Long-Term Results of the Mini Maze Standalone Bi-Atrial Surgical Ablation: A 10-Year Follow-Up
Abstract
1. Introduction
2. Materials and Methods
2.1. Population
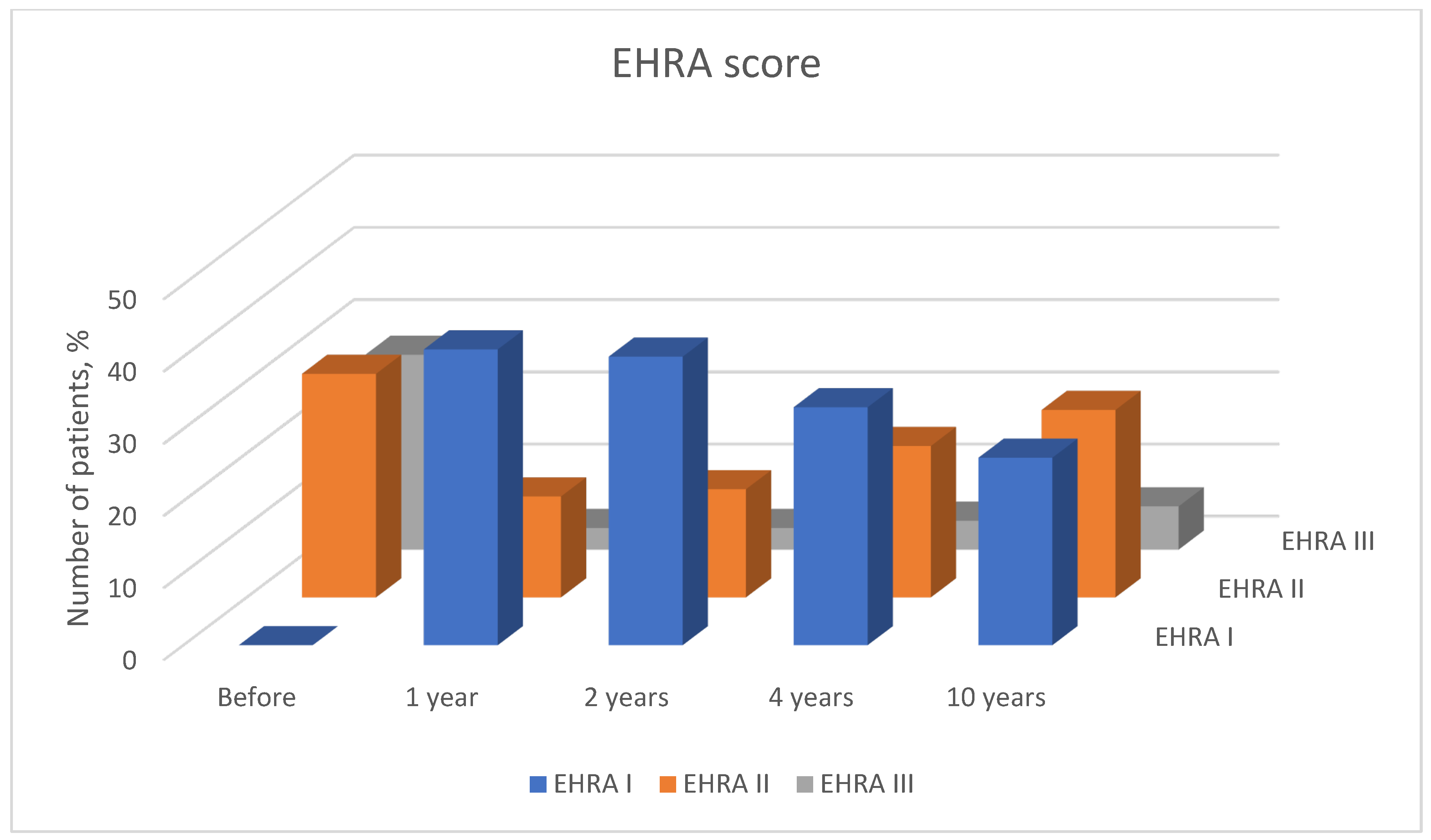
2.2. Patient Follow-Up
- EHRA I—‘No symptoms’.
