Sexual Function in Women with Polycystic Ovary Syndrome Living in Stable Heterosexual Relationships: A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Questionnaires and Scales
2.2. Statistical Analyses
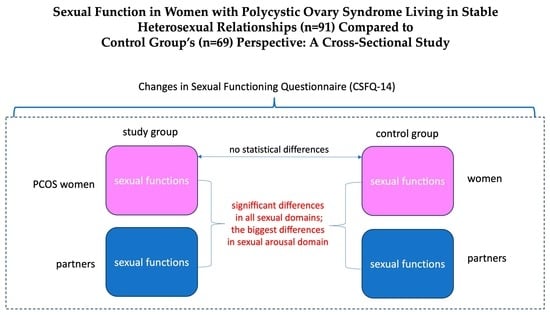
3. Results
3.1. Participants
3.2. Sexual Functioning—Descriptive Statistics
3.3. Sexual Functioning—Differences between Women with PCOS, Their Partners, and Women without PCOS
3.4. Sexual Functioning of Women with PCOS—Correlation between CSFQ and VAS
3.5. Demographic and Physical Correlates of Sexual Functioning of Women with PCOS
3.6. Associations between VAS Scores and Physical Features (WHR, Acne, and Hirsutism)
3.7. Association between Sexual Activity in Last Week and Sexual Functioning in Women Diagnosed with PCOS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joham, A.E.; Norman, R.J.; Stener-Victorin, E.; Legro, R.S.; Franks, S.; Moran, L.J.; Boyle, J.; Teede, H.J. Polycystic ovary syndrome. Lancet Diabetes Endocrinol. 2022, 10, 668–680, Erratum in Lancet Diabetes Endocrinol. 2022, 10, e11. [Google Scholar] [CrossRef] [PubMed]
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef]
- Lizneva, D.; Suturina, L.; Walker, W.; Brakta, S.; Gavrilova-Jordan, L.; Azziz, R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil. Steril. 2016, 106, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Hamilton-Fairley, D.; Taylor, A. Anovulation. BMJ 2003, 327, 546–549. [Google Scholar] [CrossRef]
- Kakoly, N.S.; Earnest, A.; Teede, H.J.; Moran, L.J.; Joham, A.E. The Impact of Obesity on the Incidence of Type 2 Diabetes Among Women With Polycystic Ovary Syndrome. Diabetes Care 2019, 42, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Dokras, A.; Stener-Victorin, E.; Yildiz, B.O.; Li, R.; Ottey, S.; Shah, D.; Epperson, N.; Teede, H. Androgen Excess-Polycystic Ovary Syndrome Society: Position statement on depression, anxiety, quality of life, and eating disorders in polycystic ovary syndrome. Fertil. Steril. 2018, 109, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Coffey, S.; Mason, H. The effect of polycystic ovary syndrome on health-related quality of life. Gynecol. Endocrinol. 2003, 17, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Deeks, A.A.; Gibson-Helm, M.E.; Teede, H.J. Anxiety and depression in polycystic ovary syndrome: A comprehensive investigation. Fertil. Steril. 2010, 93, 2421–2423. [Google Scholar] [CrossRef] [PubMed]
- Tay, C.T.; Teede, H.J.; Hill, B.; Loxton, D.; Joham, A.E. Increased prevalence of eating disorders, low self-esteem, and psychological distress in women with polycystic ovary syndrome: A community-based cohort study. Fertil. Steril. 2019, 112, 353–361. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; APA: Arlington, VA, USA, 2013. [Google Scholar]
