Abstract
Optimal myocardial reperfusion during primary percutaneous coronary intervention (pPCI) is increasingly recognized to be beyond restoring epicardial coronary flow. Both invasive and non-invasive tools have highlighted the limitation of using this metric, and more efforts are focused towards achieving optimal reperfusion at the level of the microcirculation. Recent data highlighted the close relationship between thrombus burden and impaired microcirculation in patients presenting with ST-segment elevation myocardial infarction (STEMI). Moreover, distal embolization was an independent predictor of mortality in patients with STEMI. Likewise, the development of no-reflow phenomenon has been directly linked with worse clinical outcomes. Adjunctive thrombus aspiration during pPCI is intuitively intended to remove atherothrombotic material to mitigate the risk of distal embolization and the no-reflow phenomenon (NRP). However, prior trials on the use of thrombectomy during pPCI did not support its routine use, with comparable clinical endpoints to patients who underwent PCI alone. This article aims to review the existing literature highlighting the limitation on the use of thrombectomy and provide future insights into trials investigating the role of thrombectomy in contemporary pPCI.
1. Introduction
Myocardial reperfusion with a restoration of normal epicardial coronary blood flow during primary percutaneous coronary intervention (pPCI) for patients presenting with ST-segment elevation myocardial infarction (STEMI) is fundamental to salvage myocardium and improve survival [1,2]. However, despite adequate epicardial coronary flow, a significant proportion of patients (up to 50%) fail to achieve optimal myocardial reperfusion and develop a phenomenon referred to as no reflow (NRP) [3,4,5]. This phenomenon is described as reduced coronary antegrade flow (less than Thrombolysis In Myocardial Infarction (TIMI) III grade flow) without visible obstruction or dissection of the infarct-related artery (IRA), which occurs as a result of microvascular obstruction (MVO) with a subsequent increase in myocardial tissue impedance, leading to poor epicardial antegrade TIMI flow [6,7,8].
NRP can be assessed invasively using myocardial blush grade (MBG) or index microcirculatory resistance (IMR) [4,9,10]. It can also be detected using a wide range of non-invasive tools such as myocardial contrast echocardiography (MCE), myocardial perfusion scintigraphy (MPS), positron emission tomography (PET), and cardiac magnetic resonance imaging (MRI), which remains the gold standard of identifying and assessing the status of the microcirculation [11,12,13,14,15,16]. Contrast-enhanced cardiac MRI allows the measurement of MVO and is the modality of choice for assessing infarct size. Infarct size is a strong predictor of future clinical events beyond left ventricular (LV) ejection fraction and end-systolic volume index (ESVI) [17,18]. In fact, MVO has been proposed to be a surrogate of death and re-admission with heart failure in patients presenting with STEMI [15,19]. Irrespective of the way to assess coronary microcirculation, clinical outcomes are related to its impaired status [19,20], notwithstanding that poor microcirculatory function was also associated with in-hospital major adverse events, persistent ST-segment elevation, impaired LV function, and large infarct size [4,21,22].
Adjunctive tools during pPCI have been used to mitigate this risk and to preserve coronary microcirculation, which include a thrombectomy catheter. The direct association between thrombus burden and clinical outcomes, as well as microcirculation, would support the concept of removing the clot during pPCI. However, contemporary clinical trials failed to support the use of thrombectomy in patients presenting with STEMI. In this review, we aim to examine existing evidence on the use of thrombectomy for acute myocardial infarction (AMI) and provide future insights into upcoming technologies that may address the concept of thrombectomy in the STEMI population.
2. The Detrimental Role of Thrombus Burden in STEMI
The primary pathophysiologic process leading to AMI is related to the acute erosion or rupture of a vulnerable atheromatous plaque with the formation of an occlusive thrombus of epicardial coronary flow [20,23]. Despite the restoration of coronary epicardial flow by opening the IRA with pPCI (>90% of patients), optimal myocardial reperfusion is not always guaranteed, owing to distal microembolisation, myocyte necrosis, and changes in the integrity of the microvasculature [24]. Previous studies have highlighted the direct relationship between coronary microcirculation and thrombus burden. A well-validated scoring system was developed to predict the status of coronary microcirculation. The ATI score includes age, thrombus burden, and pre-stent index microcirculatory resistance, which was highly predictive of impaired microcirculation, defined as IMR > 40, with an area under the curve of 0.87 [25]. Importantly, thrombus burden carried the largest weight in predicting high IMR post-stenting. Furthermore, patients without a very large thrombus burden (i.e., a thrombus score less than 5; see Table 1 for classification) were less likely to develop impaired microcirculation in this study. Moreover, the ATI scoring system was validated in predicting MVO and infarct size on cardiac MRI and may identify patients at higher risk of adverse long-term events [26,27]. Collectively, this highlights the central role of thrombus burden on the functional status of coronary microcirculation in patients presenting with STEMI.

Table 1.
Thrombus burden classification [28].
In addition to microcirculation, previous studies have assessed the relationship between thrombus burden and clinical outcomes. A meta-analysis of an individual patient level of more than 18,000 patients from the large thrombus aspiration (TA) trials (TAPAS, TASTE, and TOTAL) showed that patients with large (TIMI thrombus grade ≥ 3) compared to small thrombus burden (<3) had significantly higher cardiovascular (3.1% vs. 2.2%, p = 0.02), but not all-cause, mortality at 30 days (3.1% vs. 2.4%, p = 0.07) [29]. This difference remained evident after one-year follow-up, and likewise, cardiovascular (4.5% vs. 3.2%, p = 0.006) but not all-cause mortality (5.3% vs. 4.4%, p = 0.08) was significantly higher in patients with a large thrombus score. Interestingly, when categorizing patients using a thrombus score cut-off of 4, all-cause mortality was higher compared to patients with a thrombus score of <4 at 30 days (3.3% vs. 2.4%, p = 0.01) but not at 1-year follow-up (5.4% vs. 4.6%, p = 0.08) [29]. Collectively, this supports the notion that thrombus burden is a risk marker in patients presenting with STEMI.
Mechanistically, the prognostic value of thrombus burden is beyond its role at the site of plaque rupture. Previous studies have also highlighted the detrimental role of distal embolization during pPCI. This may occur spontaneously or by the mechanical breakage of thrombi during pPCI and was estimated to affect 20–40% of STEMI cases. Thrombus score is known to be a predictor of angiographically visible distal embolisation [30]. Histopathological data revealed that distal embolization is mainly plaque fragments and partially organized thrombi which are unlikely to respond to anti-thrombotic medications [4,30,31]. The data also showed that a high thrombus burden was a significant predictor of distal embolization, with an almost 3-fold increase in mortality in the TOTAL trial [4,30,31]. In addition to mechanical plugging, the microembolisation of atherothrombotic debris may result in an inflammatory response and the recruitment of additional inflammatory cells, leading to further oedema and vasospasm that inevitably contribute to a reduction in coronary flow [24].
Overall, existing evidence highlights the adverse role of thrombus burden in patients with STEMI. Its role as a risk marker is well established; however, whether reducing the thrombus burden will translate into better clinical outcomes remains very controversial. The results of clinical outcomes from trials on using thrombus aspiration have not been consistent and will be discussed in the following section.