- EHRA II—‘Mild symptoms’; normal daily activity.
- EHRA III—‘Severe symptoms’; limited daily activity.
- EHRA IV—‘Disabling symptoms’; unable to carry out daily activities.
2.3. Surgical Technique
2.3.1. Patient Positioning and Anaesthesia
2.3.2. Right-Side Access
2.3.3. Left-Side Access
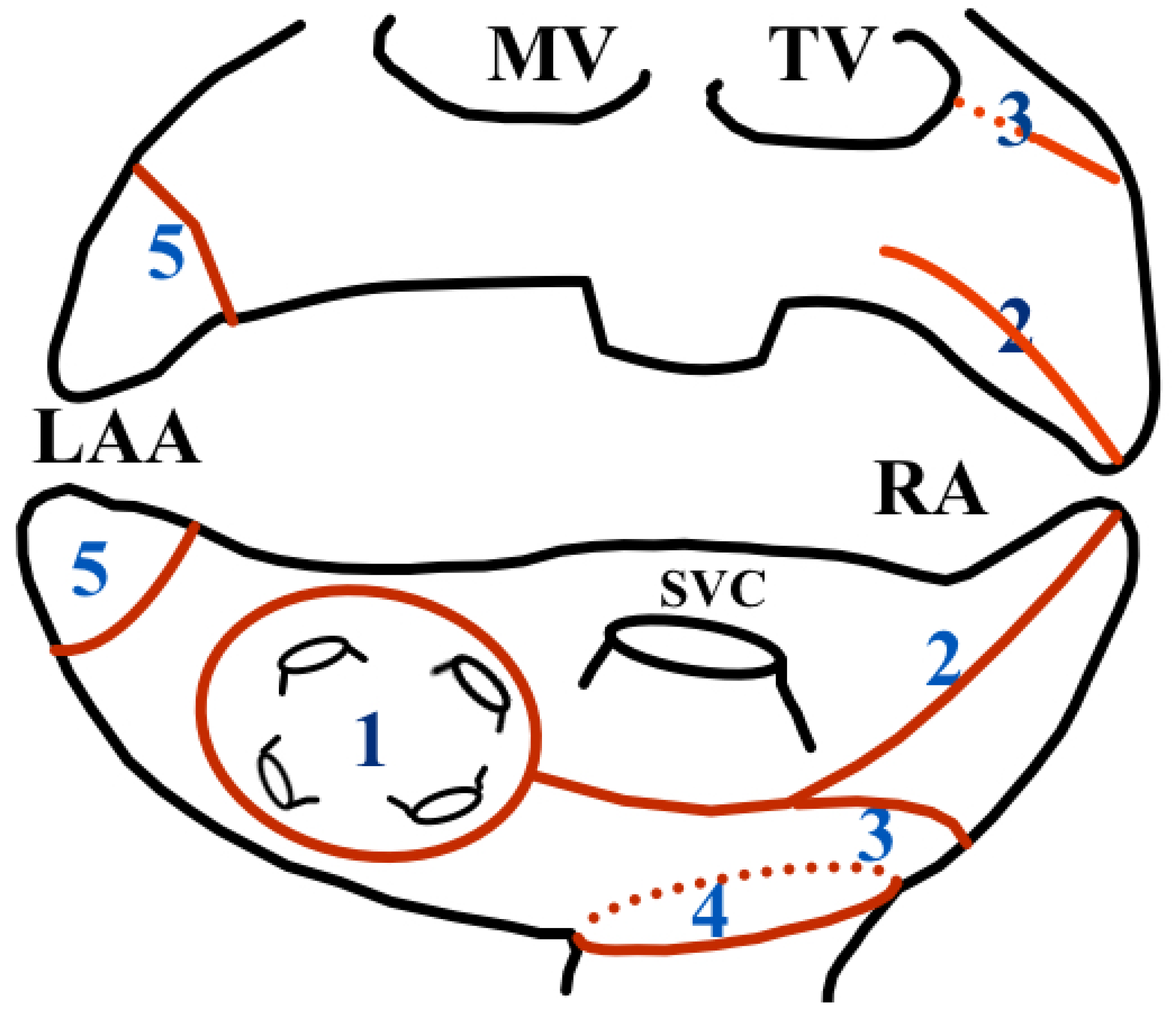
2.3.4. Right Atrial Ablation
- Pulmonary vein isolation (“Box lesion”).
