The Postoperative Paradoxical Septum (POPS): A Comprehensive Review on Physio-Pathological Mechanisms
Abstract
:1. Introduction
2. Epidemiology
| Author/Year | N. TOT | Male (%) | Mean Age of Patient | Type of Surgery | % POPS * | Imaging other than TTE M-Mode and 2d | Explanation Theories | |
|---|---|---|---|---|---|---|---|---|
| <3 Months ** | Follow-Up | |||||||
| Burggraf, 1975 [2] | 50 | 50 | 38 | 19 AVR 17 MVR 14 Other VHS | 51 § | 15 | - | Related to CPB |
| Righetti, 1977 [3] | 40 | 77 | 57 | 40 CABG | 57 | 20 | Radionuclide angiography | Transient ischemic injury and exaggerated cardiac mobility due to pericardiotomy |
| Vignola, 1979 [4] | 45 | - | 51 | 7 CABG 14 AVR 14 MVR 10 Others | 53 | - | GBPS | Related to CPB |
| Matsumoto, 1980 [5] | 24 | 67 | 58 | 12 CABG 7 AVR 5 MVR | 10 | - | Intraoperative TEE | Exaggerated cardiac mobility due to pericardiotomy |
| Waggoner, 1982 [38] | 17 | 56 | 56 ± 13 | 12 CABG 3 AVR 2 Others | 60 | - | Intraoperative direct-M-Mode post-operative 2D TTE | Exaggerated cardiac mobility due to pericardiotomy |
| Rubenson, 1982 [23] | 20 | 90 | 62 ± 15 | 20 CABG | 58 | - | TTE 2D | - |
| Kerber, 1982 [32] | 25 | - | - | 4 AVR 14 MVR 6 Others | 56 | 28 | TEE 2D | Exaggerated cardiac mobility due to limited RV free wall mobility |
| Gourdier, 1982 [30] | 256 | / | / | 256 VHS | 44 | - | - | Exaggerated cardiac mobility |
| Force, 1983 [39] | 20 | 20 | 59 | 17 CABG 2 AVR + CABG 1 AVR | 68 | - | TTE 2D (floating axis) radionuclide ventriculography | Exaggerated cardiac mobility |
| Akins, 1984 [40] | 22 | 68 | 52 | 22 CABG | 50 | - | Resting GBPS or ventricular angiography | Related to CPB and/or myocardial preservation techniques. |
| Schroeder, 1985 [31] | 324 | / | / | 110 CABG 214 HVS | 69 | 14 | - | Exaggerated cardiac mobility due to pericardiotomy |
| Schnittger, 1985 [41] | 21 | - | - | 14 CABG 6 HSV 1 Other | 76 | - | Intraoperative direct M-Mode | Exaggerated cardiac mobility due to pericardiotomy |
| Feneley, 1987 [20] | 16 | 87 | 52 | 15 CABG 1 Other | 56 | 0 | Intraoperative direct M-Mode post-operative TEE | Exaggerated cardiac mobility |
| De Nardo, 1989 [35] | 34 | 88 | 55.2± 7.0 | 34 CABG | 41 (Radionuclide angiocardiography) 29 (echocardiography) | - | Radionuclide angiocardiography | Exaggerated cardiac mobility due to pericardiotomy |
| van der Wall, 1990 [34] | 12 | 75 | 41 | 12 AVR | - | 92 | Radionuclide angiography | Rigid ring of prosthesis limiting septal excursion |
| Lehmann, 1990 [21] | 21 | 76 | 59.6 ± 9.6 | 18 CABG 2 HVS 1 Other | 100 qualitatively 76 quantitatively | - | Intraoperative TEE | Related to CPB |
| Okada, 1992 [33] | 16 | 100 | 59 | 16 CABG | 100 | 100 | Thallium-201 Scintigraphy Gated blood pool scan Tc 99m | Excluded ischemic injury |
| Wranne, 1993 [25] | 19 | 52 | 54 | 4 CABG 4 CABG + HVS 6 MVR/Rep 3 AVR 2 Others | 29 (before chest closure) 84 (after chest closure) | - | Intraoperative TEE | Recruitments of IVS to maintain RV global performance |
| Gigli, 1995 [42] | 10 | 80 | 60 ± 9 | 10 CABG | 50 | - | TEE cyclic gray-level variation study | Excluded ischemic injury |
| Giubbini, 2004 [22] | 82 | 86 | 67.8 ± 9.6 | 82 CABG | - | 93 | SPECT Tc 99 | Excluded ischemic injury |
| Hedman, 2004 [43] | 99 | 86 | 65 ± 9 | 99 CABG | - | 96 | - | Recruitments of IVS to maintain RV global performance |
