Organ-Dysfunction Markers in Mild-to-Moderate COVID-19 Convalescents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Control Group and Study Group
2.2. Methods
2.3. Statistical Analysis
2.4. Bioethical Committee Opinion
3. Results
3.1. Markers of Lung Fibrosis and Pulmonary Macrophages Activation
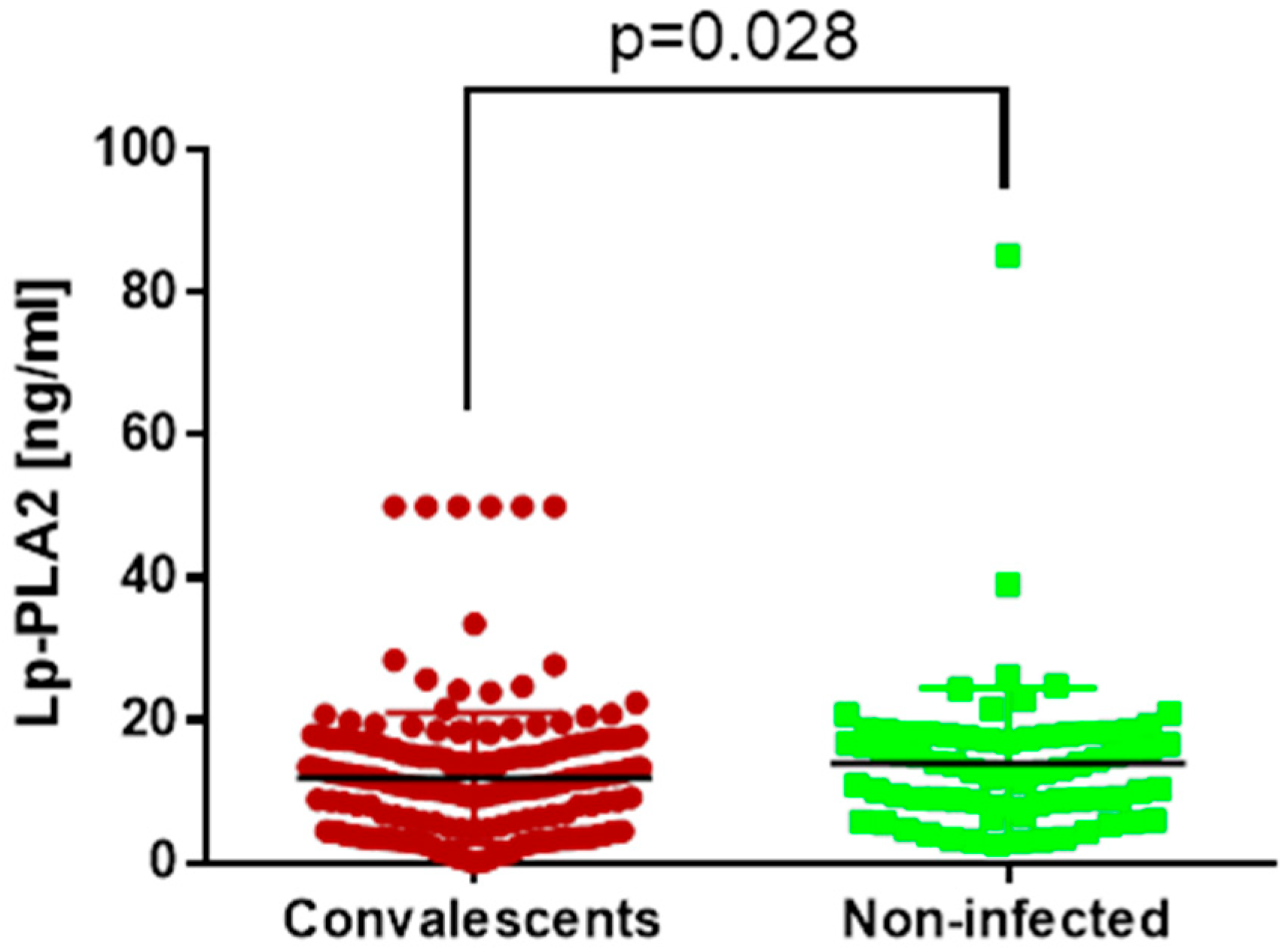
3.2. Vascular Dysfunction Marker
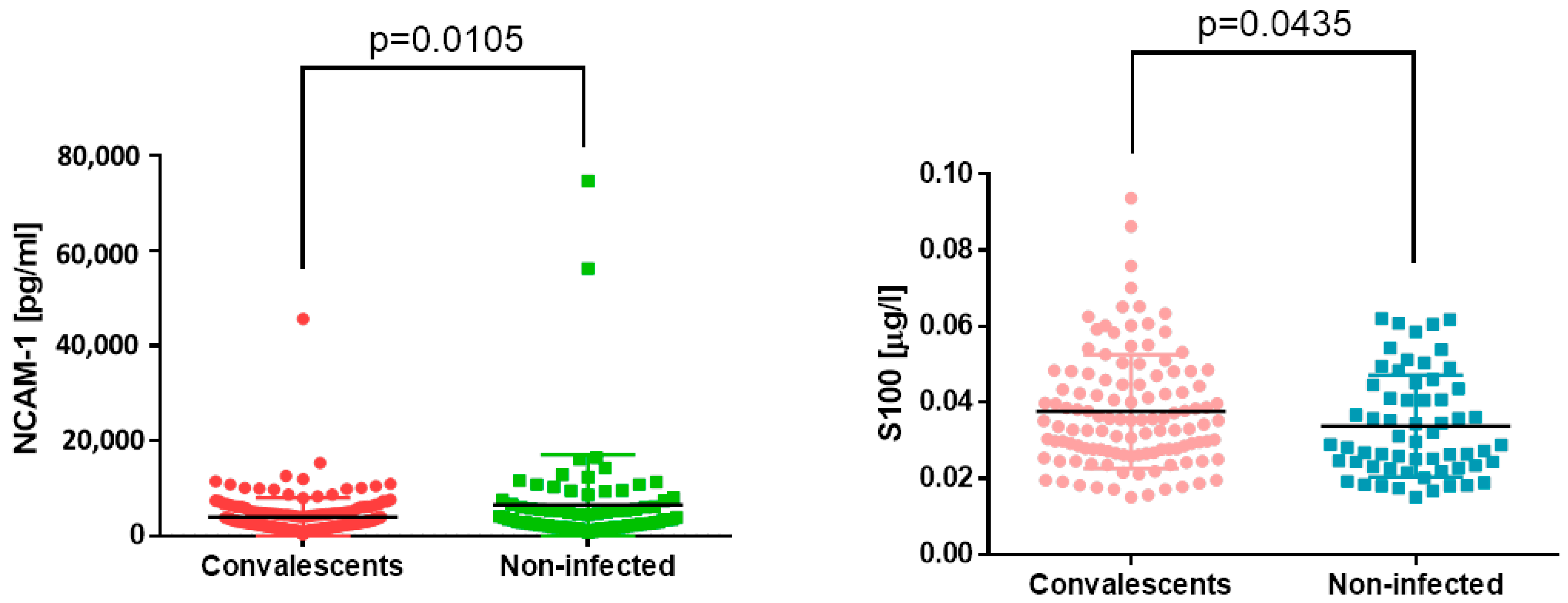
3.3. Nervous System Markers
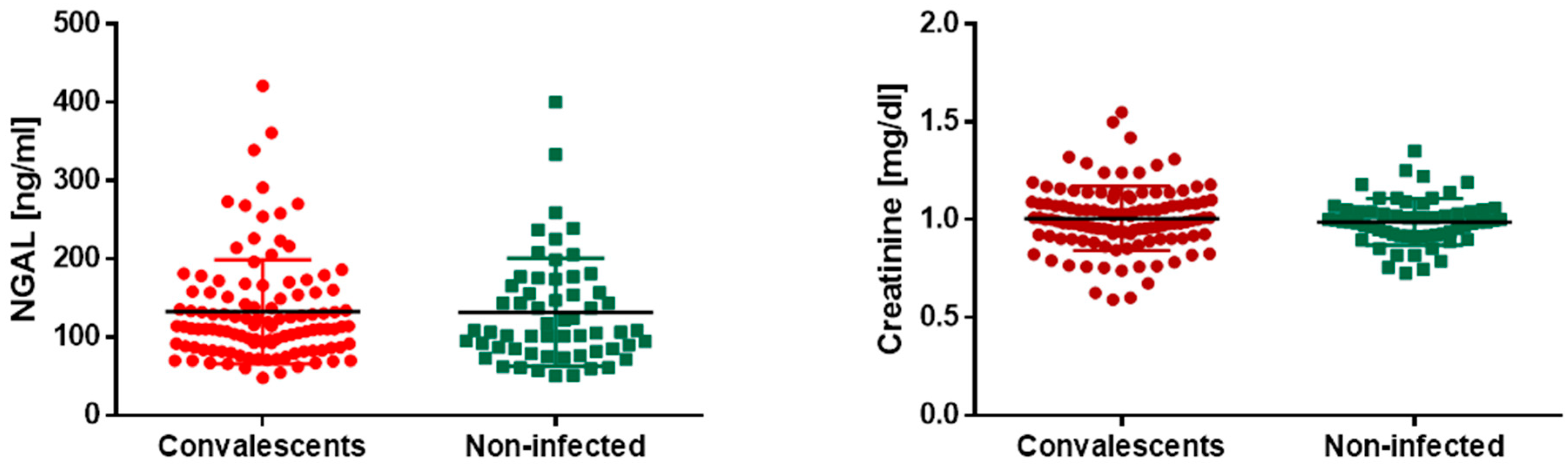
3.4. Kidney Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kozłowski, P.; Leszczyńska, A.; Ciepiela, O. Long COVID definition, symptoms, risk factors, epidemiology and autoimmunity—A narrative review. Am. J. Med. Open 2024, in press. [Google Scholar] [CrossRef]
- Guziejko, K.; Tałataj, J.; Czupryna, P.; Moniuszko-Malinowska, A. Long COVID. Epidemiol. Rev. 2022, 76, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Sanchis-Gomar, F.; Henry, B.M. COVID-19 and its long-term sequelae: What do we know in 2023? Pol. Arch. Intern. Med. 2023, 133, 16402. [Google Scholar] [CrossRef] [PubMed]
- John, A.E.; Joseph, C.; Jenkins, G.; Tatler, A.L. COVID-19 and pulmonary fibrosis: A potential role for lung epithelial cells and fibroblasts. Immunol. Rev. 2021, 302, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Hirawat, R.; Jain, N.; Aslam Saifi, M.; Rachamalla, M.; Godugu, C. Lung fibrosis: Post-COVID-19 complications and evidences. Int. Immunopharmacol. 2023, 116, 109418. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Balan, I.; Yadav, S.; Matos, W.F.; Kharawala, A.; Gaddam, M.; Sarabia, N.; Koneru, S.C.; Suddapalli, S.K.; Marzban, S. Post-COVID-19 Pulmonary Fibrosis. Cureus 2022, 14, e22770. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, H.K.; Libby, P.; Ridker, P.M. COVID-19—A vascular disease. Trends Cardiovasc. Med. 2021, 31, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Vassiliou, A.G.; Vrettou, C.S.; Keskinidou, C.; Dimopoulou, I.; Kotanidou, A.; Orfanos, S.E. Endotheliopathy in Acute COVID-19 and Long COVID. Int. J. Mol. Sci. 2023, 24, 8237. [Google Scholar] [CrossRef] [PubMed]