- From RA appendage to “box lesion”.
- From mid of RA to “box lesion”.
- IVC ostia.
- 5.
- Ligation of left atrial appendage and cutting of the Ligament of Marshall.
2.3.5. Additional Procedures
2.4. Statistical Analysis
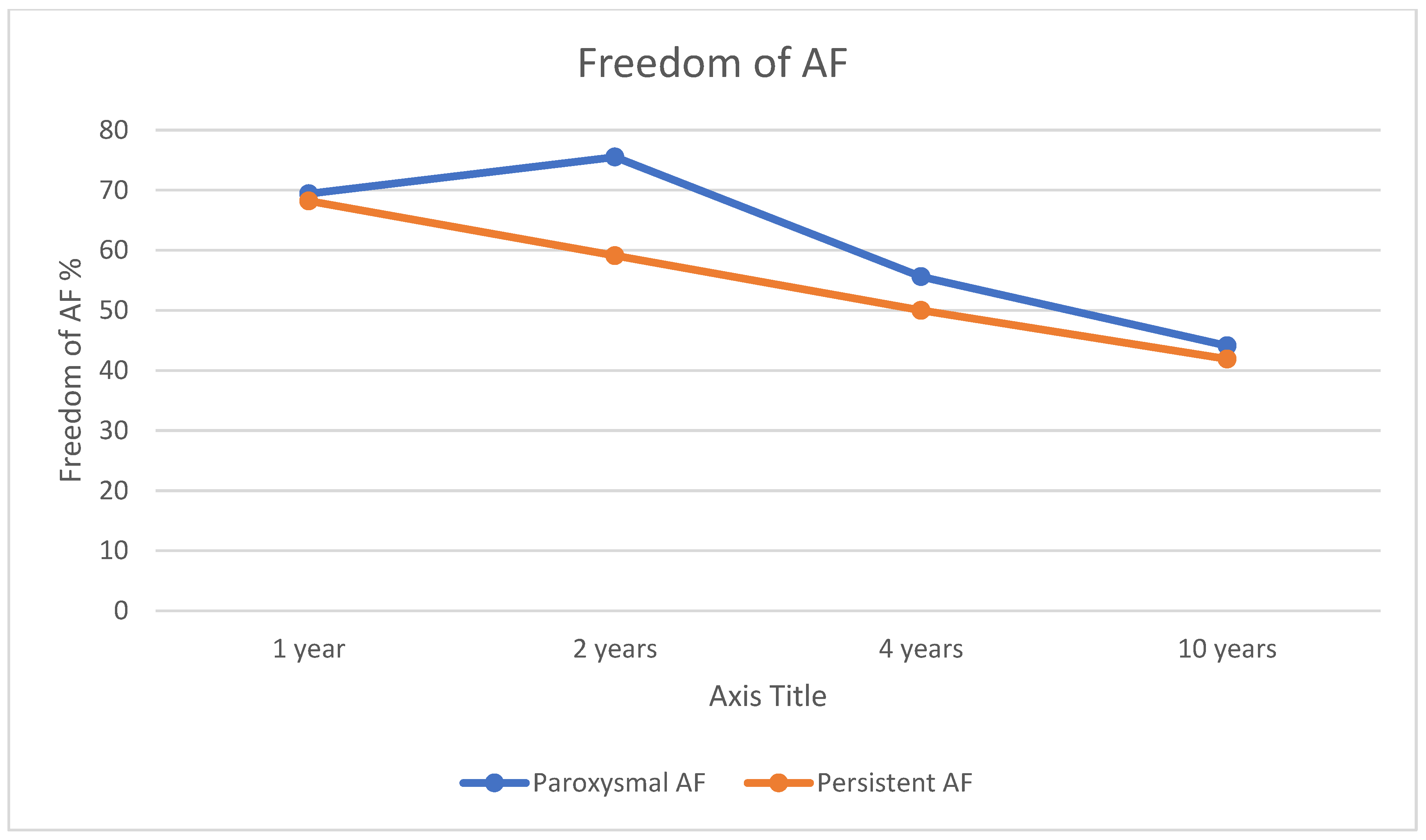
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morillo, C.A.; Banerjee, A.; Perel, P.; Wood, D.; Jouven, X. Atrial fibrillation: The current epidemic. J. Geriatr. Cardiol. 2017, 14, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2016 (GBD 2016) Results; Institute for Health Metrics and Evaluation (IHME): Seattle, WA, USA, 2017; Available online: http://ghdx.healthdata.org/gbd-results-tool (accessed on 20 April 2018).
- Krijthe, B.P.; Kunst, A.; Benjamin, E.J.; Lip, G.Y.; Franco, O.H.; Hofman, A.; Witteman, J.C.; Stricker, B.H.; Heeringa, J. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur. Heart J. 2013, 34, 2746–2751. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Luc, J.G.; Phan, K. Atrial fibrillation: Review of current treatment strategies. J Thorac. Dis. 2016, 8, E886–E900. [Google Scholar] [CrossRef] [PubMed]
- Ad, N.; Holmes, S.D.; Friehling, T. Minimally Invasive Standalone Cox Maze Procedure for Persistent and Long-Standing Persistent Atrial Fibrillation Perioperative Safety and 5-Year Outcomes. Circ. Arrhythmia Electrophysiol. 2017, 10, e005352. [Google Scholar] [CrossRef] [PubMed]
- De Asmundis, C.; Chierchia, G.B.; Mugnai, G.; Van Loo, I.; Nijs, J.; Czapla, J.; Conte, G.; Velagic, V.; Rodrigues Mañero, M.; Ciconte, G.; et al. Midterm clinical outcomes of concomitant thoracoscopic epicardial and transcatheter endocardial ablation for persistent and long-standing persistent atrial fibrillation: A single-centre experience. Europace 2017, 19, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Moulton, L.C. A Minimally Invasive Approach to the Treatment of Atrial Fibrillation: The Mini-Maze. Order and Disorder Electrophysiology Training Program. EP Lab Dig. 2005, 5, 1–6. [Google Scholar]
- Nakamura, Y.; Kiaii, B.; Chu, M.W. Minimally Invasive Surgical Therapies for Atrial Fibrillation. Int. Sch. Res. Not. 2012, 2012, 606324. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yan, T.; Zhu, S.J.; Zhu, M.; Guo, C.F. An off-pump biatrial mini-maze procedure for treatment of long-standing persistent atrial fibrillation. Eur. Heart J. 2021, 42 (Suppl. S1), ehab724.0540. [Google Scholar] [CrossRef]
