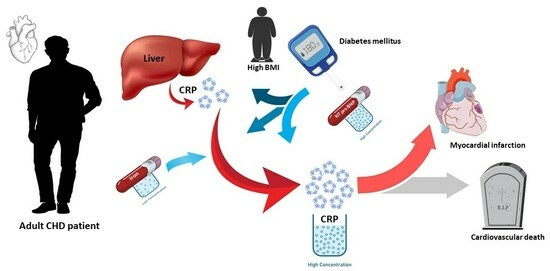
C-Reactive Protein and Long-Term Prognosis in Adult Patients with Congenital Heart Disease
Abstract
:1. Introduction
2. Methods
2.1. Clinical Data
2.2. Blood Test
2.3. Follow-Up
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.1.1. Clinical and Blood Test Data in Patients with CHD and the Control Population
3.1.2. Clinical and Blood Test Data in CHD Patients according to Their Hs-CRP Levels
3.2. Predictors of High CRP Levels in Patients with CHD
3.3. MACE in Patients with CHD
4. Discussion
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Majdalany, D.S. Congenital Heart Disease: A Growing Population with Challenges to Patients and Providers. J. Pers. Med. 2023, 13, 1442. [Google Scholar] [CrossRef] [PubMed]
- Pepys, M.B.; Hirschfield, G.M. C-reactive protein: A critical update. J. Clin. Investig. 2003, 111, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Calle, M.C.; Fernandez, M.L. Inflammation and type 2 diabetes. Diabetes Metab. 2012, 38, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Fung, E.; Xu, A.; Lan, H.Y. C-reactive protein and ageing. Clin. Exp. Pharmacol. Physiol. 2017, 44 (Suppl. S1), 9–14. [Google Scholar] [CrossRef] [PubMed]
- Emerging Risk Factors Collaboration; Kaptoge, S.; Di Angelantonio, E.; Lowe, G.; Pepys, M.B.; Thompson, S.G.; Collins, R.; Danesh, J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: An individual participant meta-analysis. Lancet 2010, 375, 132–140. [Google Scholar] [CrossRef]
- Liu, C.; Li, C. C-reactive protein and cardiovascular diseases: A synthesis of studies based on different designs. Eur. J. Prev. Cardiol. 2023, 30, 1593–1596. [Google Scholar] [CrossRef] [PubMed]
- Geenen, L.W.; Baggen, V.J.M.; van den Bosch, A.E.; Eindhoven, J.A.; Kauling, R.M.; Cuypers, J.A.A.E.; Roos-Hesselink, J.W.; Boersma, E. Prognostic value of C-reactive protein in adults with congenital heart disease. Heart 2020, 107, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Opotowsky, A.R.; Valente, A.M.; Alshawabkeh, L.; Cheng, S.; Bradley, A.; Rimm, E.B.; Landzberg, M.J. Prospective cohort study of C-reactive protein as a predictor of clinical events in adults with congenital heart disease: Results of the Boston adult congenital heart disease biobank. Eur. Heart J. 2018, 39, 3253–3261. [Google Scholar] [CrossRef] [PubMed]
- Aldweib, N.; Elia, E.G.; Brainard, S.B.; Wu, F.; Sleeper, L.A.; Rodriquez, C.; Valente, A.M.; Landzberg, M.J.; Singh, M.; Mullen, M.; et al. Serial cardiac biomarker assessment in adults with congenital heart disease hospitalized for decompensated heart failure. Int. J. Cardiol. Congenit. Heart Dis. 2022, 7, 100336. [Google Scholar] [CrossRef] [PubMed]
- Webb, D.G.; Roberta, G. Care of the Adult with Congenital Heart Disease. Presented at the 32nd Bethesda Conference, Bethesda, Maryland, October 2–3, 2000. J. Am. Coll. Cardiol. 2001, 37, 1161–1198. [Google Scholar]
- Martínez-Quintana, E.; Rodríguez-Hernández, J.L.; Rodríguez-González, F.; Riaño-Ruiz, M.; Fraguela-Medina, C.; Girolimetti, A.; Jiménez-Rodríguez, S. Cardiovascular risk factors and arterial thrombotic events in congenital heart disease patients. Int. J. Clin. Pract. 2019, 73, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.A.; Coresh, J.; Greene, T.; Levey, A.S. Assessing kidney function—Measured and estimated glomerular filtration rate. N. Engl. J. Med. 2006, 354, 2473–2483. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [PubMed]
- Nehring, S.M.; Goyal, A.; Patel, B.C. C Reactive Protein. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441843/ (accessed on 22 January 2024).