- Tellis, C.C.; Tselepis, A.D. Pathophysiological role and clinical significance of lipoprotein-associated phospholipase A(2) (Lp-PLA(2)) bound to LDL and HDL. Curr. Pharm. Des. 2014, 20, 6256–6269. [Google Scholar] [CrossRef] [PubMed]
- Dua, P.; Mishra, A.; Reeta, K.H. Lp-PLA2 as a biomarker and its possible associations with SARS-CoV-2 infection. Biomark. Med. 2022, 16, 821–832. [Google Scholar] [CrossRef]
- Monje, M.; Iwasaki, A. The neurobiology of long COVID. Neuron 2022, 110, 3484–3496. [Google Scholar] [CrossRef]
- Leng, A.; Shah, M.; Ahmad, S.A.; Premraj, L.; Wildi, K.; Li Bassi, G.; Pardo, C.A.; Choi, A.; Cho, S.M. Pathogenesis Underlying Neurological Manifestations of Long COVID Syndrome and Potential Therapeutics. Cells 2023, 12, 816. [Google Scholar] [CrossRef] [PubMed]
- Leone, M.A.; Helbok, R.; Bianchi, E.; Yasuda, C.L.; Konti, M.; Ramankulov, D.; Lolich, M.; Lovrencic-Huzjan, A.; Kovacs, T.; Armon, C.; et al. Outcome predictors of post-COVID conditions in the European Academy of Neurology COVID-19 registry. J. Neurol. 2024, 1–16. [Google Scholar] [CrossRef]
- Bowe, B.; Xie, Y.; Xu, E.; Al-Aly, Z. Kidney Outcomes in Long COVID. J. Am. Soc. Nephrol. 2021, 32, 2851–2862. [Google Scholar] [CrossRef] [PubMed]
- Rai, V. COVID-19 and Kidney: The Importance of Follow-Up and Long-Term Screening. Life 2023, 13, 2137. [Google Scholar] [CrossRef] [PubMed]
- Avotins, L.; Kroica, J.; Petersons, A.; Zentina, D.; Kravale, Z.; Saulite, A.; Racenis, K. eGFR(cystatinC)/eGFR(creatinine) ratio < 0.6 in patients with SARS-CoV-2 pneumonia: A prospective cohort study. BMC Nephrol. 2023, 24, 269. [Google Scholar]
- Bergersen, K.V.; Pham, K.; Li, J.; Ulrich, M.T.; Merrill, P.; He, Y.; Alaama, S.; Qiu, X.; Harahap-Carrillo, I.S.; Ichii, K.; et al. Health disparities in COVID-19: Immune and vascular changes are linked to disease severity and persist in a high-risk population in Riverside County, California. BMC Public Health 2023, 23, 1584. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Parimon, T.; Espindola, M.S.; Hohmann, M.S.; Konda, B.; Hogaboam, C.M.; Stripp, B.R.; Chen, P. Maladaptive TGF-beta Signals to the Alveolar Epithelium Drive Fibrosis after COVID-19 Infection. Am. J. Respir. Crit. Care Med. 2023, 208, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Martinez, G.; Jimenez-Alvarez, L.A.; Cruz-Lagunas, A.; Ignacio-Cortes, S.; Gomez-Garcia, I.A.; Rodriguez-Reyna, T.S.; Choreno-Parra, J.A.; Zuniga, J. Possible Role of Matrix Metalloproteinases and TGF-beta in COVID-19 Severity and Sequelae. J. Interferon Cytokine Res. 2022, 42, 352–368. [Google Scholar] [CrossRef] [PubMed]
- Frischbutter, S.; Durek, P.; Witkowski, M.; Angermair, S.; Treskatsch, S.; Maurer, M.; Radbruch, A.; Mashreghi, M.F. Serum TGF-beta as a predictive biomarker for severe disease and fatality of COVID-19. Eur. J. Immunol. 2023, 53, e2350433. [Google Scholar] [CrossRef] [PubMed]
- Susak, F.; Vrsaljko, N.; Vince, A.; Papic, N. TGF Beta as a Prognostic Biomarker of COVID-19 Severity in Patients with NAFLD-A Prospective Case-Control Study. Microorganisms 2023, 11, 1571. [Google Scholar] [CrossRef]
- Zivancevic-Simonovic, S.; Minic, R.; Cupurdija, V.; Stanojevic-Pirkovic, M.; Milosevic-Djordjevic, O.; Jakovljevic, V.; Mihaljevic, O. Transforming growth factor beta 1 (TGF-beta1) in COVID-19 patients: Relation to platelets and association with the disease outcome. Mol. Cell. Biochem. 2023, 478, 2461–2471. [Google Scholar] [CrossRef] [PubMed]
- Sbierski-Kind, J.; Schlickeiser, S.; Feldmann, S.; Ober, V.; Gruner, E.; Pleimelding, C.; Gilberg, L.; Brand, I.; Weigl, N.; Ahmed, M.I.M.; et al. Persistent immune abnormalities discriminate post-COVID syndrome from convalescence. Infection 2024, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Volfovitch, Y.; Tsur, A.M.; Gurevitch, M.; Novick, D.; Rabinowitz, R.; Mandel, M.; Achiron, A.; Rubinstein, M.; Shoenfeld, Y.; Amital, H. The intercorrelations between blood levels of ferritin, sCD163, and IL-18 in COVID-19 patients and their association to prognosis. Immunol. Res. 2022, 70, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Cardelli, M.; Pierpaoli, E.; Marchegiani, F.; Marcheselli, F.; Piacenza, F.; Giacconi, R.; Recchioni, R.; Casoli, T.; Stripoli, P.; Provinciali, M.; et al. Biomarkers of cell damage, neutrophil and macrophage activation associated with in-hospital mortality in geriatric COVID-19 patients. Immun. Ageing 2022, 19, 65. [Google Scholar] [CrossRef] [PubMed]