| Toyoda, 2004 [44] | 12 | 83 | 62 ± 11 | 12 CABG | 75 (not specified the interval time from the intervention) | TDI | Exaggerated cardiac mobility | |
| Reynolds, 2007 [9] | 2979 | - | - | 1808 CABG 687 AVR 759 MVR/Rep 45 Others | 40 (not specified the interval time from the intervention) | None | Related to the type of surgery and surgical approach | |
| Joshi, 2008 [26] | 23 | 73 | 64 | 23 CABG | - | 100 | NMR | Recruitments of IVS to maintain RV global performance |
| Roshanali, 2008 [36] | 240 | 79 | 58.3 ± 11.3 | 240 CABG | - | 97 | TDI | Recruitments of IVS to maintain RV global performance |
| Choi, 2010 [45] | 18 | 56 | 58 ± 12 | 18 CABG | - | 56 | NMR rest/stress | Exaggerated cardiac mobility due to pericardiotomy |
| Codreanu, 2011 [46] | 18 | 100 | 67 ± 7 | 18 CABG | - | 100 | NMR high temporal resolution tissue phase mapping | Adhesion limiting the rotational motion of LV pushing IVS anteriorly during systole |
| Michaux, 2011 [47] | 50 | 65 ±8 cohort A 61 ± 9 cohort B | 50 CABG | 32 | - | TDI | No correlation with CPB | |
| Kang, 2014 [24] | 165 | 56 | 60 ± 13 | 59 CABG 99 VHS 7 Other | 73 | 25 | TOE-VVI | Related to subtle conduction disturbance |
| Moya Mur, 2018 [37] | 30 | 60 | 69.9 ± 13.3 | 7 CABG 11 AVR 2 MVR 10 Other | 50 in PTV 43 in 4CHV | - | TTE-STI | Exaggerated cardiac mobility due to limited RV free wall mobility |
3. Normal and Paradoxical Interventricular Septum
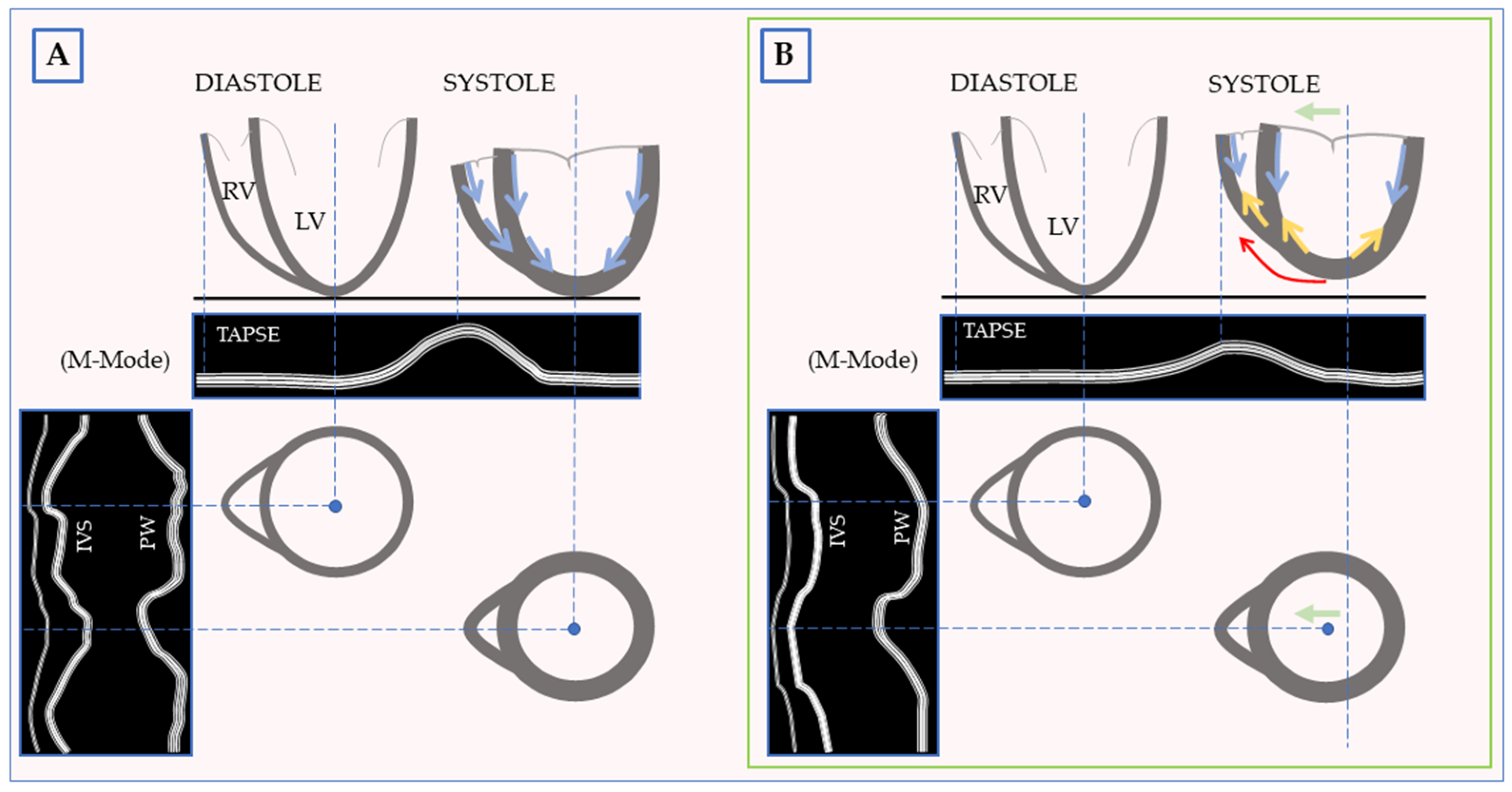
4. The Septal Injury Theory
5. The Timing
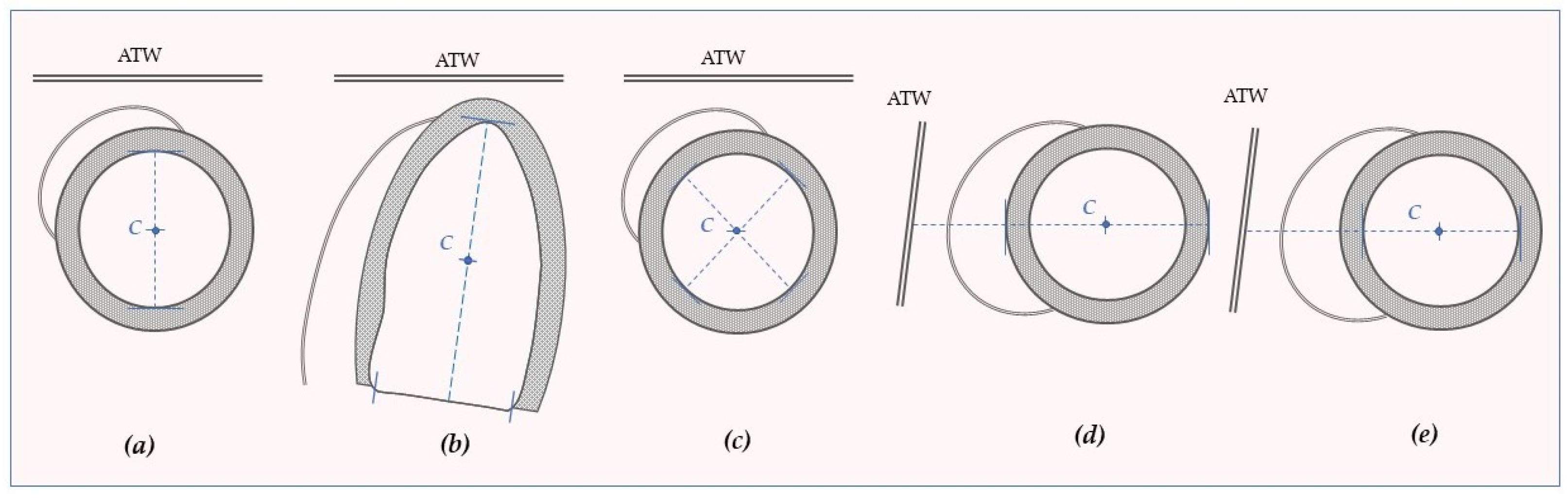
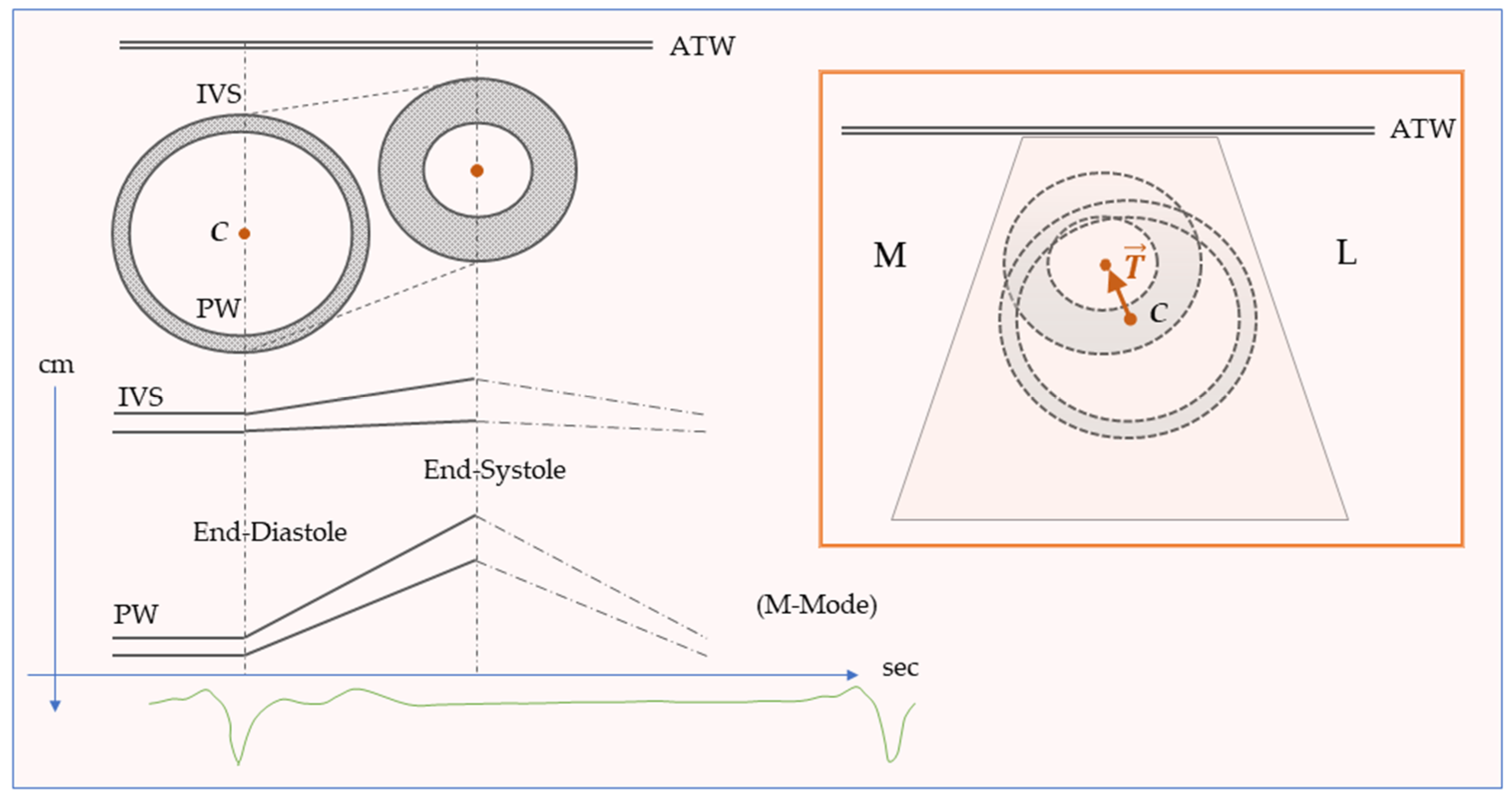
6. The Reference System
7. Curvature and Deformation
8. The Role of the Pericardium
9. The Right Ventricle: The Other Side of The Coin
10. A New Heuristic Hypothesis for POPS
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, H.C.; Gibson, D.G.; Stephens, J.D. Role of echocardiography and phonocardiography in diagnosis of mitral paraprosthetic regurgitation with Starr-Edwards prostheses. Br. Heart J. 1973, 35, 1217–1225. [Google Scholar] [CrossRef]
- Burggraf, G.W.; Craige, E. Echocardiographic studies of left ventricular wall motion and dimensions after valvular heart surgery. Am. J. Cardiol. 1975, 35, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Righetti, A.; Crawford, M.H.; O’Rourke, R.A.; Schelbert, H.; Daily, P.O.; Ross, J., Jr. Interventricular septal motion and left ventricular function after coronary bypass surgery: Evaluation with echocardiography and radionuclide angiography. Am. J. Cardiol. 1977, 39, 372–377. [Google Scholar] [CrossRef]
- Vignola, P.A.; Boucher, C.A.; Curfman, G.D.; Walker, H.J.; Shea, W.H.; Dinsmore, R.E.; Pohost, G.M. Abnormal interventricular septal motion following cardiac surgery: Clinical, surgical, echocardiographic and radionuclide correlates. Am. Heart J. 1979, 97, 27–34. [Google Scholar] [CrossRef]
- Matsumoto, M.; Oka, Y.; Strom, J.; Frishman, W.; Kadish, A.; Becker, R.M.; Frater, R.W.; Sonnenblick, E.H. Application of transesophageal echocardiography to continuous intraoperative monitoring of left ventricular performance. Am. J. Cardiol. 1980, 46, 95–105. [Google Scholar] [CrossRef]