- Pastoor, H.; Timman, R.; de Klerk, C.; Bramer, W.M.; Laan, E.T.; Laven, J.S. Sexual function in women with polycystic ovary syndrome: A systematic review and meta-analysis. Reprod. Biomed. Online 2018, 37, 750–760. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, J.; Xie, Q.; Luo, L.; Zhu, Z.; Liu, Y.; Luo, J.; Zhao, Z. Is polycystic ovary syndrome associated with risk of female sexual dysfunction? A systematic review and meta-analysis. Reprod. Biomed. Online 2019, 38, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Loh, H.H.; Yee, A.; Loh, H.S.; Kanagasundram, S.; Francis, B.; Lim, L.L. Sexual dysfunction in polycystic ovary syndrome: A systematic review and meta-analysis. Hormones 2020, 19, 413–423. [Google Scholar] [CrossRef]
- Albers, C.; Lakens, D. 2018 When power analyses based on pilot data are biased: Inaccurate effect size estimators and follow-up bias. J. Exp. Soc. Psychol. 2018, 74, 187–195. [Google Scholar] [CrossRef]
- Forbes, M.K.; Baillie, A.J.; Schniering, C.A. Critical flaws in the Female Sexual Function Index and the international index of Erectile Function. J. Sex Res. 2014, 51, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Janssen, E.; McBride, K.R.; Yarber, W.; Hill, B.J.; Butler, S.M. Factors that influence sexual arousal in men: A focus group study. Arch. Sex Behav. 2008, 37, 252–265. [Google Scholar] [CrossRef]
- Brotto, L.A.; Heiman, J.R.; Tolman, D.L. Narratives of desire in mid-age women with and without arousal difficulties. J. Sex Res. 2009, 46, 387–398. [Google Scholar] [CrossRef]
- Stovall, D.W.; Scriver, J.L.; Clayton, A.H.; Williams, C.D.; Pastore, L.M. Sexual function in women with polycystic ovary syndrome. J. Sex Med. 2012, 9, 224–230. [Google Scholar] [CrossRef]
- Regan, P.C.; Berscheid, E. Gender differences in beliefs about the cause of male and female sexual desire. Pers. Relatsh. 1995, 2, 345–358. [Google Scholar] [CrossRef]
- Harris, E.A.; Hornsey, M.J.; Hofmann, W.; Jern, P.; Murphy, S.C.; Hedenborg, F.; Barlow, F.K. Does Sexual Desire Fluctuate More Among Women than Men? Arch. Sex Behav. 2023, 52, 1461–1478. [Google Scholar] [CrossRef] [PubMed]
- Basson, R. Human sexual response. Handb. Clin. Neurol. 2015, 130, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Sand, M.; Fisher, W.A. Women’s endorsement of models of female sexual response: The nurses’ sexuality study. J. Sex Med. 2007, 4, 708–719. [Google Scholar] [CrossRef] [PubMed]
- Basson, R. The female sexual response: A different model. J. Sex Marital Ther. 2000, 26, 51–65. [Google Scholar] [CrossRef] [PubMed]
- de Jong, D.C. The role of attention in sexual arousal: Implications for treatment of sexual dysfunction. J. Sex Res. 2009, 46, 237–248. [Google Scholar] [CrossRef]
- Basson, R. Human sex-response cycles. J. Sex Marital Ther. 2001, 27, 33–43. [Google Scholar] [CrossRef]
- Spiering, M.; Everaerd, W.; Janssen, E. Priming the sexual system: Implicit versus explicit activation. J. Sex Res. 2003, 40, 134–145. [Google Scholar] [CrossRef]
- Parish, S.J.; Meston, C.M.; Althof, S.E.; Clayton, A.H.; Goldstein, I.; Goldstein, S.W.; Heiman, J.R.; McCabe, M.P.; Segraves, R.T.; Simon, J.A. Toward a More Evidence-Based Nosology and Nomenclature for Female Sexual Dysfunctions-Part III. J. Sex Med. 2019, 16, 452–462. [Google Scholar] [CrossRef]