- Attia, H.; El Nagdy, M.; Halim, R.M.A. Preliminary Study of sCD14 and sCD163 as Predictors of Disease Severity and ICU Admission in COVID-19: Relation to Hematological Parameters, Blood Morphological Changes and Inflammatory Biomarkers. Mediterr. J. Hematol. Infect. Dis. 2023, 15, e2023046. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, G.A.; Ibrahim, H.M.; Al Sayed Shehab, A.; Gendy, Y.G.E.; Aly, D.M.M.; Shousha, G.A.H. Up-regulated serum levels of soluble CD25 and soluble CD163 in pediatric patients with SARS-CoV-2. Eur. J. Pediatr. 2022, 181, 2299–2309. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Dean, L.S.; Jiyarom, B.; Gangcuangco, L.M.; Shah, P.; Awamura, T.; Ching, L.L.; Nerurkar, V.R.; Chow, D.C.; Igno, F.; et al. Elevated circulating monocytes and monocyte activation in COVID-19 convalescent individuals. Front. Immunol. 2023, 14, 1151780. [Google Scholar] [CrossRef] [PubMed]
- Rajamanickam, A.; Kumar, N.P.; Pandiarajan, A.N.; Selvaraj, N.; Munisankar, S.; Renji, R.M.; Venkatramani, V.; Murhekar, M.; Thangaraj, J.W.V.; Kumar, M.S.; et al. Dynamic alterations in monocyte numbers, subset frequencies and activation markers in acute and convalescent COVID-19 individuals. Sci. Rep. 2021, 11, 20254. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Guo, L.; Wang, Q.; Yu, X.B.; Li, L.; Wei, Q. Association between lipoprotein-associated phospholipase A(2) and lower extremity arterial disease in type 2 diabetes mellitus. Clin. Chim. Acta 2020, 510, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, Y.; Zhang, Y.; Li, N.; Yin, Q.; Liu, L.; Lv, X.; Liu, Y.; Li, A.; Fang, B.; et al. Abnormal upregulation of cardiovascular disease biomarker PLA2G7 induced by proinflammatory macrophages in COVID-19 patients. Sci. Rep. 2021, 11, 6811. [Google Scholar] [CrossRef] [PubMed]