3. Concept of Thrombectomy/Thrombus Aspiration
The primary intention of thrombectomy or TA is to remove the clot that has developed at the site of acute plaque rupture. This improves flow down the infarcted artery and may achieve effective tissue reperfusion by preventing distal embolisation during pPCI [32,33]. It is apparent that thrombus burden, distal embolisation, and impairment of the microcirculation are correlated with poor patient clinical outcomes, including all-cause mortality and heart failure [5,7,19,30,34,35].
Early studies assessed the role of TA in improving myocardial reperfusion using surrogates of patients’ clinical outcomes. The prospective single-centre REMEDIA (Randomised Evaluation of the Effect of Mechanical Reduction of Distal Embolisation by Thrombus-Aspiration in Primary and Rescue Angioplasty) trial randomised 100 consecutive patients before coronary angiography into manual TA using a 6-French Diver CE catheter (Invatec, Brescia, Italy) or standard. The primary endpoints were post-procedural angiographic (MBG ≥ 2) and electrocardiographic (ECG) (ST-segment resolution (STR) ≥ 70%). The authors showed better myocardial reperfusion parameters using TA (46%) compared to PCI alone (24.5%) (odds ratio (OR) of 2.6 (95% confidence interval [CI], 1.1–6.2), p = 0.025) [36]. These results were reinforced by the findings from the EXPIRA (Thrombectomy With Export Catheter in Infarct-Related Artery During pPCI) study, which included 175 STEMI patients. It highlighted the benefits of TA in achieving MBG ≥ 2 (80% vs. 60%, p = 0.001) and STR ≥ 70% (64% vs. 39%, p = 0.001) compared to no TA [37]. The contrast-enhanced cardiac MRI sub-study of the EXPIRA study recruited 75 patients and showed a significant reduction in MVO and infarct size at 3 months with the use of TA [37].
Collectively, the evidence from the REMEDIA and the EXPIRA studies supports the potential beneficial role of TA as an adjunctive therapy to pPCI, although both studies had a small sample size. TAPAS (Thrombus Aspiration during Percutaneous Coronary Intervention in Acute Myocardial Infarction) was a prospective randomized controlled trial (RCT) which recruited a relatively large sample size of 1071 patients and used a 6-French Export Aspiration Catheter [38]. The use of TA resulted not only in better microvascular reperfusion (MBG 0 or 1, 17.1% vs. 26.3%, p < 0.001) but also in a cardiovascular mortality reduction at 1 year (3.6% vs. 6.7%, hazard ratio 1.93, 95% confidence interval 1.11–3.37, p = 0.02) [39]. A subsequent meta-analysis by De Luca et al. reported a significant reduction in 30-day mortality with adjunctive manual TA during pPCI for AMI patients (1.7 vs. 3.1%, OR 0.58; 95% CI (0.34–0.98), p = 0.01) [40,41]. Similarly, 6–12-month mortality benefits were reported in an updated meta-analysis of 18 trials (including TAPAS) [40,41]. Given the findings from these studies, this was reflected in previous guidelines in 2008/2009 of the European Society of Cardiology (ESC) and the American Society of Cardiology (ACC)/American Heart Association (AHA), which recommended TA as a class IIa recommendation for routine use during pPCI [42,43].
However, larger studies including TASTE (Thrombus Aspiration in ST-Elevation Myocardial Infarction in Scandinavia) and TOTAL (Thrombectomy with PCI vs. PCI Alone in patients with STEMI) did not support the use of routine TA in patients presenting with STEMI. TASTE was conducted in 2013 and included 7244 STEMI patients within the Swedish Coronary Angiography and Angioplasty Registry (SCAAR) to investigate the primary endpoint of all-cause mortality at 30 days [44,45]. It showed no significant difference in the primary outcome of 30-day mortality between the two groups (2.8% vs. 3.0%, hazard ratio (HR) 0.94, [95% CI, 0.72–1.22]). It also showed no difference in stent thrombosis (HR 0.57, [95% CI, 0.20–1.02]) or recurrent MI reduction (HR 0.61, [95% CI, 0.34–1.07]) with the use of TA [44], with similar results at 1-year follow-up [46]. More importantly, TOTAL was the largest (n = 10,732) multi-centre prospective RCT comparing routine upfront manual TA with pPCI versus pPCI alone in STEMI [47,48]. The TOTAL study showed no difference in the incidence of the primary endpoints of cardiovascular death, recurrent MI, cardiogenic shock, and New York Heart Association (NYHA IV) heart failure at 180 days with the routine use of TA (6.9% vs. 7.0%; p = 0.86) and CV mortality (3.1% vs. 3.5%; p = 0.34) [48]. Importantly, there was a signal of harm given the small but significant increased risk of stroke in the TA arm (30-day stroke rates: 0.7% vs. 0.3%; p = 0.02) [48]. At 1-year follow-up, the primary endpoint occurred in 8% of patients undergoing routine TA as well as PCI alone (HR 1.00 [95% CI, 0.87–1.15], p = 0.99) [49]. Similarly, 1-year cardiovascular death was 4% in both groups (HR 0.93 [95% CI, 0.76–1.14]; p = 0.48) [49]. Interestingly, the difference in stroke continues to increase past 1-year follow-up, challenging the causal relationship with the use of TA (1.2% vs. 0.7%, HR 1.66 [95% CI, 1.10–2.51]; p = 0.015) [49]. Similarly, the INFUSE-AMI (Intracoronary Abciximab and Aspiration Thrombectomy in Patients with Large Anterior Myocardial Infarction) trial assessed the role of TA in 452 STEMI patients with occluded proximal or mid-left anterior descending artery to discern any benefits in patients with large infarcts [50,51]. TA did not result in a reduction in infarct size at 30 days, as assessed by MRI [52]. Subsequently, the 2021 ACC/AHA guidelines for Coronary Artery Revascularization downgraded the class of recommendation for routine TA to class III (no benefit) with a level of evidence of A [53]. Likewise, the most recent (2023) ESC guidelines for the management of acute coronary syndromes did not recommend the use of routine TA (class IIIA recommendation) [2]. TA studies are summarized and presented in Table 2.

Table 2.
Brief summary of previous manual thrombus aspiration trials *.
4. The Disconnect between Mechanistic and Clinical Evidence
Previous data highlighted thrombus burden as a risk marker in patients presenting with STEMI. Subsequently, small RCTs showed benefits in angiographic surrogates of clinical outcomes. Nonetheless, large RCTs did not support the use of routine TA in patients undergoing pPCI. Of interest, a recently published meta-analysis of 28 published studies by Bianchini et al. attempted to provide some insights into variables that were associated with an improvement in left-ventricle function in response to TA [54]. This included total ischemic time, left anterior descending artery (LAD) involvement, and TA technique [54]. Therefore, it is important to understand the limitations within these recent RCTs, which may explain this disconnect and may provide a platform to design future studies that could address the use of TA in patients presenting with STEMI.
Firstly, there was a lack of standard technical steps to guide TA during pPCI. Thrombectomy was used by crossing the thrombotic lesion and attempted to retrieve the thrombus by applying negative pressure during the pullback manoeuvre. This does not only reduce the efficacy of TA but also increases the risk of distal embolization by pushing the thrombus distally. Moreover, the volume of vacuum syringes was not standardized, and frequently, a single syringe was only used during thrombectomy. Furthermore, deep intubation of the guiding catheter was not recommended. This may, in theory, reduce the risk of thrombus dislodgement, causing peripheral embolization and stroke [41]. Overall, there is no consensus on the optimal use of TA in patients presenting with STEMI.