- Guaricci, A.I.; Bulzis, G.; Pontone, G.; Scicchitano, P.; Carbonara, R.; Rabbat, M.; De Santis, D.; Ciccone, M.M. Current interpretation of myocardial stunning. Trends Cardiovasc. Med. 2018, 28, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Todiere, G.; Barison, A.; Baritussio, A.; Cipriani, A.; Guaricci, A.I.; Pica, S.; Indolfi, C.; Pontone, G.; Dellegrottaglie, S. Acute clinical presentation of nonischemic cardiomyopathies: Early detection by cardiovascular magnetic resonance. J. Cardiovasc. Med. 2023, 24, e36–e46. [Google Scholar] [CrossRef]
- Guaricci, A.I.; Basso, C.; Tarantini, G. Recurrent syncope on effort due to concealed constrictive pericarditis. Eur. Heart J. 2013, 34, 1817. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, H.R.; Tunick, P.A.; Grossi, E.A.; Dilmanian, H.; Colvin, S.B.; Kronzon, I. Paradoxical septal motion after cardiac surgery: A review of 3,292 cases. Clin. Cardiol. 2007, 30, 621–623. [Google Scholar] [CrossRef]
- Mori, S.; Nakatani, S.; Kanzaki, H.; Yamagata, K.; Take, Y.; Matsuura, Y.; Kyotani, S.; Nakanishi, N.; Kitakaze, M. Patterns of the interventricular septal motion can predict conditions of patients with pulmonary hypertension. J. Am. Soc. Echocardiogr. 2008, 21, 386–393. [Google Scholar] [CrossRef]
- Perone, F.; Bernardi, M.; Redheuil, A.; Mafrica, D.; Conte, E.; Spadafora, L.; Ecarnot, F.; Tokgozoglu, L.; Santos-Gallego, C.G.; Kaiser, S.E.; et al. Role of Cardiovascular Imaging in Risk Assessment: Recent Advances, Gaps in Evidence, and Future Directions. J. Clin. Med. 2023, 12, 5563. [Google Scholar] [CrossRef] [PubMed]
- Ghio, S.; Acquaro, M.; Agostoni, P.; Ambrosio, G.; Carluccio, E.; Castiglione, V.; Colombo, D.; D’Alto, M.; Delle Grottaglie, S.; Dini, F.L.; et al. Right heart failure in left heart disease: Imaging, functional, and biochemical aspects of right ventricular dysfunction. Heart Fail. Rev. 2023, 28, 1009–1022. [Google Scholar] [CrossRef] [PubMed]
- Gaibazzi, N.; Porter, T.; Lorenzoni, V.; Pontone, G.; De Santis, D.; De Rosa, A.; Guaricci, A.I. Effect of Coronary Revascularization on the Prognostic Value of Stress Myocardial Contrast Wall Motion and Perfusion Imaging. J. Am. Heart Assoc. 2017, 6, e006202. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Martini, C.; Gatti, M.; Dell’Aversana, S.; Ricci, F.; Guglielmo, M.; Baggiano, A.; Fusini, L.; Bracciani, A.; Scafuri, S.; et al. Feasibility of late gadolinium enhancement (LGE) in ischemic cardiomyopathy using 2D-multisegment LGE combined with artificial intelligence reconstruction deep learning noise reduction algorithm. Int. J. Cardiol. 2021, 343, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Merlo, M.; Gagno, G.; Baritussio, A.; Bauce, B.; Biagini, E.; Canepa, M.; Cipriani, A.; Castelletti, S.; Dellegrottaglie, S.; Guaricci, A.I.; et al. Clinical application of CMR in cardiomyopathies: Evolving concepts and techniques: A position paper of myocardial and pericardial diseases and cardiac magnetic resonance working groups of Italian society of cardiology. Heart Fail. Rev. 2023, 28, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Neglia, D.; Liga, R.; Gimelli, A.; Podlesnikar, T.; Cvijić, M.; Pontone, G.; Miglioranza, M.H.; Guaricci, A.I.; Seitun, S.; Clemente, A.; et al. Use of cardiac imaging in chronic coronary syndromes: The EURECA Imaging registry. Eur. Heart J. 2022, 44, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Connolly, H.M.; Click, R.L.; Schattenberg, T.T.; Seward, J.B.; Tajik, A.J. Congenital absence of the pericardium: Echocardiography as a diagnostic tool. J. Am. Soc. Echocardiogr. 