- Lönnerholm, S.; Blomström, P.L.; Nilsson, S.; Jideus, O.L.; Blomström-Lundqvist, C. Effects of the Maze Operation on Health-Related Quality of Life in Patients with Atrial Fibrillation. Circulation 2000, 101, 2607–2611. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Barakat, A.F.; Saliba, W.I.; Rehman, K.A.; Tarakji, K.G.; Rickard, J.; Bassiouny, M.; Baranowski, B.; Tchou, P.; Bhargava, M.; et al. Recurrent Atrial Fibrillation After Initial Long-Term Ablation Success. Electrophysiological Findings and Outcomes of Repeat Ablation Procedures. Circ. Arrhythmia Electrophysiol. 2018, 11, e005785. [Google Scholar] [CrossRef]
- Ferre-Vallverdu, M.; Ligero, C.; Vidal-Perez, R.; Martinez-Rubio, A.; Vinolas, X.; Alegret, J.M. Improvement in Atrial Fibrillation-Related Symptoms After Cardioversion: Role of NYHA Functional Class and Maintenance of Sinus Rhythm. Clin. Interv. Aging 2021, 16, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Wazni, O.; Dandamudi, G.; Sood, N.; Hoyt, R.; Tyler, J.; Durrani, S.; Niebauer, M.; Makati, K.; Halperin, B.; Gauri, A.; et al. Quality of life after the initial treatment of atrial fibrillation with cryoablation versus drug therapy. Heart Rhythm. 2022, 19, 197–205. [Google Scholar] [CrossRef] [PubMed]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2019, 16, e66–e93. [Google Scholar] [PubMed]
- Verma, A.; Haines, D.E.; Boersma, L.V.; Sood, N.; Natale, A.; Marchlinski, F.E.; Calkins, H.; Sanders, P.; Packer, D.L.; Kuck, K.H.; et al. Pulsed Field Ablation for the Treatment of Atrial Fibrillation: PULSED AF Pivotal Trial. Circulation 2023, 147, 1422–1432. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.L.; Jaquiss, R.D.; Schuessler, R.B.; Boineau, J.P. Modification of the maze procedure for atrial flutter and atrial fibrillation: II. Surgical technique of the maze III procedure. J. Thorac. Cardiovasc. Surg. 1995, 110, 473–484. [Google Scholar] [CrossRef]
- Wolf, R.K.; Schneeberger, E.W.; Osterday, R.; Miller, D.; Merrill, W.; Fiege, J.B.; Gillinov, A.M. Video-assisted bilateral pulmonary vein isolationand left atrial appendage exclusion for atrial fibrillation. J Thorac. Cardiovasc. Surg. 2005, 130, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Mariani, M.A.; Pozzoli, A.; Maat, G.; Alfieri, O.R.; Benussi, S. What Does the Blanking Period Blank? J. Atr. Fibrillation 2015, 8, 1268. [Google Scholar]