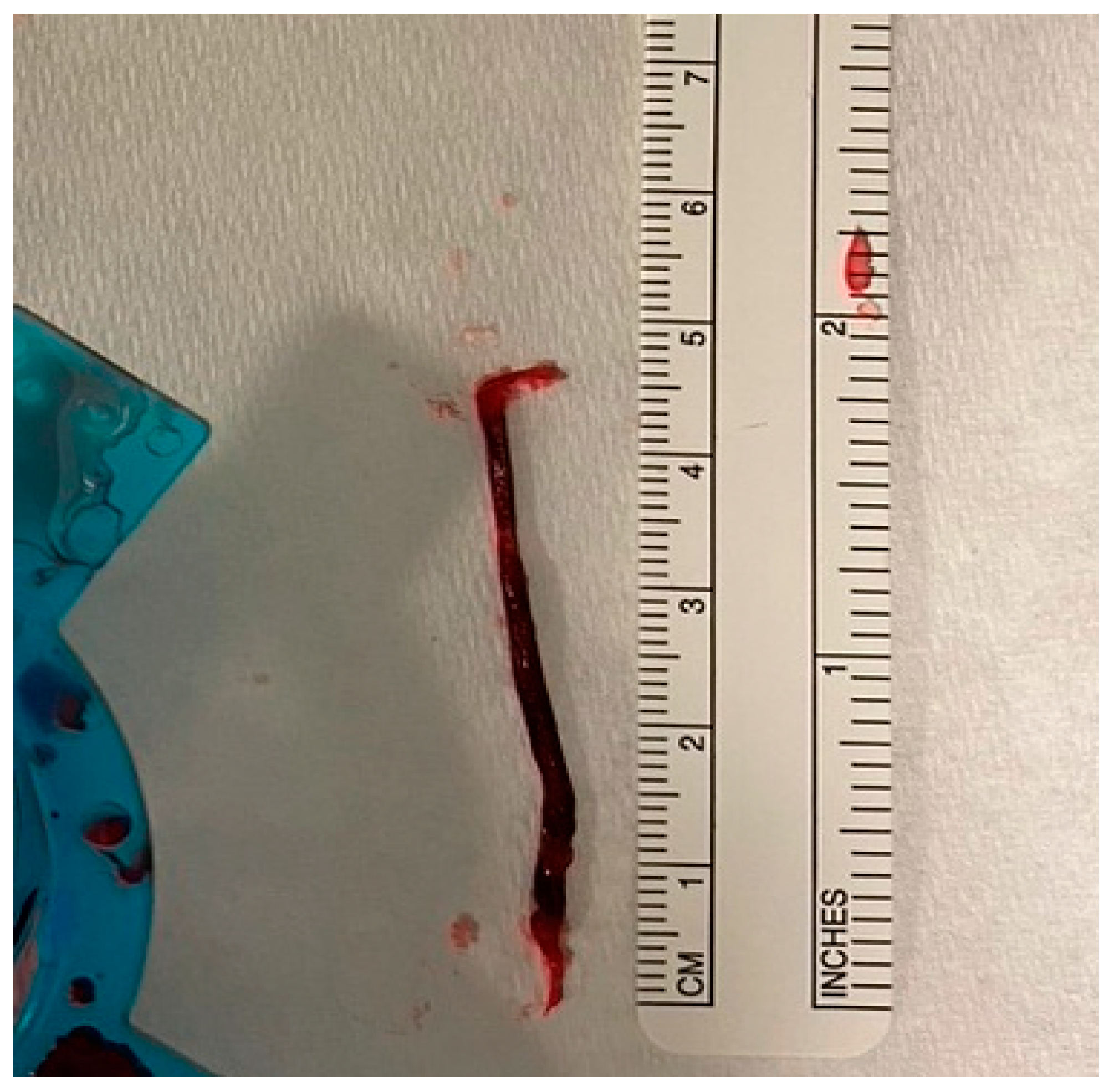
Secondly, large RCTs included all-comers of AMI cases and tested the benefits of routinely using TA in patients presenting with acute MI. Those patients have variable degrees of thrombus burden, and the purpose of thrombectomy is to extract the clot from the infarct site. Therefore, the rationale behind the use of TA in patients with a small thrombus burden is mechanistically not valid. On the other hand, thrombectomy should be considered in patients who have a large thrombus burden. This selective approach may allow a better assessment of the role of thrombectomy and whether it reduces cardiovascular risk. More importantly, the use of TA in patients with a small thrombus burden may dilute the result and mask any potential benefits of using TA in patients presenting with STEMI. In fact, an individual patient-level meta-analysis of the three large thrombectomy studies (TOTAL, TASTE, and TAPAS) showed that TA was associated with a small reduction in cardiovascular death in the high-thrombus-burden patient subgroup (TIMI thrombus grade ≥ 3) (2.5% vs. 3.1%; HR, 0.80; [95% CI, 0.65–0.98]; p = 0.03) [29] (Figure 1 illustrates an example of a large extracted thrombus). This finding was not present in patients with a low thrombus burden or in the whole cohort, highlighting the importance of a selective approach when applying TA in patients presenting with STEMI.
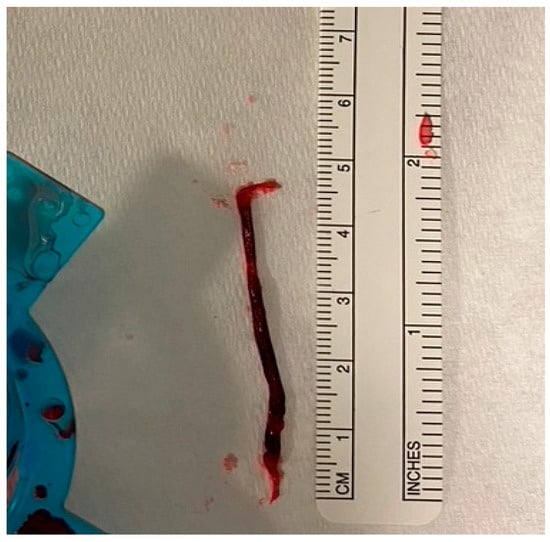
Figure 1.
Large thrombus aspirated with manual TA during pPCI for anterior STEMI.
Thirdly, the currently used thrombectomy catheters are not very effective in retrieving thrombi. Manual thrombectomy catheters such as Export (Medtronic, crossing profile 0.068 in), Eliminate (Terumo Medical Corporation, Tokyo, Japan, crossing profile 0.068 in), OXT (Vascular Solutions, Minneapolis, MN, USA crossing profile 0.067 in), and Pronto (Vascular Solution, crossing profile 0.065 in) share the same concept of applying a vacuum at the proximal end in order to extract the thrombus from the plaque rupture site [44]. In fact, angiographic and imaging data suggest a large residual thrombus burden following the use of currently available thrombectomy catheters. The ineffective removal of a thrombus with a large residual thrombus burden (rTB) increases the risk of distal embolisation, NRP, and poor clinical outcomes [31,55]. Data from the TOTAL trial showed that a large rTB, as quantified by TIMI thrombus grade, was present in one third of patients who underwent TA [55]. Importantly, there was more than an 80% increase in the risk of the primary endpoint and cardiovascular death in patients with a large compared to small rTB following TA. Moreover, the risk of cardiogenic shock was doubled in this subgroup [55]. Similarly, the OCT sub-study from TOTAL, which included 214 STEMI patients, did not reveal any reduction in pre-stent thrombus burden with the adjunctive use of TA compared to PCI alone (2.36%, [95% CI, 1.73–3.22] vs. 2.88% [95% CI, 2.12–3.90], respectively, p = 0.373) [56].