- Althof, S.E.; Meston, C.M.; Perelman, M.A.; Handy, A.B.; Kilimnik, C.D.; Stanton, A.M. Opinion Paper: On the Diagnosis/Classification of Sexual Arousal Concerns in Women. J. Sex Med. 2017, 14, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Skakoon-Sparling, S.; Cramer, K.M.; Shuper, P.A. The Impact of Sexual Arousal on Sexual Risk-Taking and Decision-Making in Men and Women. Arch. Sex Behav. 2016, 45, 33–42. [Google Scholar] [CrossRef]
- Meston, C.M.; Buss, D.M. Why humans have sex. Arch. Sex Behav. 2007, 36, 477–507. [Google Scholar] [CrossRef] [PubMed]
- McNulty, J.K.; Wenner, C.A.; Fisher, T.D. Longitudinal Associations Among Relationship Satisfaction, Sexual Satisfaction, and Frequency of Sex in Early Marriage. Arch. Sex Behav. 2016, 45, 85–97. [Google Scholar] [CrossRef]
- Hackbert, L.; Heiman, J.R. Acute dehydroepiandrosterone (DHEA) effects on sexual arousal in postmenopausal women. J. Womens Health Gend.-Based Med. 2002, 11, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Schmid, Y.; Hysek, C.M.; Preller, K.H.; Bosch, O.G.; Bilderbeck, A.C.; Rogers, R.D.; Quednow, B.B.; Liechti, M.E. Effects of methylphenidate and MDMA on appraisal of erotic stimuli and intimate relationships. Eur. Neuropsychopharmacol. 2015, 25, 17–25. [Google Scholar] [CrossRef]
- Sipski, M.L.; Rosen, R.C.; Alexander, C.J.; Hamer, R.M. Sildenafil effects on sexual and cardiovascular responses in women with spinal cord injury. Urology 2000, 55, 812–815. [Google Scholar] [CrossRef] [PubMed]
- Handy, A.B.; Stanton, A.M.; Meston, C.M. Understanding Women’s Subjective Sexual Arousal Within the Laboratory: Definition, Measurement, and Manipulation. Sex Med. Rev. 2018, 6, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Velten, J.; Milani, S.; Margraf, J.; Brotto, L.A. Visual attention and sexual arousal in women with and without sexual dysfunction. Behav. Res. Ther. 2021, 144, 103915. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, J.; Graham, C.A.; Janssen, E.; Sanders, S.A. The dual control model: Current status and future directions. J. Sex Res. 2009, 46, 121–142. [Google Scholar] [CrossRef] [PubMed]
- Janssen, E.; Bancroft, J. The Dual Control Model of Sexual Response: A Scoping Review, 2009–2022. J. Sex Res. 2023, 60, 948–968. [Google Scholar] [CrossRef]
- Davis, S.R.; Guay, A.T.; Shifren, J.L.; Mazer, N.A. Endocrine aspects of female sexual dysfunction. J. Sex Med. 2004, 1, 82–86. [Google Scholar] [CrossRef]
- Kingsberg, S.A.; Clayton, A.H.; Pfaus, J.G. The Female Sexual Response: Current Models, Neurobiological Underpinnings and Agents Currently Approved or Under Investigation for the Treatment of Hypoactive Sexual Desire Disorder. CNS Drugs 2015, 29, 915–933. [Google Scholar] [CrossRef]
- Månsson, M.; Norström, K.; Holte, J.; Landin-Wilhelmsen, K.; Dahlgren, E.; Landén, M. Sexuality and psychological wellbeing in women with polycystic ovary syndrome compared with healthy controls. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 155, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Bazarganipour, F.; Ziaei, S.; Montazeri, A.; Foroozanfard, F.; Kazemnejad, A.; Faghihzadeh, S. Sexual Functioning among Married Iranian Women with Polycystic Ovary Syndrome. Int. J. Fertil. Steril. 2014, 8, 273–280. [Google Scholar] [PubMed]