- Hicks, K.A.; Mahaffey, K.W.; Mehran, R.; Nissen, S.E.; Wiviott, S.D.; Dunn, B.; Solomon, S.D.; Marler, J.R.; Teerlink, J.R.; Farb, A.; et al. 2017 Cardiovascular and Stroke Endpoint Definitions for Clinical Trials. Circulation 2018, 137, 961–972. [Google Scholar] [CrossRef]
- Ong, K.L.; Allison, M.A.; Cheung, B.M.; Wu, B.J.; Barter, P.J.; Rye, K.A. Trends in C-reactive protein levels in US adults from 1999 to 2010. Am. J. Epidemiol. 2013, 177, 1430–1442. [Google Scholar] [CrossRef] [PubMed]
- Stanimirovic, J.; Radovanovic, J.; Banjac, K.; Obradovic, M.; Essack, M.; Zafirovic, S.; Gluvic, Z.; Gojobori, T.; Isenovic, E.R. Role of C-Reactive Protein in Diabetic Inflammation. Mediat. Inflamm. 2022, 2022, 3706508. [Google Scholar] [CrossRef] [PubMed]
- Thorand, B.; Löwel, H.; Schneider, A.; Kolb, H.; Meisinger, C.; Fröhlich, M.; Koenig, W. C-reactive protein as a predictor for incident diabetes mellitus among middle-aged men: Results from the MONICA Augsburg cohort study, 1984–1998. Arch. Intern. Med. 2003, 163, 93–99. [Google Scholar] [CrossRef]
- Kamath, D.Y.; Xavier, D.; Sigamani, A.; Pais, P. High sensitivity C-reactive protein (hsCRP) & cardiovascular disease: An Indian perspective. Indian. J. Med. Res. 2015, 142, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef]
- Maierean, S.; Webb, R.; Banach, M.; Mazidi, M. The role of inflammation and the possibilities of inflammation reduction to prevent cardiovascular events. Eur. Heart J. Open 2022, 2, oeac039. [Google Scholar] [CrossRef] [PubMed]
- Anand, I.S.; Latini, R.; Florea, V.G.; Kuskowski, M.A.; Rector, T.; Masson, S.; Signorini, S.; Mocarelli, P.; Hester, A.; Glazer, R.; et al. C-reactive protein in heart failure: Prognostic value and the effect of valsartan. Circulation 2005, 112, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Cappellini, M.D.; Comin-Colet, J.; de Francisco, A.; Dignass, A.; Doehner, W.; Lam, C.S.; Macdougall, I.C.; Rogler, G.; Camaschella, C.; Kadir, R.; et al. Iron deficiency across chronic inflammatory conditions: International expert opinion on definition, diagnosis, and management. Am. J. Hematol. 2017, 92, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, T.; Stein, J.; Sharma, N.; Kulnigg-Dabsch, S.; Vel, S.; Gasche, C. Clinical significance of C-reactive protein levels in predicting responsiveness to iron therapy in patients with inflammatory bowel disease and iron deficiency anemia. Dig. Dis. Sci. 2015, 60, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Wessling-Resnick, M. Iron homeostasis and the inflammatory response. Annu. Rev. Nutr. 2010, 30, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Hackam, D.G.; Shumak, S.L. C-reactive protein for the prediction of cardiovascular risk: Ready for prime-time? CMAJ 2004, 170, 1563–1565. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, D.; Zhao, Y.; Liu, D.; Li, Q.; Guo, C.; Tian, G.; Han, M.; Qie, R.; Huang, S.; et al. Association between serum level of C-reactive protein and risk of cardiovascular events based on cohort studies. J. Hum. Hypertens. 2021, 35, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Yeboah, J.; McClelland, R.L.; Polonsky, T.S.; Burke, G.L.; Sibley, C.T.; O’Leary, D.; Carr, J.J.; Goff, D.C.; Greenland, P.; Herrington, D.M. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA 2012, 308, 788–795. [Google Scholar] [CrossRef]
- Hendricks, S.; Dykun, I.; Balcer, B.; Totzeck, M.; Rassaf, T.; Mahabadi, A.A. Higher BNP/NT-pro BNP levels stratify prognosis equally well in patients with and without heart failure: A meta-analysis. ESC Heart Fail. 2022, 9, 3198–3209. [Google Scholar] [CrossRef] [PubMed]

| Types of CHD according to Complexity | Number of Patients |
|---|---|
| Simple complexity | 222 |
| Aortic valve disease | 26 |
| Pulmonary valve disease | 41 |
| Atrial septal defect | 49 |
| Ventricular septal defect | 70 |
| Ductus | 10 |
| Anomalous pulmonary venous drainage | 2 |
| Other simple defects | 24 |
| Moderate complexity | 131 |
| Subvalvular or supravalvular aortic stenosis | 15 |
| Coarctation of the aorta | 39 |
| Subvalvular or supravalvular pulmonary stenosis | 7 |
| Tetralogy of Fallot | 37 |
| Ebstein | 5 |
| Atrioventricular septal defects | 28 |
| Great complexity | 81 |
| Dextro transposition of the great arteries | 17 |
| Levo transposition of the great arteries | 9 |
| Pulmonary atresia | 4 |
| Single ventricle | 10 |
| Double outlet right ventricle | 7 |
| Tricuspid atresia | 3 |
| Trucus arteriosus | 2 |
| CHD with pulmonary arterial hypertension (Eisenmenger) | 29 |
| Total of patients with CHD | 434 |
| Control | CHD | p * | |
|---|---|---|---|
| CHD patients, n | 820 | 434 | |
| Age, years | 33 (19–49) | 30 (18–62) | 0.702 |
| Sex (male), n | 515 (63) | 256 (59) | 0.186 |
| Arterial hypertension, n | 83 (10) | 59 (14) | 0.065 |
| Diabetes mellitus | 0.232 | ||
| Type 1, n | 8 (1) | 3 (1) | |
| Type 2 diabetes oral hypoglycemic agents, n | 29 (4) | 15 (3) | |
| Type 2 with insulin, n | 4 (0.5) | 7 (2) | |
| Dyslipidemia, n | 182 (22) | 81 (19) | 0.144 |
| Smoking, n | 152 (19) | 23 (5) | <0.001 |
| Laboratory results | |||
| Glucose, mg/dL | 94 (81–120) | 94 (81–117) | 0.134 |
| Creatinine, mg/dL | 0.8 (0.5–1.0) | 0.9 (0.6–1.2) | <0.001 |
| GFR, mL/min/1.73 m2 | 111 (83–153) | 91 (61–154) | <0.001 |
| Hemoglobin, mg/dL | 14 (12–17) | 15 (12–17) | 0.083 |
| Total bilirubin, mg/dL | 0.6 (0.3–1.5) | 0.7 (0.3–2.1) | <0.001 |