To overcome the presence of a large thrombus burden, the ‘mother-in-child’ thrombectomy technique was developed to reduce the burden of thrombus in patients presenting with AMI. This technique is performed using a 5-French ‘Heartrail’ catheter (Terumo Medical) inside 6-French guiding system. In a small study of 13 patients, this technique reduced angiographic thrombus burden and improved coronary flow in 85% of patients.
5. Emerging Thrombectomy Technologies
Given the above inherent limitations in the design of thrombectomy catheters alongside their use in all-comer AMI patients, new technologies were developed to overcome these limitations, aiming to improve their efficacy in reducing thrombus burden and possibly clinical outcomes (Figure 2). Nonetheless, these tools had to be initially assessed to ensure safety during pPCI.
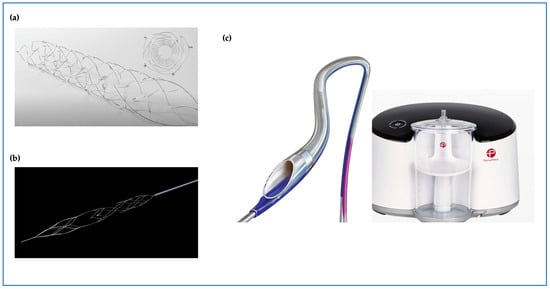
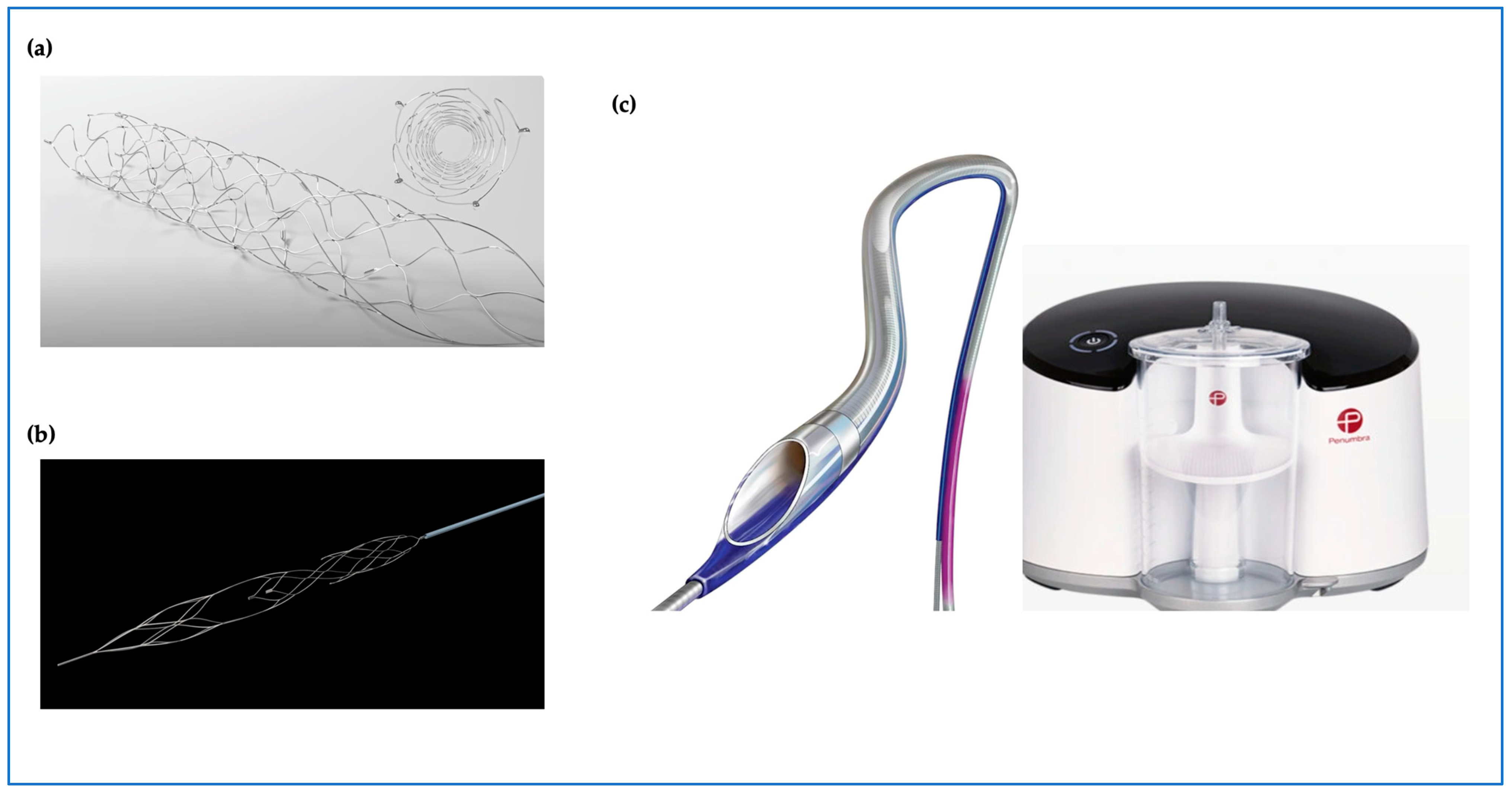
Figure 2.
Emerging thrombectomy tool technologies with new designs; (a) Solitaire™ device stent retriever (Medtronic). (b) enVastTM stent retriever (Vesalio). (c) CAT RX powered by Penumbra Engine.
Mechanical thrombectomy using the Indigo CAT RX Aspiration System (Penumbra Inc., Alameda, CA, USA) was designed to provide continuous aspiration using a larger aspiration port and lumen (compared to manual aspiration catheters) and works with a Penumbra aspiration pump (Figure 2) [57]. The CAT RX is intended to provide a higher and more sustained vacuum to retrieve larger thrombus burdens and is currently used to treat ischemic stroke during neurovascular intervention. The CHEETAH Study (Sustained Mechanical Aspiration Thrombectomy for High Thrombus Burden Coronary Vessel Occlusion) investigated the safety of continuous mechanical thrombectomy prior to PCI in TIMI-thrombus-grade-4 and -5 AMI patients [57]. This single-arm registry enrolled 400 patients between August 2019 and December 2020 across 25 hospitals in the United States. It demonstrated the feasibility and safety of sustained mechanical thrombectomy in selected patients with a large thrombus burden. This was reflected in the low adverse CV event rate in this study with the primary endpoint of cardiovascular death, recurrent MI, cardiogenic shock, and NYHA IV heart failure of 3.60% [95% CI, 2.0–6.0%]. Additionally, the study reported a significant rate of final MBG grade 3 in 99.75% at the end of the procedure [57]. A previous meta-analysis reported increased mortality associated with mechanical thrombectomy [58]. It is important to highlight that the CAT RX catheter was not part of this analysis [58]. This meta-analysis referenced an ‘old’ technology whereby saline jets or rotating catheters were used to break up the thrombus prior to aspiration [58]. Importantly, there are no previous RCTs supporting the use of mechanical thrombectomy, and therefore, their efficacy as well as safety remain to be determined.