- Carmo, H.R.P.; Yoshinaga, M.Y.; Castillo, A.R.; Britto Chaves-Filho, A.; Bonilha, I.; Barreto, J.; Muraro, S.P.; de Souza, G.F.; Davanzo, G.G.; Perroud, M.W., Jr.; et al. Phenotypic changes in low-density lipoprotein particles as markers of adverse clinical outcomes in COVID-19. Mol. Genet. Metab. 2023, 138, 107552. [Google Scholar] [CrossRef] [PubMed]
- Gravrand, V.; Mellot, F.; Ackermann, F.; Ballester, M.C.; Zuber, B.; Kirk, J.T.; Navalkar, K.; Yager, T.D.; Petit, F.; Pascreau, T.; et al. Stratification of COVID-19 Severity Using SeptiCyte RAPID, a Novel Host Immune Response Test. Viruses 2023, 15, 419. [Google Scholar] [CrossRef]
- Batsika, C.S.; Gerogiannopoulou, A.D.; Mantzourani, C.; Vasilakaki, S.; Kokotos, G. The design and discovery of phospholipase A(2) inhibitors for the treatment of inflammatory diseases. Expert Opin. Drug Discov. 2021, 16, 1287–1305. [Google Scholar] [CrossRef] [PubMed]
- Suto, R.; Pocsi, M.; Fagyas, M.; Kalina, E.; Fejes, Z.; Szentkereszty, Z.; Kappelmayer, J.; Nagy, B., Jr. Comparison of Different Vascular Biomarkers for Predicting In-Hospital Mortality in Severe SARS-CoV-2 Infection. Microorganisms 2024, 12, 229. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Tang, S.; Cao, Y.; Fu, C. Relationship between Plasma Lipoprotein-Associated Phospholipase A2 Concentrations and Apolipoprotein in Stable Coronary Artery Disease Patients. Dis. Markers 2020, 2020, 8818358. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, H.H.; Capsomidis, A.; Smits, E.L.; Van Tendeloo, V.F. CD56 in the Immune System: More Than a Marker for Cytotoxicity? Front. Immunol. 2017, 8, 892. [Google Scholar] [CrossRef] [PubMed]
- Laudanski, K.; Hajj, J.; Restrepo, M.; Siddiq, K.; Okeke, T.; Rader, D.J. Dynamic Changes in Central and Peripheral Neuro-Injury vs. Neuroprotective Serum Markers in COVID-19 Are Modulated by Different Types of Anti-Viral Treatments but Do Not Affect the Incidence of Late and Early Strokes. Biomedicines 2021, 9, 1791. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Tang, N.; Peluso, M.J.; Iyer, N.S.; Torres, L.; Donatelli, J.L.; Munter, S.E.; Nixon, C.C.; Rutishauser, R.L.; Rodriguez-Barraquer, I.; et al. Characterization and Biomarker Analyses of Post-COVID-19 Complications and Neurological Manifestations. Cells 2021, 10, 386. [Google Scholar] [CrossRef] [PubMed]