- Bahadori, F.; Jahanian Sadatmahalleh, S.; Montazeri, A.; Nasiri, M. Sexuality and psychological well-being in different polycystic ovary syndrome phenotypes compared with healthy controls: A cross-sectional study. BMC Womens Health 2022, 22, 390. [Google Scholar] [CrossRef]
- Anger, J.T.; Brown, A.J.; Amundsen, C.L. Sexual Dysfunction in Women With Polycystic Ovary Syndrome: The Effects of Testosterone, Obesity, and Depression. J. Pelvic Med. Surg. 2007, 13, 119–124. [Google Scholar] [CrossRef]
- Elsenbruch, S.; Hahn, S.; Kowalsky, D.; Offner, A.H.; Schedlowski, M.; Mann, K.; Janssen, O.E. Quality of life, psychosocial well-being, and sexual satisfaction in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2003, 88, 5801–5807. [Google Scholar] [CrossRef] [PubMed]
- Stapinska-Syniec, A.; Grabowska, K.; Szpotanska-Sikorska, M.; Pietrzak, B. Depression, sexual satisfaction, and other psychological issues in women with polycystic ovary syndrome. Gynecol. Endocrinol. 2018, 34, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Ruan, X.; Du, J.; Cheng, J.; Ju, R.; Mueck, A.O. Sexual function in Chinese women with different clinical phenotypes of polycystic ovary syndrome. Gynecol. Endocrinol. 2023, 39, 2221736. [Google Scholar] [CrossRef] [PubMed]
- Morotti, E.; Persico, N.; Battaglia, B.; Fabbri, R.; Meriggiola, M.C.; Venturoli, S.; Battaglia, C. Body imaging and sexual behavior in lean women with polycystic ovary syndrome. J. Sex Med. 2013, 10, 2752–2760. [Google Scholar] [CrossRef]
- Sills, E.S.; Perloe, M.; Tucker, M.J.; Kaplan, C.R.; Genton, M.G.; Schattman, G.L. Diagnostic and treatment characteristics of polycystic ovary syndrome: Descriptive measurements of patient perception and awareness from 657 confidential self-reports. BMC Womens Health 2001, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Eftekhar, T.; Sohrabvand, F.; Zabandan, N.; Shariat, M.; Haghollahi, F.; Ghahghaei-Nezamabadi, A. Sexual dysfunction in patients with polycystic ovary syndrome and its affected domains. Iran. J. Reprod. Med. 2014, 12, 539–546. [Google Scholar] [PubMed]
- Ercan, C.M.; Coksuer, H.; Aydogan, U.; Alanbay, I.; Keskin, U.; Karasahin, K.E.; Baser, I. Sexual dysfunction assessment and hormonal correlations in patients with polycystic ovary syndrome. Int. J. Impot. Res. 2013, 25, 127–132. [Google Scholar] [CrossRef]
- Veras, A.B.; Bruno, R.V.; de Avila, M.A.; Nardi, A.E. Sexual dysfunction in patients with polycystic ovary syndrome: Clinical and hormonal correlations. Compr. Psychiatry 2011, 52, 486–489. [Google Scholar] [CrossRef]
- Rellini, A.H.; Stratton, N.; Tonani, S.; Santamaria, V.; Brambilla, E.; Nappi, R.E. Differences in sexual desire between women with clinical versus biochemical signs of hyperandrogenism in polycystic ovarian syndrome. Horm. Behav. 2013, 63, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.R.; Davison, S.L.; Donath, S.; Bell, R.J. Circulating androgen levels and self-reported sexual function in women. JAMA 2005, 294, 91–96. [Google Scholar] [CrossRef]