More recently, Spirito et al. reported the results of the first in-human use of the EnVast stent retrieval catheter (previously known as NeVa device) [59]. The authors included 61 patients presenting with AMI and a large thrombus burden and reported excellent safety data, with a single patient suffering from side-branch embolization. This phenomenon was subsequently overcome using the vacuum-assisted aspiration technique. Another side effect was reversible coronary spasm in 23% of patients, but there were no reports of coronary dissection or perforation. The results of EnVast on its own (prior to stenting) were excellent, with only 10% of patients having TIMI flow < 3 and almost one quarter having MBG < 2 [59]. EnVast received CE marking for use in AMI in 2019 [60].
Other stent retrieval technologies are also available for use in AMI patients. Few cases have been reported in the literature highlighting the benefits of using the nitinol stent-based self-expanding device (Solitaire, Medtronic) to retrieve a refractory thrombus from ectatic large coronary arteries [61,62]. The RETRIEVE AMI is a pilot RCT designed to assess the safety and efficacy of stent retrieval technology using Solitaire compared to conventional PCI or manual TA in patients with a large thrombus burden [63]. The primary endpoint will be the change in thrombus burden following thrombectomy and will be quantified using OCT. The study is planned to complete recruitment in 2024.
Overall, the safety profile of existing and emerging thrombectomy catheters is excellent. Given that these tools are delivered using a monorail system, there has not been any signal to suggest an increased risk of coronary dissection or perforation. Side-branch embolization has been reported with the use of stent retrieval catheters, but with increased experience and the use of a guide extension catheter, this risk could be minimized. Stroke has been reported as a potential side effect associated with existing thrombectomy catheters. This has not been consistent across all studies and is likely to reflect the used technique when deploying thrombectomy.
6. Conclusions
Thrombus burden is recognized to be a risk marker for patients presenting with AMI. Previous thrombectomy catheters have failed to show significant clinical benefits when tested in large RCTs. This may be related to the design of these studies or the used thrombectomy tools. Emerging thrombectomy technologies may provide more insights into the role of thrombus aspiration in patients presenting with AMI [64]. They could address the question as to whether decreasing thrombus burden is directly linked to reducing adverse clinical outcomes.
Author Contributions
Conceptualization: M.A.; methodology: Z.S. and M.A.; resources: M.E., M.F. and M.A.; writing—original draft preparation: Z.S. and M.A.; review and editing: Z.S., M.O., B.B., T.C., M.E., M.F. and M.A.; visualization: Z.S., M.O. and M.A.; supervision: M.A.; project administration: Z.S. and M.A.; funding acquisition: M.E., M.F. and M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author M.A. declares that he received a research grant from Vesalio. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
References
- Libby, P. Mechanisms of Acute Coronary Syndromes and Their Implications for Therapy. N. Engl. J. Med. 2013, 368, 2004–2013. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes: Developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Szummer, K.; Wallentin, L.; Lindhagen, L.; Alfredsson, J.; Erlinge, D.; Held, C.; James, S.; Kellerth, T.; Lindahl, B.; Ravn-Fischer, A.; et al. Improved outcomes in patients with ST-elevation myocardial infarction during the last 20 years are related to implementation of evidence-based treatments: Experiences from the SWEDEHEART registry 1995–2014. Eur. Heart J. 2017, 38, 3056–3065. [Google Scholar] [CrossRef] [PubMed]
- Van’t Hof, A.W.J.; Liem, A.; Suryapranata, H.; Hoorntje, J.C.A.; De Boer, M.J.; Zijlstra, F. Angiographic Assessment of Myocardial Reperfusion in Patients Treated with Primary Angioplasty for Acute Myocardial Infarction. Circulation 1998, 97, 2302–2306. [Google Scholar] [CrossRef] [PubMed]
- Henriques, J.P.S.; Zijlstra, F.; Ottervanger, J.P.; De Boer, M.J.; Van’T Hof, A.W.J.; Hoorntje, J.C.A.; Suryapranata, H. Incidence and clinical significance of distal embolization during primary angioplasty for acute myocardial infarction. Eur. Heart J. 2002, 23, 1112–1117. [Google Scholar] [CrossRef] [PubMed]
- Piana, R.N.; Paik, G.Y.; Moscucci, M.; Cohen, D.J.; Gibson, C.M.; Kugelmass, A.D.; Carrozza, J.P.; Kuntz, R.E.; Baim, D.S. Incidence and treatment of “no-reflow” after percutaneous coronary intervention. Circulation 1994, 89, 2514–2518. [Google Scholar] [CrossRef] [PubMed]
- Morishima, I.; Sone, T.; Mokuno, S.; Taga, S.; Shimauchi, A.; Oki, Y.; Kondo, J.; Tsuboi, H.; Sassa, H. Clinical significance of no-reflow phenomenon observed on angiography after successful treatment of acute myocardial infarction with percutaneous transluminal coronary angioplasty. Am. Heart J. 1995, 130, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Iwakura, K.; Ito, H.; Takiuchi, S.; Taniyama, Y.; Nakatsuchi, Y.; Negoro, S.; Higashino, Y.; Okamura, A.; Masuyama, T.; Hori, M.; et al. Alternation in the Coronary Blood Flow Velocity Pattern in Patients With No Reflow and Reperfused Acute Myocardial Infarction. Circulation 1996, 94, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Fearon, W.F.; Balsam, L.B.; Farouque, H.M.O.; Robbins, R.C.; Fitzgerald, P.J.; Yock, P.G.; Yeung, A.C. Novel Index for Invasively Assessing the Coronary Microcirculation. Circulation 2003, 107, 3129–3132. [Google Scholar] [CrossRef]
- De Maria, G.L.; Alkhalil, M.; Wolfrum, M.; Fahrni, G.; Borlotti, A.; Gaughran, L.; Dawkins, S.; Langrish, J.P.; Lucking, A.J.; Choudhury, R.P.; et al. Index of Microcirculatory Resistance as a Tool to Characterize Microvascular Obstruction and to Predict Infarct Size Regression in Patients with STEMI Undergoing Primary PCI. JACC Cardiovasc. Imaging 2019, 12, 837–848. [Google Scholar] [CrossRef]
- Ragosta, M.; Camarano, G.; Kaul, S.; Powers, E.R.; Sarembock, I.J.; Gimple, L.W. Microvascular integrity indicates myocellular viability in patients with recent myocardial infarction. New insights using myocardial contrast echocardiography. Circulation 1994, 89, 2562–2569. [Google Scholar] [CrossRef] [PubMed]