1995, 8, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Walmsley, J.; Huntjens, P.R.; Prinzen, F.W.; Delhaas, T.; Lumens, J. Septal flash and septal rebound stretch have different underlying mechanisms. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H394–H403. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.L.; Abbara, S.; Agler, D.A.; Appleton, C.P.; Asher, C.R.; Hoit, B.; Hung, J.; Garcia, M.J.; Kronzon, I.; Oh, J.K.; et al. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease: Endorsed by the Society for Cardiovascular Magnetic Resonance and Society of Cardiovascular Computed Tomography. J. Am. Soc. Echocardiogr. 2013, 26, 965–1012.e1015. [Google Scholar] [CrossRef]
- Feneley, M.; Kearney, L.; Farnsworth, A.; Shanahan, M.; Chang, V. Mechanisms of the development and resolution of paradoxical interventricular septal motion after uncomplicated cardiac surgery. Am. Heart J. 1987, 114, 106–114. [Google Scholar] [CrossRef]
- Lehmann, K.G.; Lee, F.A.; McKenzie, W.B.; Barash, P.G.; Prokop, E.K.; Durkin, M.A.; Ezekowitz, M.D. Onset of altered interventricular septal motion during cardiac surgery. Assessment by continuous intraoperative transesophageal echocardiography. Circulation 1990, 82, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Giubbini, R.; Rossini, P.; Bertagna, F.; Bosio, G.; Paghera, B.; Pizzocaro, C.; Canclini, S.; Terzi, A.; Germano, G. Value of gated SPECT in the analysis of regional wall motion of the interventricular septum after coronary artery bypass grafting. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Rubenson, D.S.; Tucker, C.R.; London, E.; Miller, D.C.; Stinson, E.B.; Popp, R.L. Two-dimensional echocardiographic analysis of segmental left ventricular wall motion before and after coronary artery bypass surgery. Circulation 1982, 66, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.K.; Chang, H.J.; Cho, I.J.; Shin, S.; Shim, C.Y.; Hong, G.R.; Yu, K.J.; Chang, B.C.; Chung, N. Echocardiographic investigation of the mechanism underlying abnormal interventricular septal motion after open heart surgery. J. Cardiovasc. Ultrasound 2014, 22, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Wranne, B.; Pinto, F.J.; Siegel, L.C.; Miller, D.C.; Schnittger, I. Abnormal postoperative interventricular motion: New intraoperative transesophageal echocardiographic evidence supports a novel hypothesis. Am. Heart J. 1993, 126, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.B.; Salah, A.K.; Mendoza, D.D.; Goldstein, S.A.; Fuisz, A.R.; Lindsay, J. Mechanism of paradoxical ventricular septal motion after coronary artery bypass grafting. Am. J. Cardiol. 2009, 103, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Buckberg, G.D.; The RESTORE Group. The ventricular septum: The lion of right ventricular function, and its impact on right ventricular restoration. Eur. J. Cardiothorac. Surg. 2006, 29 (Suppl. S1), S272–S278. [Google Scholar] [CrossRef]
- Korshin, A.; Gronlykke, L.; Holmgaard, F.; Kjoller, S.M.; Gustafsson, F.; Nilsson, J.C.; Ravn, H.B. Right ventricular transverse displacement increases following cardiac surgery: Possibly compensating loss in tricuspid annular plane systolic excursion (TAPSE). J. Clin. Monit. Comput. 2020, 34, 1139–1148. [Google Scholar] [CrossRef]