- Wierman, M.E.; Arlt, W.; Basson, R.; Davis, S.R.; Miller, K.K.; Murad, M.H.; Rosner, W.; Santoro, N. Androgen therapy in women: A reappraisal: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2014, 99, 3489–3510. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.; Janssen, O.E.; Tan, S.; Pleger, K.; Mann, K.; Schedlowski, M.; Kimmig, R.; Benson, S.; Balamitsa, E.; Elsenbruch, S. Clinical and psychological correlates of quality-of-life in polycystic ovary syndrome. Eur. J. Endocrinol. 2005, 153, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Ferraresi, S.R.; Lara, L.A.; Reis, R.M.; Rosa e Silva, A.C. Changes in sexual function among women with polycystic ovary syndrome: A pilot study. J. Sex Med. 2013, 10, 467–473. [Google Scholar] [CrossRef]
- Yaylali, G.F.; Tekekoglu, S.; Akin, F. Sexual dysfunction in obese and overweight women. Int. J. Impot. Res. 2010, 22, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Brody, S.; Weiss, P. Slimmer women’s waist is associated with better erectile function in men independent of age. Arch. Sex Behav. 2013, 42, 1191–1198. [Google Scholar] [CrossRef]
- Zueff, L.N.; Lara, L.A.; Vieira, C.S.; Martins, W.P.; Ferriani, R.A. Body composition characteristics predict sexual functioning in obese women with or without PCOS. J. Sex Marital Ther. 2015, 41, 227–237. [Google Scholar] [CrossRef]
- De Frène, V.; Verhofstadt, L.; Loeys, T.; Stuyver, I.; Buysse, A.; De Sutter, P. Sexual and relational satisfaction in couples where the woman has polycystic ovary syndrome: A dyadic analysis. Hum. Reprod. 2015, 30, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Benetti-Pinto, C.L.; Ferreira, S.R.; Antunes, A., Jr.; Yela, D.A. The influence of body weight on sexual function and quality of life in women with polycystic ovary syndrome. Arch. Gynecol. Obstet. 2015, 291, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Nohr, E.A.; Hansen, A.B.; Andersen, M.S.; Hjorth, S. Sexual health in parous women with a history of polycystic ovary syndrome: A national cross-sectional study in Denmark. Int. J. Gynaecol. Obstet. 2022, 157, 702–709. [Google Scholar] [CrossRef] [PubMed]
- March, W.A.; Moore, V.M.; Willson, K.J.; Phillips, D.I.; Norman, R.J.; Davies, M.J. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum. Reprod. 2010, 25, 544–551. [Google Scholar] [CrossRef] [PubMed]
| M | SD | Min | Max | Skewness | |
|---|---|---|---|---|---|
| PCOS group—women | |||||
| Pleasure | 3.92 | 0.96 | 1.00 | 5.00 | −0.78 |
| Desire—frequency | 6.95 | 1.37 | 3.00 | 10.00 | −0.35 |
| Desire—interest | 8.75 | 2.06 | 3.00 | 12.00 | −0.34 |
| Arousal | 10.56 | 2.05 | 5.00 | 14.00 | −0.37 |
| Orgasm | 10.88 | 2.46 | 3.00 | 15.00 | −0.40 |
| Total CSFQ | 49.59 | 7.47 | 27.00 | 63.00 | −0.34 |
| Control group—women | |||||
| Pleasure | 3.93 | 0.99 | 1.00 | 5.00 | −0.98 |
| Desire—frequency | 6.97 | 1.40 | 4.00 | 10.00 | −0.28 |
| Desire—interest | 8.58 | 2.45 | 3.00 | 13.00 | −0.29 |
| Arousal | 11.16 | 2.10 | 6.00 | 15.00 | −0.83 |
| Orgasm | 11.15 | 2.94 | 3.00 | 15.00 | −1.02 |
| Total CSFQ | 50.68 | 8.63 | 24.00 | 64.00 | −1.31 |
| PCOS group—men | |||||
| Pleasure | 4.28 | 0.82 | 1.00 | 5.00 | −1.55 |
| Desire—frequency | 7.42 | 1.39 | 4.00 | 10.00 | −0.18 |