- Schofer, J.; Montz, R.; Mathey, D.G. Scintigraphic evidence of the “No reflow” phenomenon in human beings after coronary thrombolysis. J. Am. Coll. Cardiol. 1985, 5, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Lepper, W.; Hoffmann, R.; Kamp, O.; Franke, A.; De Cock, C.C.; Kühl, H.P.; Sieswerda, G.T.; Vom Dahl, J.; Janssens, U.; Voci, P.; et al. Assessment of Myocardial Reperfusion by Intravenous Myocardial Contrast Echocardiography and Coronary Flow Reserve After Primary Percutaneous Transluminal Coronary Angiography in Patients with Acute Myocardial Infarction. Circulation 2000, 101, 2368–2374. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.C.; Kim, R.J.; Bluemke, D.A.; Rochitte, C.E.; Zerhouni, E.A.; Becker, L.C.; Lima, J.A.C. Quantification and time course of microvascular obstruction by contrast-enhanced echocardiography and magnetic resonance imaging following acute myocardial infarction and reperfusion. J. Am. Coll. Cardiol. 1998, 32, 1756–1764. [Google Scholar] [CrossRef] [PubMed]
- Ghobrial, M.; Bawamia, B.; Cartlidge, T.; Spyridopoulos, I.; Kunadian, V.; Zaman, A.; Egred, M.; McDiarmid, A.; Williams, M.; Farag, M.; et al. Microvascular Obstruction in Acute Myocardial Infarction, a Potential Therapeutic Target. J. Clin. Med. 2023, 12, 5934. [Google Scholar] [CrossRef] [PubMed]
- Alkhalil, M.; Borlotti, A.; De Maria, G.L.; Wolfrum, M.; Dawkins, S.; Fahrni, G.; Gaughran, L.; Langrish, J.P.; Lucking, A.; Ferreira, V.M.; et al. Hyper-acute cardiovascular magnetic resonance T1 mapping predicts infarct characteristics in patients with ST elevation myocardial infarction. J. Cardiovasc. Magn. Reson. 2020, 22, 3. [Google Scholar] [CrossRef] [PubMed]
- Wu, E.; Ortiz, J.T.; Tejedor, P.; Lee, D.C.; Bucciarelli-Ducci, C.; Kansal, P.; Carr, J.C.; Holly, T.A.; Lloyd-Jones, D.; Klocke, F.J.; et al. Infarct size by contrast enhanced cardiac magnetic resonance is a stronger predictor of outcomes than left ventricular ejection fraction or end-systolic volume index: Prospective cohort study. Heart 2008, 94, 730–736. [Google Scholar] [CrossRef] [PubMed]
- De Carlo, M.; Aquaro, G.D.; Palmieri, C.; Guerra, E.; Misuraca, L.; Giannini, C.; Lombardi, M.; Berti, S.; Petronio, A.S. A prospective randomized trial of thrombectomy versus no thrombectomy in patients with ST-segment elevation myocardial infarction and thrombus-rich lesions: MUSTELA (MUltidevice Thrombectomy in Acute ST-Segment ELevation Acute Myocardial Infarction) trial. JACC Cardiovasc. Interv. 2012, 5, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- De Waha, S.; Patel, M.R.; Granger, C.B.; Ohman, E.M.; Maehara, A.; Eitel, I.; Ben-Yehuda, O.; Jenkins, P.; Thiele, H.; Stone, G.W. Relationship between microvascular obstruction and adverse events following primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: An individual patient data pooled analysis from seven randomized trials. Eur. Heart J. 2017, 38, 3502–3510. [Google Scholar] [CrossRef]
- Alkhalil, M.; De Maria, G.L.; Akbar, N.; Ruparelia, N.; Choudhury, R.P. Prospects for Precision Medicine in Acute Myocardial Infarction: Patient-Level Insights into Myocardial Injury and Repair. J. Clin. Med. 2023, 12, 4668. [Google Scholar] [CrossRef]
- Poli, A.; Fetiveau, R.; Vandoni, P.; Del Rosso, G.; D’Urbano, M.; Seveso, G.; Cafiero, F.; De Servi, S. Integrated Analysis of Myocardial Blush and ST-Segment Elevation Recovery After Successful Primary Angioplasty. Circulation 2002, 106, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Fahrni, G.; Wolfrum, M.; De Maria, G.L.; Cuculi, F.; Dawkins, S.; Alkhalil, M.; Patel, N.; Forfar, J.C.; Prendergast, B.D.; Choudhury, R.P.; et al. Index of Microcirculatory Resistance at the Time of Primary Percutaneous Coronary Intervention Predicts Early Cardiac Complications: Insights from the OxAMI (Oxford Study in Acute Myocardial Infarction) Cohort. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2017, 6, e005409. [Google Scholar] [CrossRef] [PubMed]
- Falk, E. Plaque rupture with severe pre-existing stenosis precipitating coronary thrombosis. Characteristics of coronary atherosclerotic plaques underlying fatal occlusive thrombi. Br. Heart J. 1983, 50, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, R.; Charron, T.; Puley, G.; Dick, A.; Strauss, B.H. Microvascular Obstruction and the No-Reflow Phenomenon After Percutaneous Coronary Intervention. Circulation 2008, 117, 3152–3156. [Google Scholar] [CrossRef]
- De Maria, G.L.; Fahrni, G.; Alkhalil, M.; Cuculi, F.; Dawkins, S.; Wolfrum, M.; Choudhury, R.P.; Forfar, J.C.; Prendergast, B.D.; Yetgin, T.; et al. A tool for predicting the outcome of reperfusion in ST-elevation myocardial infarction using age, thrombotic burden and index of microcirculatory resistance (ATI score). EuroIntervention 2016, 12, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- De Maria, G.L.; Alkhalil, M.; Wolfrum, M.; Fahrni, G.; Borlotti, A.; Gaughran, L.; Dawkins, S.; Langrish, J.P.; Lucking, A.J.; Choudhury, R.P.; et al. The ATI score (age-thrombus burden-index of microcirculatory resistance) determined during primary percutaneous coronary intervention predicts final infarct size in patients with ST-elevation myocardial infarction: A cardiac magnetic resonance validation study. EuroIntervention 2017, 13, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Montalto, C.; Kotronias, R.A.; Marin, F.; Terentes-Printzios, D.; Shanmuganathan, M.; Emfietzoglou, M.; Scalamera, R.; Porto, I.; Langrish, J.; Lucking, A.; et al. Pre-procedural ATI score (age-thrombus burden-index of microcirculatory resistance) predicts long-term clinical outcomes in patients with ST elevation myocardial infarction treated with primary percutaneous coronary intervention. Int. J. Cardiol. 2021, 339, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.M.; de Lemos, J.A.; Murphy, S.A.; Marble, S.J.; Mccabe, C.H.; Cannon, C.P.; Antman, E.M.; Braunwald, E. Combination Therapy with Abciximab Reduces Angiographically Evident Thrombus in Acute Myocardial Infarction A TIMI 14 Substudy. 2021. Available online: http://www.circulationaha.org (accessed on 5 March 2024).