- Björkström, N.K.; Strunz, B.; Ljunggren, H.G. Natural killer cells in antiviral immunity. Nat. Rev. Immunol. 2022, 22, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Saresella, M.; Trabattoni, D.; Marventano, I.; Piancone, F.; La Rosa, F.; Caronni, A.; Lax, A.; Bianchi, L.; Banfi, P.; Navarro, J.; et al. NK Cell Subpopulations and Receptor Expression in Recovering SARS-CoV-2 Infection. Mol. Neurobiol. 2021, 58, 6111–6120. [Google Scholar] [CrossRef] [PubMed]
- Maucourant, C.; Filipovic, I.; Ponzetta, A.; Aleman, S.; Cornillet, M.; Hertwig, L.; Strunz, B.; Lentini, A.; Reinius, B.; Brownlie, D.; et al. Natural killer cell immunotypes related to COVID-19 disease severity. Sci. Immunol. 2020, 5, eabd6832. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Liu, Y.; Yuan, J.; Wen, Y.; Xu, G.; Zhao, J.; Cheng, L.; Li, J.; Wang, X.; Wang, F.; et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020, 26, 842–844. [Google Scholar] [CrossRef]
- Leem, G.; Cheon, S.; Lee, H.; Choi, S.J.; Jeong, S.; Kim, E.S.; Jeong, H.W.; Jeong, H.; Park, S.H.; Kim, Y.S.; et al. Abnormality in the NK-cell population is prolonged in severe COVID-19 patients. J. Allergy Clin. Immunol. 2021, 148, 996–1006.e18. [Google Scholar] [CrossRef] [PubMed]
- Casado, J.L.; Moraga, E.; Vizcarra, P.; Velasco, H.; Martin-Hondarza, A.; Haemmerle, J.; Gomez, S.; Quereda, C.; Vallejo, A. Expansion of CD56(dim)CD16(neg) NK Cell Subset and Increased Inhibitory KIRs in Hospitalized COVID-19 Patients. Viruses 2021, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Aceti, A.; Margarucci, L.M.; Scaramucci, E.; Orsini, M.; Salerno, G.; Di Sante, G.; Gianfranceschi, G.; Di Liddo, R.; Valeriani, F.; Ria, F.; et al. Serum S100B protein as a marker of severity in COVID-19 patients. Sci. Rep. 2020, 10, 18665. [Google Scholar] [CrossRef] [PubMed]