- Ozdemir, S.; Yener, A.U.; Barutcu, A.; Tan, Y.Z.; Celik, F. The assessment of septal wall motion in patients undergoing CABG by myocardial perfusion-gated SPECT. Nucl. Med. Commun. 2015, 36, 738–746. [Google Scholar] [CrossRef]
- Gourdier, M.; Jouannot, P.; Hatt, P.Y. Echocardiographic anomaly of septal contraction after open heart surgery. Arch. Mal. Coeur Vaiss. 1982, 75, 73–83. [Google Scholar]
- Schroeder, E.; Marchandise, B.; Schoevaerdts, J.C.; Kremer, R. Paradoxical ventricular septal motion after cardiac surgery. Analysis of M-mode echocardiograms and follow-up in 324 patients. Acta Cardiol. 1985, 40, 315–324. [Google Scholar] [PubMed]
- Kerber, R.E.; Litchfield, R. Postoperative abnormalities of interventricular septal motion: Two-dimensional and M-mode echocardiographic correlations. Am. Heart J. 1982, 104, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Okada, R.D.M.J.; Boucher, C.A.; Pohost, G.M.; Strauss, H.W.; Johnson, G., 3rd; Daggett, W.M. Relationship between septal perfusion, viability, and motion before and after coronary artery bypass surgery. Am. Heart J. 1992, 124, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- van der Wall, E.E.; Kasim, M.; Camps, J.A.; van Rijk-Zwikker, G.; Voogd, P.J.; Pauwels, E.K.; Bruschke, A.V. Abnormal septal motion after aortic valve replacement for chronic aortic regurgitation: No evidence for myocardial ischaemia by exercise radionuclide angiography. Eur. J. Nucl. Med. 1990, 17, 252–256. [Google Scholar] [CrossRef] [PubMed]
- De Nardo, D.C.Q.; Mercanti, C.; Alessandri, N.; Scibilia, G.; Chiavarelli, R.; Antolini, M.; Pitucco, G.; Caputo, V.; Marino, B. Effects of Uncomplicated Coronary Artery Bypass Graft Surgery on Global and Regional Left Ventricular Function at Rest Study by Equilibrium Radionuclide Angiocardiography. Cardiology 1989, 76, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Roshanali, F.; Yousefnia, M.A.; Mandegar, M.H.; Rayatzadeh, H.; Alinejad, S. Decreased right ventricular function after coronary artery bypass grafting. Tex. Heart Inst. J. 2008, 35, 250–255. [Google Scholar] [PubMed]
- Moya Mur, J.L.; Garcia Martin, A.; Garcia Lledo, A.; Lazaro Rivera, C.; Rincon Diaz, L.M.; Miguelena Hycka, J.; Boretti, I.; Gimaraes, C.; Casas Rojo, E.; Jimenez Nacher, J.J.; et al. Geometrical and functional cardiac changes after cardiac surgery: A phisiopatological explanation based on speckle tracking. Int. J. Cardiovasc. Imaging 2018, 34, 1905–1915. [Google Scholar] [CrossRef] [PubMed]
- Waggoner, A.D.; Shah, A.A.; Schuessler, J.S.; Crawford, E.S.; Nelson, J.G.; Miller, R.R.; Quinones, M.A. Effect of cardiac surgery on ventricular septal motion: Assessment by intraoperative echocardiography and cross-sectional two-dimensional echocardiography. Am. Heart J. 1982, 104, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Force, T.; Bloomfield, P.; O’Boyle, J.E.; Pietro, D.A.; Dunlap, R.W.; Khuri, S.F.; Parisi, A.F. Quantitative two-dimensional echocardiographic analysis of motion and thickening of the interventricular septum after cardiac surgery. Circulation 1983, 68, 1013–1020. [Google Scholar] [CrossRef]
- Akins, C.W.; Boucher, C.A.; Pohost, G.M. Preservation of interventricular septal function in patients having coronary artery bypass grafts without cardiopulmonary bypass. Am. Heart J. 1984, 107, 304–309. [Google Scholar] [CrossRef]