- Jolly, S.S.; James, S.; Džavík, V.; Cairns, J.A.; Mahmoud, K.D.; Zijlstra, F.; Yusuf, S.; Olivecrona, G.K.; Renlund, H.; Gao, P.; et al. Thrombus Aspiration in ST-Segment-Elevation Myocardial Infarction: An Individual Patient Meta-Analysis: Thrombectomy Trialists Collaboration. Circulation 2017, 135, 143–152. [Google Scholar] [CrossRef]
- Sharma, V.; Jolly, S.S.; Hamid, T.; Sharma, D.; Chiha, J.; Chan, W.; Fuchs, F.; Bui, S.; Gao, P.; Kassam, S.; et al. Myocardial blush and microvascular reperfusion following manual thrombectomy during percutaneous coronary intervention for ST elevation myocardial infarction: Insights from the TOTAL trial. Eur. Heart J. 2016, 37, 1891–1898. [Google Scholar] [CrossRef]
- Limbruno, U.; De Carlo, M.; Pistolesi, S.; Micheli, A.; Petronio, A.S.; Camacci, T.; Fontanini, G.; Balbarini, A.; Mariani, M.; De Caterina, R. Distal embolization during primary angioplasty: Histopathologic features and predictability. Am. Heart J. 2005, 150, 102–108. [Google Scholar] [CrossRef]
- Mongeon, F.P.; Bélisle, P.; Joseph, L.; Eisenberg, M.J.; Rinfret, S. Adjunctive thrombectomy for acute myocardial infarction a Bayesian meta-analysis. Circ. Cardiovasc. Interv. 2010, 3, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Tamhane, U.U.; Chetcuti, S.; Hameed, I.; Grossman, P.M.; Moscucci, M.; Gurm, H.S. Safety and efficacy of thrombectomy in patients undergoing primary percutaneous coronary intervention for acute ST elevation MI: A meta-analysis of randomized controlled trials. BMC Cardiovasc. Disord. 2010, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Fearon, W.F.; Low, A.F.; Yong, A.S.; McGeoch, R.; Berry, C.; Shah, M.G.; Ho, M.Y.; Kim, H.S.; Loh, J.P.; Oldroyd, K.G. Prognostic Value of the Index of Microcirculatory Resistance Measured After Primary Percutaneous Coronary Intervention. Circulation 2013, 127, 2436–2441. [Google Scholar] [CrossRef] [PubMed]
- Carrick, D.; Haig, C.; Ahmed, N.; Carberry, J.; Yue May, V.T.; McEntegart, M.; Petrie, M.C.; Eteiba, H.; Lindsay, M.; Hood, S.; et al. Comparative Prognostic Utility of Indexes of Microvascular Function Alone or in Combination in Patients with an Acute ST-Segment-Elevation Myocardial Infarction. Circulation 2016, 134, 1833–1847. [Google Scholar] [CrossRef] [PubMed]
- Burzotta, F.; Trani, C.; Romagnoli, E.; Mazzari, M.A.; Rebuzzi, A.G.; De Vita, M.; Garramone, B.; Giannico, F.; Niccoli, G.; Biondi-Zoccai, G.G.L.; et al. Manual Thrombus-Aspiration Improves Myocardial Reperfusion: The Randomized Evaluation of the Effect of Mechanical Reduction of Distal Embolization by Thrombus-Aspiration in Primary and Rescue Angioplasty (REMEDIA) Trial. J. Am. Coll. Cardiol. 2005, 46, 371–376. [Google Scholar] [CrossRef]
- Sardella, G.; Mancone, M.; Bucciarelli-Ducci, C.; Agati, L.; Scardala, R.; Carbone, I.; Francone, M.; Di Roma, A.; Benedetti, G.; Conti, G.; et al. Thrombus Aspiration During Primary Percutaneous Coronary Intervention Improves Myocardial Reperfusion and Reduces Infarct Size. The EXPIRA (Thrombectomy with Export Catheter in Infarct-Related Artery During Primary Percutaneous Coronary Intervention) Prospective, Randomized Trial. J. Am. Coll. Cardiol. 2009, 53, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Svilaas, T.; Vlaar, P.J.; van der Horst, I.C.; Diercks, G.F.; JGL de Smet, B.; van den Heuvel, A.F.; Anthonio, R.L.; Jessurun, G.A.; Tan, E.-S.; Suurmeijer, A.J.; et al. Thrombus Aspiration during Primary Percutaneous Coronary Intervention. N. Engl. J. Med. 2008, 358, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Vlaar, P.J.; Svilaas, T.; van der Horst, I.C.; Diercks, G.F.; Fokkema, M.L.; de Smet, B.J.; van den Heuvel, A.F.; Anthonio, R.L.; Jessurun, G.A.; Tan, E.S.; et al. Cardiac death and reinfarction after 1 year in the Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS): A 1-year follow-up study. Lancet 2008, 371, 1915–1920. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Dudek, D.; Sardella, G.; Marino, P.; Chevalier, B.; Zijlstra, F. Adjunctive manual thrombectomy improves myocardial perfusion and mortality in patients undergoing primary percutaneous coronary intervention for ST-elevation myocardial infarction: A meta-analysis of randomized trials. Eur. Heart J. 2008, 29, 3002–3010. [Google Scholar] [CrossRef]
- Kumbhani, D.J.; Bavry, A.A.; Desai, M.Y.; Bangalore, S.; Bhatt, D.L. Role of aspiration and mechanical thrombectomy in patients with acute myocardial infarction undergoing primary angioplasty: An updated meta-analysis of randomized trials. J. Am. Coll. Cardiol. 2013, 62, 1409–1418. [Google Scholar] [CrossRef]
- Van De Werf, F.; Bax, J.; Betriu, A.; Blomstrom-Lundqvist, C.; Crea, F.; Falk, V.; Filippatos, G.; Fox, K.; Huber, K.; Kastrati, A.; et al. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: The Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology. Eur. Heart J. 2008, 29, 2909–2945. [Google Scholar] [CrossRef]
- Kushner, F.G.; Hand, M.; Smith, S.C.; King, S.B.; Anderson, J.L.; Antman, E.M.; Bailey, S.R.; Bates, E.R.; Blankenship, J.C.; Casey, D.E.; et al. 2009 Focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update). Catheter. Cardiovasc. Interv. 2009, 74, E25–E68. [Google Scholar] [CrossRef]
- Fröbert, O.; Lagerqvist, B.; Olivecrona, G.K.; Omerovic, E.; Gudnason, T.; Maeng, M.; Aasa, M.; Angerås, O.; Calais, F.; Danielewicz, M.; et al. Thrombus Aspiration during ST-Segment Elevation Myocardial Infarction. N. Engl. J. Med. 2013, 369, 1587–1597. [Google Scholar] [CrossRef]
- Fröbert, O.; Lagerqvist, B.; Gudnason, T.; Thuesen, L.; Svensson, R.; Olivecrona, G.K.; James, S.K. Thrombus aspiration in ST-elevation myocardial infarction in Scandinavia (TASTE trial). A multicenter, prospective, randomized, controlled clinical registry trial based on the Swedish angiography and angioplasty registry (SCAAR) platform. study design and rationale. Am. Heart J. 2010, 160, 1042–1048. [Google Scholar] [CrossRef]