- Edgerton, J.R.; McClelland, J.H.; Duke, D.; Gerdisch, M.W.; Steinberg, B.M.; Bronleewe, S.H.; Prince, S.L.; Herbert, M.A.; Hoffman, S.; Mack, M.J.; et al. Minimally invasive surgical ablation of atrial fibrillation: Six-month results. J. Thorac. Cardiovasc. Surg. 2009, 138, 109–113; discussion 114. [Google Scholar] [CrossRef]
- Bagge, L.; Blomström, P.; Nilsson, L.; Einarsson, G.M.; Jidéus, L.; Blomström-Lundqvist, C. Epicardial off-pump pulmonary vein isolation and vagal denervation improve long-term outcome and quality of life in patients with atrial fibrillation. J. Thorac. Cardiovasc. Surg. 2009, 137, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J. Current results of minimally invasive surgical ablation for isolated atrial fibrillation. Heart Rhythm. 2009, 6, S46–S49. [Google Scholar] [CrossRef] [PubMed]
- Santini, M.; Loiaconi, V.; Tocco, M.P.; Mele, F.; Pandozi, C. Feasibility and efficacy of minimally invasive standalone surgical ablation of atrial fibrillation. A single-center experience. J. Interv. Card. Electrophysiol. 2012, 34, 79–87. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Willems, S.; Khairy, P.; Andrade, J.; Hoffmann, B.; Levesque, S.; Verma, A.; Weerasooriya, R.; Novak, P.; Arentz, T.; Deisenhofer, I.; et al. Redefining the Blanking Period After Catheter Ablation for Paroxysmal Atrial Fibrillation: Insights from the ADVICE (Adenosine Following Pulmonary Vein Isolation to Target Dormant Conduction Elimination) Trial. Circ. Arrhythmia Electrophysiol. 2016, 9, e003909. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Mei, J.; Lu, R.X.; Jiang, Z.L.; Tang, M.; Ding, F.B. Clinical results of Mei mini maze procedure for atrial fibrillation patients with previously failed catheter ablation. Zhonghua Xin Xue Guan Bing Za Zhi 2018, 46, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Navaratnarajah, M.; Suvitesh, L.; Sunil, O. Surgical Treatment of Atrial Fibrillation. In Epidemiology and Treatment of Atrial Fibrillation; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar] [CrossRef]