- Schnittger, I.; Keren, A.; Yock, P.G.; Allen, M.D.; Modry, D.L.; Zusman, D.R.; Mitchell, R.S.; Miller, D.C.; Popp, R.L. Timing of abnormal interventricular septal motion after cardiopulmonary bypass operations. Lack of injury proved by preoperative, intraoperative, and postoperative echocardiography. J. Thorac. Cardiovasc. Surg. 1986, 91, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Gigli, G.; Maffei, S.; Picano, E.; Paterni, M.; Baroni, M.; Terrazzi, M.; Rovai, D.; Biagini, A. Cardiac cycle-dependent gray-level variation is not distorted by abnormal septal motion after cardiac surgery: A transesophageal videodensitometric study in humans. J. Am. Soc. Echocardiogr. 1995, 8, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Hedman, A.; Alam, M.; Zuber, E.; Nordlander, R.; Samad, B.A. Decreased right ventricular function after coronary artery bypass grafting and its relation to exercise capacity: A tricuspid annular motion-based study. J. Am. Soc. Echocardiogr. 2004, 17, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, T.; Akasaka, T.; Watanabe, N.; Akiyama, M.; Neishi, Y.; Kume, T.; Komuro, I.; Yoshida, K. Evaluation of abnormal motion of interventricular septum after coronary artery bypass grafting operation: Assessment by ultrasonic strain rate imaging. J. Am. Soc. Echocardiogr. 2004, 17, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Choi, S.I.; Chun, E.J.; Chang, H.J.; Park, K.H.; Lim, C.; Kim, S.J.; Kang, J.W.; Lim, T.H. Abnormal motion of the interventricular septum after coronary artery bypass graft surgery: Comprehensive evaluation with MR imaging. Korean J. Radiol. 2010, 11, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Codreanu, I.; Pegg, T.J.; Selvanayagam, J.B.; Robson, M.D.; Rider, O.J.; Dasanu, C.A.; Jung, B.A.; Taggart, D.P.; Clarke, K.; Holloway, C.J. Details of left ventricular remodeling and the mechanism of paradoxical ventricular septal motion after coronary artery bypass graft surgery. J. Invasive Cardiol. 2011, 23, 276–282. [Google Scholar] [PubMed]
- Michaux, I.; Filipovic, M.; Skarvan, K.; Bolliger, D.; Schumann, R.; Bernet, F.; Seeberger, M.D. A randomized comparison of right ventricular function after on-pump versus off-pump coronary artery bypass graft surgery. J. Thorac. Cardiovasc. Surg. 2011, 141, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Buckberg, G.; Hoffman, J.I. Right ventricular architecture responsible for mechanical performance: Unifying role of ventricular septum. J. Thorac. Cardiovasc. Surg. 2014, 148, 3166–3171.e4. [Google Scholar] [CrossRef]
- Azhari, H.; Beyar, R.; Sideman, S. On the human left ventricular shape. Comput. Biomed. Res. 1999, 32, 264–282. [Google Scholar] [CrossRef]
- Clancy, D.J.; McLean, A.; Slama, M.; Orde, S.R. Paradoxical septal motion: A diagnostic approach and clinical relevance. Australas. J. Ultrasound Med. 2018, 21, 79–86. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology/American College of Cardiology/American Heart Association/World Heart Federation Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.; Kale, S.C.; Chowdhury, U.K.; Ramakrishnan, L.; Chauhan, S.; Kiran, U. Myocardial injury in coronary artery bypass grafting: On-pump versus off-pump comparison by measuring heart-type fatty-acid-binding protein release. Tex. Heart Inst. J. 2006, 33, 321–327. [Google Scholar] [PubMed]
