rate | 0.98 | 0.97–0.99 |
| Systolic blood pressure | 0.99 | 0.98–1.01 |
| STEMI (reference: NSTEMI) | 1.96 | 1.10–3.68 |
| Factors | Adjusted OR | 95% CI |
|---|---|---|
| Invasive intervention | 0.25 | 0.10–0.67 |
| Available catheterization laboratory in the hospital where in-hospital AMI was diagnosed (reference: no) | 8.75 | 2.68–25.39 |
| Urban region (reference: rural) | 0.22 | 0.06–1.20 |
| Age ≤ 75 years | 0.28 | 0.14–3.01 |
| Age > 75 years | 4.60 | 0.32–6.7 |
| Hypertension | 0.53 | 0.23–1.37 |
| Heart failure | 1.91 | 0.84–4.40 |
| Heart rate upon admission | 1.01 | 0.99–1.02 |
| STEMI (reference: NSTEMI) | 2.85 | 1.19–6.84 |
| Number of Patients Who Survived beyond 30 Days after In-Hospital AMI Onset: N = 340 | Conservative Treatment: N = 103 | Invasive Intervention: N = 237 | ||
|---|---|---|---|---|
| N (%) | 95% CI | N (%) | 95% CI | |
| ASS | 87 (84.5) | 76.6–90.5 | 216 (91.1) | 87.0–94.3 |
| P2Y12 receptor inhibitor | 52 (50.5) | 40.9–60.0 | 192 (81.0) | 75.7–85.6 |
| Anticoagulant | 39 (37.9) | 28.9–47.5 | 50 (21.1) | 16.3–26.6 |
| ACE/ARB | 68 (66.0) | 56.5–74.6 | 173 (73.0) | 67.1–78.3 |
| Beta-blocker | 72 (69.9) | 60.6–78.1 | 204 (86.1) | 81.2–90.0 |
| Statin | 47 (45.6) | 36.2–55.3 | 179 (75.5) | 69.8–80.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assaf, M.; Costa, D.; Efremov, L.; Holland, K.; Mikolajczyk, R. Comparison between Invasive Intervention and Conservative Treatment in Patients with In-Hospital Myocardial Infarctions: Results from the Regional Myocardial Infarction Registry of Saxony-Anhalt (RHESA) Study. J. Clin. Med. 2024, 13, 2194. https://doi.org/10.3390/jcm13082194
Assaf M, Costa D, Efremov L, Holland K, Mikolajczyk R. Comparison between Invasive Intervention and Conservative Treatment in Patients with In-Hospital Myocardial Infarctions: Results from the Regional Myocardial Infarction Registry of Saxony-Anhalt (RHESA) Study. Journal of Clinical Medicine. 2024; 13(8):2194. https://doi.org/10.3390/jcm13082194
Chicago/Turabian StyleAssaf, Mohamad, Daniela Costa, Ljupcho Efremov, Karen Holland, and Rafael Mikolajczyk. 2024. "Comparison between Invasive Intervention and Conservative Treatment in Patients with In-Hospital Myocardial Infarctions: Results from the Regional Myocardial Infarction Registry of Saxony-Anhalt (RHESA) Study" Journal of Clinical Medicine 13, no. 8: 2194. https://doi.org/10.3390/jcm13082194
APA StyleAssaf, M., Costa, D., Efremov, L., Holland, K., & Mikolajczyk, R. (2024). Comparison between Invasive Intervention and Conservative Treatment in Patients with In-Hospital Myocardial Infarctions: Results from the Regional Myocardial Infarction Registry of Saxony-Anhalt (RHESA) Study. Journal of Clinical Medicine, 13(8), 2194. https://doi.org/10.3390/jcm13082194