- Krul, S.P.; Driessen, A.H.; Zwinderman, A.H.; van Boven, W.J.; Wilde, A.A.; de Bakker, J.M.; de Groot, J.R. Navigating the mini-maze: Systematic review of the first results and progress of minimally-invasive surgery in the treatment of atrial fibrillation. Int. J. Cardiol. 2013, 166, 132–140. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Björkenheim, A.; Brandes, A.; Magnuson, A.; Chemnitz, A.; Svedberg, L.; Edvardsson, N.; Poçi, D. Assessment of Atrial Fibril-lation–Specific Symptoms Before and 2 Years After Atrial Fibrillation Ablation: Do Patients and Physicians Differ in Their Per-ception of Symptom Relief? JACC Clin. Electrophysiol. 2017, 3, 1168–1176. [Google Scholar] [CrossRef] [PubMed]

| Paroxysmal AF (n = 36) | Persistent AF (n = 22) | p-Value | |
|---|---|---|---|
| Age | 51.36 ± 9.19 | 49.73 ± 8.59 | 0.504 |
| Female | 5 | 4 | 0.98 |
| CHA2DS2-VAsc Score 0 | 2 | 6 | 0.94 |
| CHA2DS2-VASc Score 1 | 27 | 12 | |
| CHA2DS2-VAsc Score 2 | 5 | 3 | |
| CHA2DS2-VASc Score ≥ 3 | 2 | 1 | |
| NYHA functional class I | 13 | 8 | 0.946 |
| NYHA functional class II | 16 | 9 | |
| NYHA functional class III | 7 | 5 | |
| LA area (cm2) | 23.91 ± 4.11 | 26.77 ± 7.03 | 0.095 |
| LA volume index (mL/m2) | 38.89 ± 10.66 | 45.57 ± 14.50 | 0.05 |
| LA inferior–superior diameter (mm) | 59.2 ± 6.3 | 60.1 ± 8.9 | 0.671 |
| LA medial–lateral diameter (mm) | 46.6 ± 4.4 | 4.6 ± 5.4 | 0.636 |
| AF duration (months) | 68.84 ± 52 | 71.53 ± 49 | 0.414 |
| Paroxysmal AF | Persistent AF | p-Value | |
|---|---|---|---|
| LA area (cm2) before | 23.91 ± 4.11 | 26.77 ± 7.03 | 0.095 |
| LA area (cm2) 4 years after | 27.72 ± 5.74 | 28.86 ± 6.08 | 0.76 |
| LA area (cm2) 10 years after | 30 ± 5.28 | 31.03 ± 6.17 | 0.50 |
| LA inferior–superior diameter (mm) before | 59.2 ± 6.3 | 60.1 ± 8.9 | 0.671 |
| LA inferior–superior diameter (mm) 4 years after | 64.5 ± 6.4 | 65.9 ± 7.5 | 0.463 |
| LA inferior–superior diameter (mm) 10 years after | 68.6 ± 5.8 | 69.5 ± 7.4 | 0.581 |
| LA medial–lateral diameter (mm) before | 46.6 ± 4.4 | 46.0 ± 5.4 | 0.636 |
| LA medial–lateral diameter (mm) 4 years after | 48.0 ± 5.3 | 50.2 ± 5.3 | 0.127 |
| LA medial–lateral diameter (mm) 10 years after | 50.0 ± 4.5 | 53.5 ± 5.8 | 0.016 |
| LA volume index (mL/m2) before | 38.89 ± 10.66 | 45.57 ± 14.50 | 0.05 |