- Khan, N.E.; De Souza, A.; Mister, R.; Flather, M.; Clague, J.; Davies, S.; Collins, P.; Wang, D.; Sigwart, U.; Pepper, J. A randomized comparison of off-pump and on-pump multivessel coronary-artery bypass surgery. N. Engl. J. Med. 2004, 350, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, M. Abnormal septal motion after coronary bypass surgery. Am. J. Cardiol. 1978, 41, 619–620. [Google Scholar] [CrossRef] [PubMed]
- Gunday, M.; Alpaslan, M.; Ciftci, O.; Ozulku, M.; Copur, G.; Aslamaci, S. Is off-pump coronary artery bypass surgery superior to on-pump coronary artery bypass surgery on postoperative paradoxical ventricular septal motion? Heart Surg. Forum 2014, 17, E191–E195. [Google Scholar] [CrossRef] [PubMed]
- Diviggiano, E.E.; Rosi, S.; Landra, F.; Marallo, C.; Scoppa, C.; Castellani, D.; Mandoli, G.E.; Pastore, M.C.; Cavigli, L.; D’Ascenzi, F.; et al. Reverse Septal Movement: A Step Forward in the Comprehension of the Underlying Causes. J. Clin. Med. 2024, 13, 928. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Respir. J. 2023, 61, 2200879. [Google Scholar] [CrossRef] [PubMed]
- Donauer, M.; Schneider, J.; Jander, N.; Beyersdorf, F.; Keyl, C. Perioperative Changes of Right Ventricular Function in Cardiac Surgical Patients Assessed by Myocardial Deformation Analysis and 3-Dimensional Echocardiography. J. Cardiothorac. Vasc. Anesth. 2020, 34, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Guaricci, A.I.; Chiarello, G.; Gherbesi, E.; Fusini, L.; Soldato, N.; Siena, P.; Ursi, R.; Ruggieri, R.; Guglielmo, M.; Muscogiuri, G.; et al. Coronary-specific quantification of myocardial deformation by strain echocardiography may disclose the culprit vessel in patients with non-ST-segment elevation acute coronary syndrome. Eur. Heart J. Open 2022, 2, oeac010. [Google Scholar] [CrossRef]
- Pontone, G.; Andreini, D.; Baggiano, A.; Bertella, E.; Mushtaq, S.; Conte, E.; Beltrama, V.; Guaricci, A.I.; Pepi, M. Functional relevance of coronary artery disease by cardiac magnetic resonance and cardiac computed tomography: Myocardial perfusion and fractional flow reserve. BioMed Res. Int. 2015, 2015, 297696. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Chiesa, M.; Baggiano, A.; Spadafora, P.; De Santis, R.; Guglielmo, M.; Scafuri, S.; Fusini, L.; Mushtaq, S.; Conte, E.; et al. Diagnostic performance of deep learning algorithm for analysis of computed tomography myocardial perfusion. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3119–3128. [Google Scholar] [CrossRef] [PubMed]
- Al’Aref, S.J.; Altibi, A.M.; Malkawi, A.; Mansour, M.; Baskaran, L.; Masri, A.; Rahmouni, H.; Abete, R.; Andreini, D.; Aquaro, G.; et al. Cardiac magnetic resonance for prophylactic implantable-cardioverter defibrillator therapy international study: Prognostic value of cardiac magnetic resonance-derived right ventricular parameters substudy. Eur. Heart J. Cardiovasc. Imaging 2022, 24, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Barutcu, A.; Ozdemir, S.; Tan, Y.Z.; Yener, A.U.; Ozcan, S.; Gazi, E. Relationship between fragmented QRS and paradoxical septal motion in coronary artery bypass graft patients. Ann. Nucl. Med. 2015, 29, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Aljaroudi, W.; Alraies, M.C.; Brunken, R.; Cerquiera, M.; Jaber, W.A. Paradoxical septal motion from prior coronary artery bypass graft surgery does not impact left ventricular mechanical dyssynchrony by gated myocardial perfusion imaging. J. Nucl. Cardiol. 