| Total cholesterol, mg/dL | 176 (122–246) | 160 (108–231) | 0.678 |
| LDL cholesterol, mg/dL | 101 (60–156) | 91 (46–149) | 0.634 |
| HDL cholesterol, mg/dL | 50 (35–75) | 48 (32–70) | 0.341 |
| ALT, IU/L | 19 (10–64) | 17 (9–50) | 0.847 |
| AST, IU/L | 22 (15–45) | 22 (14–42) | 0.702 |
| Hs-CRP, mg/dL | 0.17 (0.03–1.39) | 0.16 (0.00–1.59) | 0.404 |
| Medical treatment | |||
| Antiplatelet, n | 9 (1) | 40 (9) | <0.001 |
| Oral anticoagulation, n | 4 (0.5) | 65 (15) | <0.001 |
| Betablockers, n | 18 (2) | 62 (14) | <0.001 |
| ACE inhibitors/ARBs, n | 66 (8) | 65 (15) | <0.001 |
| Calcium channel blockers, n | 11 (1) | 16 (4) | 0.006 |
| Loop diuretics, n | 25 (3) | 61 (14) | <0.001 |
| Oral iron, n | 31 (4) | 19 (4) | 0.607 |
| Statins, n | 51 (6) | 37 (9) | 0.128 |
| CHD Patients | p * | ||
|---|---|---|---|
| Hs-CRP < 0.3 mg/dL | Hs-CRP ≥ 0.3 mg/dL | ||
| CHD patients, n | 277 | 157 | |
| Age, years | 28 (19–61) | 37 (19–64) | <0.001 |
| Sex (male), n | 172 (62) | 84 (54) | 0.080 |
| BMI, kg/m2 | 23 (18–33) | 25 (17–38) | <0.001 |
| Great CHD complexity, n | 0.131 | ||
| Mild | 147 (53) | 73 (46) | |
| Moderate | 86 (31) | 47 (30) | |
| Great | 44 (16) | 37 (24) | |
| NYHA functional class (≥2), n | 36 (13) | 42 (27) | 0.005 |
| Arterial hypertension, n | 33 (12) | 26 (17) | 0.175 |
| Diabetes mellitus, n | 7 (3) | 18 (11) | <0.001 |
| Dyslipidemia, n | 42 (15) | 39 (25) | 0.013 |
| Smoker, n | 16 (6) | 7 (4) | 0.379 |
| Laboratory results | |||
| Glucose, mg/dL | 93 (82–111) | 94 (77–139) | 0.267 |
| Creatinine, mg/dL | 0.9 (0.6–1.2) | 0.9 (0.6–1.3) | 0.247 |
| GFR, mL/min/1.73 m2 | 91 (67–152) | 90 (54–162) | 0.289 |
| Hemoglobin, mg/dL | 15 (12–17) | 14 (11–18) | 0.017 |
| Total bilirubin, mg/dL | 0.7 (0.3–2.0) | 0.6 (0.3–2.5) | 0.016 |
| Total cholesterol, mg/dL | 156 (108–231) | 166 (108–231) | 0.130 |
| LDL cholesterol, mg/dL | 90 (46–148) | 93 (47–152) | 0.078 |
| HDL cholesterol, mg/dL | 50 (34–70) | 47 (29–69) | 0.070 |
| ALT, IU/L | 16 (9–48) | 19 (10–54) | 0.019 |
| AST, IU/L | 22 (14–39) | 22 (14–43) | 0.207 |
| NT-pro-BNP, pg/mL | 58 (0–712) | 106 (6–1796) | <0.001 |
| Iron, µg/dL | 85 (29–157) | 67 (18–133) | <0.001 |
| Ferritin, ng/mL | 34 (6–184) | 38 (7–245) | 0.250 |
| Treatment | |||
| Antiplatelet, n | 22 (8) | 18 (11) | 0.223 |
| Oral anticoagulation, n | 31 (11) | 34 (22) | 0.035 |
| Beta-blockers, n | 30 (11) | 32 (20) | 0.006 |
| ACE inhibitors/ARBs, n | 33 (12) | 32 (20) | 0.018 |
| Calcium channel blockers, n | 9 (3) | 7 (4) | 0.521 |
| Loop diuretics, n | 26 (9) | 35 (22) | <0.001 |
| Statins, n | 21 (8) | 10 (6) | 0.350 |
| Oral iron, n | 7 (2) | 12 (8) | 0.012 |
| Mechanical valve prosthesis, n | 11 (4) | 5 (3) | 0.670 |