| LA volume index (mL/m2) 4 years after | 46.22 ± 14.64 | 50.20 ± 10.54 | 0.29 |
| LA volume index (mL/m2) 10 years after | 51.02 ± 12.04 | 59.58 ± 13.45 | 0.016 |
| No Recurrence of AF | Recurrence of AF | p-Value | |
|---|---|---|---|
| LA area (cm2) before | 23.72 ± 5.2 | 25.84 ± 4.59 | 0.192 |
| LA area (cm2) 4 years after | 26.83 ± 5.33 | 29.96 ± 6.91 | 0.02 |
| LA area (cm2) 10 years after | 29.34 ± 3.4 | 31.79 ± 6.07 | 0.073 |
| LA inferior–superior diameter (mm) before | 58.6 ± 7.5 | 60.7 ± 7.0 | 0.249 |
| LA inferior–superior diameter (mm) 4 years after | 63.0 ± 6.1 | 67.3 ± 7.0 | 0.027 |
| LA inferior–superior diameter (mm) 10 years after | 67.0 ± 6.1 | 71.2 ± 6.1 | 0.020 |
| LA medial–lateral diameter (mm) before | 45.6 ± 4.2 | 47.2 ± 4.9 | 0.209 |
| LA medial–lateral diameter (mm) 4 years after | 47.7 ± 5.7 | 50.0 ± 4.9 | 0.116 |
| LA medial–lateral diameter (mm) 10 years after | 52.9 ± 5.1 | 59.9 ± 4.9 | 0.027 |
| LA volume index (mL/m2) before | 27.63 ± 10.54 | 30.50 ± 15.41 | 0.544 |
| LA volume index (mL/m2) 4 years after | 42.13 ± 9.94 | 56.89 ± 16.06 | 0.000 |
| LA volume index (mL/m2) 10 years after | 51.06 ± 12.22 | 58.21 ± 13.44 | 0.035 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radauskaite, G.; Rackauskas, G.; Danilenko, S.; Janusauskas, V.; Aidietis, A. Long-Term Results of the Mini Maze Standalone Bi-Atrial Surgical Ablation: A 10-Year Follow-Up. J. Clin. Med. 2024, 13, 2195. https://doi.org/10.3390/jcm13082195
Radauskaite G, Rackauskas G, Danilenko S, Janusauskas V, Aidietis A. Long-Term Results of the Mini Maze Standalone Bi-Atrial Surgical Ablation: A 10-Year Follow-Up. Journal of Clinical Medicine. 2024; 13(8):2195. https://doi.org/10.3390/jcm13082195
Chicago/Turabian StyleRadauskaite, Greta, Gediminas Rackauskas, Svetlana Danilenko, Vilius Janusauskas, and Audrius Aidietis. 2024. "Long-Term Results of the Mini Maze Standalone Bi-Atrial Surgical Ablation: A 10-Year Follow-Up" Journal of Clinical Medicine 13, no. 8: 2195. https://doi.org/10.3390/jcm13082195
APA StyleRadauskaite, G., Rackauskas, G., Danilenko, S., Janusauskas, V., & Aidietis, A. (2024). Long-Term Results of the Mini Maze Standalone Bi-Atrial Surgical Ablation: A 10-Year Follow-Up. Journal of Clinical Medicine, 13(8), 2195. https://doi.org/10.3390/jcm13082195