- Mete, E.; Sabirli, R.; Goren, T.; Turkcuer, I.; Kurt, O.; Koseler, A. Association Between S100b Levels and COVID-19 Pneumonia: A Case Control Study. In Vivo 2021, 35, 2923–2928. [Google Scholar] [CrossRef] [PubMed]
- Sahin, B.E.; Celikbilek, A.; Kocak, Y.; Saltoglu, G.T.; Konar, N.M.; Hizmali, L. Plasma biomarkers of brain injury in COVID-19 patients with neurological symptoms. J. Neurol. Sci. 2022, 439, 120324. [Google Scholar] [CrossRef] [PubMed]
- Savarraj, J.; Park, E.S.; Colpo, G.D.; Hinds, S.N.; Morales, D.; Ahnstedt, H.; Paz, A.S.; Assing, A.; Liu, F.; Juneja, S.; et al. Brain injury, endothelial injury and inflammatory markers are elevated and express sex-specific alterations after COVID-19. J. Neuroinflamm. 2021, 18, 277. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Kido, T.; Shi, J.; McCafferty, E.; Ford, J.M.; Dal Bon, K.; Pulliam, L. Blood Markers Show Neural Consequences of LongCOVID-19. Cells 2024, 13, 478. [Google Scholar] [CrossRef] [PubMed]
- Menez, S.; Moledina, D.G.; Thiessen-Philbrook, H.; Wilson, F.P.; Obeid, W.; Simonov, M.; Yamamoto, Y.; Corona-Villalobos, C.P.; Chang, C.; Garibaldi, B.T.; et al. Prognostic Significance of Urinary Biomarkers in Patients Hospitalized With COVID-19. Am. J. Kidney Dis. 2022, 79, 257–267.e1. [Google Scholar] [CrossRef] [PubMed]
- Pode Shakked, N.; de Oliveira, M.H.S.; Cheruiyot, I.; Benoit, J.L.; Plebani, M.; Lippi, G.; Benoit, S.W.; Henry, B.M. Early prediction of COVID-19-associated acute kidney injury: Are serum NGAL and serum Cystatin C levels better than serum creatinine? Clin. Biochem. 2022, 102, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Serwin, N.; Cecerska-Heryc, E.; Pius-Sadowska, E.; Serwin, K.; Niedzwiedz, A.; Wisniewska, M.; Roszak, M.; Grygorcewicz, B.; Skwirczynska, E.; Machalinski, B.; et al. Renal and Inflammation Markers-Renalase, Cystatin C, and NGAL Levels in Asymptomatic and Symptomatic SARS-CoV-2 Infection in a One-Month Follow-Up Study. Diagnostics 2022, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, A.; Heleniak, Z.; Muchlado, M.; Slizien, Z.; Ruszkowski, J.; Biedunkiewicz, B.; Tylicki, L.; Krol, E.; Debska-Slizien, A. Changes in Kidney Graft Function in COVID-19 Convalescents. Transplant. Proc. 2022, 54, 884–887. [Google Scholar] [CrossRef] [PubMed]