| Desire—interest | 9.77 | 2.19 | 4.00 | 15.00 | −0.01 |
| Arousal | 12.88 | 1.46 | 9.00 | 15.00 | −0.84 |
| Orgasm | 12.40 | 1.67 | 7.00 | 15.00 | −0.71 |
| Total CSFQ | 56.12 | 5.55 | 40.00 | 70.00 | −0.51 |
| Control group—men | |||||
| Pleasure | 4.26 | 0.82 | 1.00 | 5.00 | −1.36 |
| Desire—frequency | 8.03 | 1.28 | 4.00 | 10.00 | −0.31 |
| Desire—interest | 10.19 | 1.84 | 6.00 | 15.00 | 0.02 |
| Arousal | 12.32 | 1.72 | 8.00 | 15.00 | −0.44 |
| Orgasm | 12.10 | 1.87 | 7.00 | 15.00 | −0.68 |
| Total CSFQ | 56.33 | 6.37 | 37.00 | 70.00 | −0.74 |
| Effect | F | df | p | ω2 | |
|---|---|---|---|---|---|
| Pleasure | Within subject | 33.80 | 1, 158 | <0.001 | 0.03 |
| Between subject | 0.00 | 1, 158 | 0.971 | 0.00 | |
| Within × between subject | 0.02 | 1, 158 | 0.877 | 0.00 | |
| Desire—frequency | Within subject | 59.42 | 1, 158 | <0.001 | 0.07 |
| Between subject | 2.70 | 1, 158 | 0.102 | 0.01 | |
| Within × between subject | 8.69 | 1, 158 | 0.004 | 0.01 | |
| Desire—interest | Within subject | 54.71 | 1, 158 | <0.001 | 0.08 |
| Between subject | 0.19 | 1, 158 | 0.668 | 0.00 | |
| Within × between subject | 2.72 | 1, 158 | 0.101 | 0.00 | |
| Arousal | Within subject | 123.57 | 1, 158 | <0.001 | 0.18 |
| Between subject | 0.01 | 1, 158 | 0.938 | 0.00 | |
| Within × between subject | 13.73 | 1, 158 | <0.001 | 0.02 | |
| Orgasm | Within subject | 42.25 | 1, 158 | <0.001 | 0.07 |
| Between subject | 0.00 | 1, 158 | 0.963 | 0.00 | |
| Within × between subject | 2.17 | 1, 158 | 0.143 | 0.00 | |
| Total CSFQ | Within subject | 130.35 | 1, 158 | <0.001 | 0.16 |
| Between subject | 0.43 | 1, 158 | 0.511 | 0.00 | |
| Within × between subject | 0.67 | 1, 158 | 0.413 | 0.00 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Pleasure | — | |||||||||||
| 2. Desire—frequency | 0.44 *** | — | ||||||||||
| 3. Desire—interest | 0.16 | 0.55 *** | — | |||||||||
| 4. Arousal | 0.64 *** | 0.55 *** | 0.49 *** | — | ||||||||
| 5. Orgasm | 0.58 *** | 0.37 *** | 0.35 *** | 0.63 *** | — | |||||||
| 6. Total CSFQ | 0.69 *** | 0.70 *** | 0.66 *** | 0.89 *** | 0.80 *** | — | ||||||
| 7. Sexual satisfaction importance (VAS1) | 0.32 ** | 0.24 * | 0.37 *** | 0.30 ** | 0.22 * | 0.35 *** | — | |||||
| 8. Sexual thoughts and fantasies—last month (VAS2) | 0.20 | 0.46 *** | 0.73 *** | 0.38 *** | 0.25 * | 0.52 *** | 0.55 *** | — | ||||
| 9. Personal sexual attractiveness (VAS3) | 0.24 * | 0.20 | 0.25 * | 0.30 ** | 0.28 ** | 0.34 *** | 0.26 * | 0.27 * | — | |||
| 10. Impact of excessive hair on personal sexuality (VAS4) | −0.26 * | −0.18 | −0.06 | −0.11 | −0.19 | −0.19 | 0.01 | 0.06 | −0.16 | — | ||
| 11. Social struggles due to appearance (VAS5) | −0.27 ** | −0.11 | 0.08 | −0.11 | −0.19 | −0.17 | 0.07 | 0.22 * | −0.18 | 0.49 *** | — | |
| 12. Painful sexual encounters (VAS6) | −0.39 *** | −0.13 | 0.05 | −0.34 *** | −0.27 * | −0.35 *** | −0.02 | 0.03 | −0.06 | 0.10 | 0.21 * | — |
| 13. Level of sexual satisfaction—last month (VAS7) | 0.67 *** | 0.48 *** | 0.14 | 0.39 *** | 0.53 *** | 0.53 *** | 0.30 ** | 0.27 ** | 0.18 | −0.18 | −0.04 | −0.14 |
| Pleasure | Desire—Frequency | Desire—Interest | Arousal | Orgasm | Total | |
|---|---|---|---|---|---|---|