- Lagerqvist, B.; Fröbert, O.; Olivecrona, G.K.; Gudnason, T.; Maeng, M.; Alström, P.; Andersson, J.; Calais, F.; Carlsson, J.; Collste, O.; et al. Outcomes 1 Year after Thrombus Aspiration for Myocardial Infarction. N. Engl. J. Med. 2014, 371, 1111–1120. [Google Scholar] [CrossRef]
- Jolly, S.S.; Cairns, J.; Yusuf, S.; Meeks, B.; Shestakovska, O.; Thabane, L.; Niemelä, K.; Steg, P.G.; Bertrand, O.F.; Rao, S.V.; et al. Design and rationale of the TOTAL trial: A randomized trial of routine aspiration ThrOmbecTomy with percutaneous coronary intervention (PCI) versus PCI ALone in patients with ST-elevation myocardial infarction undergoing primary PCI. Am. Heart J. 2014, 167, 315–321.e1. [Google Scholar] [CrossRef]
- Jolly, S.S.; Cairns, J.A.; Yusuf, S.; Meeks, B.; Pogue, J.; Rokoss, M.J.; Kedev, S.; Thabane, L.; Stankovic, G.; Moreno, R.; et al. Randomized Trial of Primary PCI with or without Routine Manual Thrombectomy. N. Engl. J. Med. 2015, 372, 1389–1398. [Google Scholar] [CrossRef]
- Jolly, S.S.; Cairns, J.A.; Yusuf, S.; Rokoss, M.J.; Gao, P.; Meeks, B.; Kedev, S.; Stankovic, G.; Moreno, R.; Gershlick, A.; et al. Outcomes after thrombus aspiration for ST elevation myocardial infarction: 1-year follow-up of the prospective randomised TOTAL trial. Lancet 2016, 387, 127–135. [Google Scholar] [CrossRef]
- Stone, G.W.; Witzenbichler, B.; Godlewski, J.; Dambrink, J.H.E.; Ochala, A.; Chowdhary, S.; El-Omar, M.; Neunteufl, T.; Metzger, D.C.; Dizon, J.M.; et al. Intralesional abciximab and thrombus aspiration in patients with large anterior myocardial infarction one-year results from the infuse-ami trial. Circ. Cardiovasc. Interv. 2013, 6, 527–534. [Google Scholar] [CrossRef]
- Stone, G.W.; Maehara, A.; Witzenbichler, B.; Godlewski, J.; Parise, H.; Dambrink, J.H.E.; Ochala, A.; Carlton, T.W.; Cristea, E.; Wolff, S.D.; et al. Intracoronary abciximab and aspiration thrombectomy in patients with large anterior myocardial infarction: The INFUSE-AMI randomized trial. JAMA 2012, 307, 1817–1826. [Google Scholar] [CrossRef]
- Stone, G.W.; Maehara, A.; Witzenbichler, B.; Godlewski, J.; Parise, H.; Dambrink, J.; Ochala, A.; Carlton, T.; Cristea, E.; Wolf, S.; et al. Intracoronary Abciximab and Aspiration Thrombectomy During Primary Pci For Anterior Stemi: One–Year Results from The Randomized Infuse–Ami Trial. J. Am. Coll. Cardiol. 2013, 61, E1853. [Google Scholar] [CrossRef]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, E18–E114. [Google Scholar] [CrossRef]
- Bianchini, E.; Lombardi, M.; Buonpane, A.; Ricchiuto, A.; Maino, A.; Laborante, R.; Anastasia, G.; D’Amario, D.; Aurigemma, C.; Romagnoli, E.; et al. Impact of thrombus aspiration on left ventricular remodeling and function in patients with ST-segment elevation myocardial infarction: A meta-analysis of randomized controlled trials. Int. J. Cardiol. 2023, 397, 131590. [Google Scholar] [CrossRef]
- Alkhalil, M.; Kuzemczak, M.; Zhao, R.; Kavvouras, C.; Cantor, W.J.; Overgaard, C.B.; Lavi, S.; Sharma, V.; Chowdhary, S.; Stanković, G.; et al. Prognostic Role of Residual Thrombus Burden Following Thrombectomy: Insights from the TOTAL Trial. Circ. Cardiovasc. Interv. 2022, 15, E011336. [Google Scholar] [CrossRef]
- Bhindi, R.; Kajander, O.A.; Jolly, S.S.; Kassam, S.; Lavi, S.; Niemelä, K.; Fung, A.; Cheema, A.N.; Meeks, B.; Alexopoulos, D.; et al. Culprit lesion thrombus burden after manual thrombectomy or percutaneous coronary intervention-alone in ST-segment elevation myocardial infarction: The optical coherence tomography sub-study of the TOTAL (ThrOmbecTomy versus PCI ALone) trial. Eur. Heart J. 2015, 36, 1892–1900. [Google Scholar] [CrossRef]
- Mathews, S.J.; Parikh, S.A.; Wu, W.; Metzger, D.C.; Chambers, J.W.; Ghali, M.G.H.; Sumners, M.J.; Kolski, B.C.; Pinto, D.S.; Dohad, S. Sustained Mechanical Aspiration Thrombectomy for High Thrombus Burden Coronary Vessel Occlusion: The Multicenter CHEETAH Study. Circ. Cardiovasc. Interv. 2023, 16, E012433. [Google Scholar] [CrossRef]
- Bavry, A.A.; Kumbhani, D.J.; Bhatt, D.L. Role of adjunctive thrombectomy and embolic protection devices in acute myocardial infarction: A comprehensive meta-analysis of randomized trials. Eur. Heart J. 2008, 29, 2989–3001. [Google Scholar] [CrossRef]
- Spirito, A.; Quagliana, A.; Coiro, M.; Melaku, G.D.; Vandenberghe, S.; Leibundgut, G.; Häner, J.; Moccetti, M.; Araco, M.; Garcia-Garcia, H.M.; et al. A prospective, first-in-human use of the NeVa mechanical thrombectomy device for patients with acute coronary syndromes. EuroIntervention 2022, 18, 242. [Google Scholar] [CrossRef]
- Akpinar, C.K.; Ozdemir, A.O.; Gurkas, E.; Bilgic, A.B.; Aykac, O.; Inanc, Y.; Giray, S. Favorable first-pass recanalization rates with NeVaTM thrombectomy device in acute stroke patients: Initial clinical experience. Interv. Neuroradiol. 2021, 27, 107–113. [Google Scholar] [CrossRef]
- Bhoopalan, K.; Rajendran, R.; Alagarsamy, S.; Kesavamoorthy, N. Successful extraction of refractory thrombus from an ectatic coronary artery using stent retriever during primary angioplasty for acute myocardial infarction: A case report. Eur. Heart J.-Case Rep. 2019, 3, yty161. [Google Scholar] [CrossRef]
- Patel, N.; Badiye, A.; Yavagal, D.R.; Mendoza, C.E. Stent-Based Mechanical Thrombectomy in Left Main Coronary Artery Thrombus Presenting as ST-Segment Elevation Myocardial Infarction. JACC Cardiovasc. Interv. 2017, 10, 302–303. [Google Scholar] [CrossRef]
- Kotronias, R.A.; Marin, F.; Emfietzoglou, M.; Langrish, J.P.; Lucking, A.J.; Channon, K.M.; Banning, A.P.; De Maria, G.L. Rationale and Design of a Randomized Controlled Pilot Trial to Assess Stent Retriever Thrombectomy for Thrombus Burden Reduction in Patients with Acute Myocardial Infarction: The RETRIEVE-AMI Study. Cardiovasc. Revascularization Med. 2023, 52, 75–85. [Google Scholar] [CrossRef] [PubMed]
- NATURE (EnVast as an Adjunct PPCI in Subjects Presenting with STEMI)—Full Text View—ClinicalTrials.gov. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04969471 (accessed on 26 January 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).