| Median | Q1 | Q3 | p | ||
|---|---|---|---|---|---|
| TGFβ [pg/mL] | C | 159.83 | 100.75 | 193.22 | p = 0.91 |
| NI | 150.66 | 106.76 | 208.55 | ||
| sCD163 [ng/mL] | C | 178.09 | 143.78 | 226.73 | p = 0.42 |
| NI | 175.13 | 138.45 | 229.02 | ||
| Lp-PLA2 [ng/mL] | C | 11.09 | 4.67 | 15.26 | p = 0.028 |
| NI | 12.99 | 8.6 | 17.81 | ||
| NCAM-1 [pg/mL] | C | 2881.78 | 1875.45 | 4755.82 | p = 0.0105 |
| NI | 3728.48 | 2212.74 | 6795.65 | ||
| S100 [µg/mL] | C | 0.040 | 0.027 | 0.046 | p = 0.04 |
| NI | 0.029 | 0.023 | 0.044 | ||
| NGAL [ng/mL] | C | 113.00 | 87.08 | 156.25 | p = 0.77 |
| NI | 107.00 | 85.13 | 163.00 | ||
| Creatinine [mg/dL] | C | 1.00 | 0.92 | 1.09 | p = 0.37 |
| NI | 0.99 | 0.92 | 1.04 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiśniewska, A.; Kijak, A.; Nowak, K.; Lulek, M.; Skwarek, A.; Małecka-Giełdowska, M.; Śmiarowski, M.; Wąsik, S.; Ciepiela, O. Organ-Dysfunction Markers in Mild-to-Moderate COVID-19 Convalescents. J. Clin. Med. 2024, 13, 2241. https://doi.org/10.3390/jcm13082241
Wiśniewska A, Kijak A, Nowak K, Lulek M, Skwarek A, Małecka-Giełdowska M, Śmiarowski M, Wąsik S, Ciepiela O. Organ-Dysfunction Markers in Mild-to-Moderate COVID-19 Convalescents. Journal of Clinical Medicine. 2024; 13(8):2241. https://doi.org/10.3390/jcm13082241
Chicago/Turabian StyleWiśniewska, Aleksandra, Aleksandra Kijak, Karolina Nowak, Michalina Lulek, Agata Skwarek, Milena Małecka-Giełdowska, Marcin Śmiarowski, Szczepan Wąsik, and Olga Ciepiela. 2024. "Organ-Dysfunction Markers in Mild-to-Moderate COVID-19 Convalescents" Journal of Clinical Medicine 13, no. 8: 2241. https://doi.org/10.3390/jcm13082241
APA StyleWiśniewska, A., Kijak, A., Nowak, K., Lulek, M., Skwarek, A., Małecka-Giełdowska, M., Śmiarowski, M., Wąsik, S., & Ciepiela, O. (2024). Organ-Dysfunction Markers in Mild-to-Moderate COVID-19 Convalescents. Journal of Clinical Medicine, 13(8), 2241. https://doi.org/10.3390/jcm13082241