| Age | 0.01 | −0.06 | −0.07 | 0.08 | 0.21 * | 0.09 |
| WHR | 0.07 | 0.02 | −0.17 | −0.05 | 0.05 | −0.03 |
| Ferriman–Gallwey score | 0.03 | 0.07 | −0.09 | −0.03 | −0.16 | −0.07 |
| Education | −0.03 | 0.02 | 0.13 | 0.02 | −0.05 | 0.05 |
| W | p | rbs | ||
|---|---|---|---|---|
| Grouping variable: Residence (alone vs. with family) | Pleasure | 64.50 | 0.429 | 0.12 |
| Desire—frequency | 72.50 | 0.099 | 0.26 | |
| Desire—interest | 77.50 | 0.030 | 0.35 | |
| Arousal | 675.50 | 0.255 | 0.19 | |
| Orgasm | 507.00 | 0.500 | −0.11 | |
| Total CSFQ | 668.00 | 0.296 | 0.17 | |
| Grouping variable: Acne presence (yes vs. no) | Pleasure | 921.50 | 0.560 | −0.07 |
| Desire—frequency | 866.00 | 0.303 | −0.13 | |
| Desire—interest | 892.50 | 0.426 | −0.10 | |
| Arousal | 926.50 | 0.604 | −0.06 | |
| Orgasm | 947.50 | 0.731 | −0.04 | |
| Total CSFQ | 921.00 | 0.578 | −0.07 | |
| Grouping variable: Hirsutism (yes vs. no) | Pleasure | 918.00 | 0.823 | −0.03 |
| Desire—frequency | 858.00 | 0.466 | −0.09 | |
| Desire—interest | 958.50 | 0.906 | 0.02 | |
| Arousal | 853.00 | 0.446 | −0.10 | |
| Orgasm | 92.50 | 0.847 | −0.03 | |
| Total CSFQ | 928.50 | 0.901 | −0.02 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| 1. WHR ratio | — | |||||||
| 2. Ferriman–Gallwey score | 0.19 | — | ||||||
| 3. Sexual satisfaction importance (VAS1) | −0.02 | 0.12 | — | |||||
| 4. Sexual thoughts and fantasies—last month (VAS2) | −0.09 | −0.03 | 0.55 *** | — | ||||
| 5. Personal sexual attractiveness (VAS3) | −0.18 | 0.03 | 0.26 * | 0.27 * | — | |||
| 6. Impact of excessive hair on personal sexuality (VAS4) | 0.07 | 0.41 *** | 0.01 | 0.06 | −0.16 | — | ||
| 7. Social struggles due to appearance (VAS5) | 0.23 * | 0.15 | 0.07 | 0.22 * | −0.18 | 0.49 *** | — | |
| 8. Painful sexual encounters (VAS6) | −0.03 | −0.07 | −0.02 | 0.03 | −0.06 | 0.10 | 0.21 * | — |
| 9. Level of sexual satisfaction—last month (VAS7) | 0.30 ** | 0.05 | 0.30 ** | 0.27 ** | 0.18 | −0.18 | −0.04 | −0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warchala, A.; Madej, P.; Kochanowicz, M.; Krzystanek, M. Sexual Function in Women with Polycystic Ovary Syndrome Living in Stable Heterosexual Relationships: A Cross-Sectional Study. J. Clin. Med. 2024, 13, 2227. https://doi.org/10.3390/jcm13082227
Warchala A, Madej P, Kochanowicz M, Krzystanek M. Sexual Function in Women with Polycystic Ovary Syndrome Living in Stable Heterosexual Relationships: A Cross-Sectional Study. Journal of Clinical Medicine. 2024; 13(8):2227. https://doi.org/10.3390/jcm13082227
Chicago/Turabian StyleWarchala, Anna, Paweł Madej, Marta Kochanowicz, and Marek Krzystanek. 2024. "Sexual Function in Women with Polycystic Ovary Syndrome Living in Stable Heterosexual Relationships: A Cross-Sectional Study" Journal of Clinical Medicine 13, no. 8: 2227. https://doi.org/10.3390/jcm13082227
APA StyleWarchala, A., Madej, P., Kochanowicz, M., & Krzystanek, M. (2024). Sexual Function in Women with Polycystic Ovary Syndrome Living in Stable Heterosexual Relationships: A Cross-Sectional Study. Journal of Clinical Medicine, 13(8), 2227. https://doi.org/10.3390/jcm13082227