| Systemic ventricular dysfunction #, n | 6 (2) | 9 (6) | 0.057 |
| Atrial fibrillation/flutter, n | 7 (2) | 13 (8) | 0.006 |
| Stroke, n | 10 (4) | 5 (3) | 0.816 |
| Myocardial infarction, n | 1 (0.4) | 4 (3) | 0.040 |
| Cardiovascular mortality, n | 7 (3) | 13 (8) | 0.006 |
| MACE, n | 18 (6) | 22 (14) | 0.009 |
| OR (Crude) (95% CI) | p | OR (Adjusted) (95%CI) | p | |
|---|---|---|---|---|
| Age, years | 1.09 (1.04–1.14) | <0.001 | 1.01 (0.99–1.04) | 0.354 |
| BMI, kg/m2 | 1.09 (1.04–1.14) | <0.001 | 1.07 (1.01–1.13) | 0.022 |
| NYHA (≥2) | 2.24 (0.91–5.53) | 0.080 | ||
| Diabetes mellitus | 4.99 (2.04–12.24) | <0.001 | 3.57 (1.07–11.97) | 0.039 |
| Dyslipidemia | 1.85 (1.13–3.01) | 0.014 | 0.62 (0.23–1.70) | 0.346 |
| Hemoglobin, mg/dL | 0.93 (0.84–1.03) | 0.193 | ||
| Bilirubin, mg/dL | 1.07 (0.94–1.21) | 0.289 | ||
| NT-pro-BNP, pg/mL | 1.01 (1.00–1.01) | 0.005 | 1.00 (1.00–1.01) | 0.021 |
| Iron, µg/dL | 0.99 (0.98–0.99) | 0.001 | 0.98 (0.97–0.99) | 0.001 |
| ALT, IU/L | 1.07 (0.98–1.02) | 0.164 | ||
| Atrial/flutter fibrillation | 3.48 (1.36–8.92) | 0.009 | 1.02 (0.21–5.01) | 0.980 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95%CI) | p | |
| Age, years | 1.05 (1.03–1.07) | <0.001 | 1.04 (1.014–1.06) | 0.001 |
| CHD complexity a | 6.17 (3.04–12.51) | <0.001 | 2.46 (1.07–5.69) | 0.035 |
| NYHA class b | 7.13 (3.19–15.39) | <0.031 | 2.07 (0.81–5.22) | 0.125 |
| Diabetes mellitus | 2.45 (0.91–6.60) | 0.076 | ||
| NT pro-BNP c | 15.87 (5.57–45.22) | <0.001 | 7.73 (2.54–23.50) | <0.001 |
| Hs-CRP d | 2.55 (1.28–5.09) | 0.005 | 1.34 (0.56–3.19) | 0.512 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Quintana, E.; Alcántara-Castellano, M.; García-Suárez, M.I.; Rodríguez-González, F. C-Reactive Protein and Long-Term Prognosis in Adult Patients with Congenital Heart Disease. J. Clin. Med. 2024, 13, 2199. https://doi.org/10.3390/jcm13082199
Martínez-Quintana E, Alcántara-Castellano M, García-Suárez MI, Rodríguez-González F. C-Reactive Protein and Long-Term Prognosis in Adult Patients with Congenital Heart Disease. Journal of Clinical Medicine. 2024; 13(8):2199. https://doi.org/10.3390/jcm13082199
Chicago/Turabian StyleMartínez-Quintana, Efrén, María Alcántara-Castellano, Marta Isabel García-Suárez, and Fayna Rodríguez-González. 2024. "C-Reactive Protein and Long-Term Prognosis in Adult Patients with Congenital Heart Disease" Journal of Clinical Medicine 13, no. 8: 2199. https://doi.org/10.3390/jcm13082199
APA StyleMartínez-Quintana, E., Alcántara-Castellano, M., García-Suárez, M. I., & Rodríguez-González, F. (2024). C-Reactive Protein and Long-Term Prognosis in Adult Patients with Congenital Heart Disease. Journal of Clinical Medicine, 13(8), 2199. https://doi.org/10.3390/jcm13082199