2012, 19, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- Payvandi, M.N.; Kerber, R.E. Echocardiography in congenital and acquired absence of the pericardium. An echocardiographic mimic of right ventricular volume overload. Circulation 1976, 53, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Eslami, B.; Roitman, D.; Karp, R.B.; Sheffield, L.T. The echocardiogram after pericardiectomy. Jpn. Heart J. 1979, 20, 1–5. [Google Scholar] [CrossRef]
- Lindqvist, P.; Holmgren, A.; Zhao, Y.; Henein, M.Y. Effect of pericardial repair after aortic valve replacement on septal and right ventricular function. Int. J. Cardiol. 2012, 155, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Starr, I.J.W.; Meade, R.H. The absence of conspicuous increments of venous pressure after severe damage to the right ventricle of the dog, with a discussion of the relation between clinical congestive failure and heart disease. Am. Heart J. 1943, 26, 291–301. [Google Scholar] [CrossRef]
- Sanz, J.; Sanchez-Quintana, D.; Bossone, E.; Bogaard, H.J.; Naeije, R. Anatomy, Function, and Dysfunction of the Right Ventricle: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 1463–1482. [Google Scholar] [CrossRef]
- Giusca, S.; Dambrauskaite, V.; Scheurwegs, C.; D’Hooge, J.; Claus, P.; Herbots, L.; Magro, M.; Rademakers, F.; Meyns, B.; Delcroix, M.; et al. Deformation imaging describes right ventricular function better than longitudinal displacement of the tricuspid ring. Heart 2010, 96, 281–288. [Google Scholar] [CrossRef]
- Rosner, A.; Avenarius, D.; Malm, S.; Iqbal, A.; Schirmer, H.; Bijnens, B.; Myrmel, T. Changes in Right Ventricular Shape and Deformation Following Coronary Artery Bypass Surgery-Insights from Echocardiography with Strain Rate and Magnetic Resonance Imaging. Echocardiography 2015, 32, 1809–1820. [Google Scholar] [CrossRef] [PubMed]
- Stanley, A.; Athanasuleas, C.; Nanda, N. Paradoxical Septal Motion after Uncomplicated Cardiac Surgery: A Consequence of Altered Regional Right Ventricular Contractile Patterns. Curr. Cardiol. Rev. 2022, 18, e060122200068. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, M.; Dangas, G.D.; Angiolillo, D.J.; Brodt, J.; Chikwe, J.; DeAnda, A.; Hameed, I.; Rodgers, M.L.; Sandner, S.; Sun, L.Y.; et al. Considerations on the Management of Acute Postoperative Ischemia After Cardiac Surgery: A Scientific Statement from the American Heart Association. Circulation 2023, 148, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Keyl, C.; Schneider, J.; Beyersdorf, F.; Ruile, P.; Siepe, M.; Pioch, K.; Schneider, R.; Jander, N. Right ventricular function after aortic valve replacement: A pilot study comparing surgical and transcatheter procedures using 3D echocardiography. Eur. J. Cardiothorac. Surg. 2016, 49, 966–971. [Google Scholar] [CrossRef]

| Characteristics of Abnormal Septal Motion | Common Causes of Abnormal Septal Motion | ||||||
|---|---|---|---|---|---|---|---|
| POPS | LBBB/RV Pacing Rhythm | Ischemia | Rv Pressure/ Volume Overload | Constrictive Pericarditis | Pericardial Tamponade | Obstructive Pulmonary Diseases/Mechanical Ventilation | |
| Systolic | + | + | + (*) | + (**) | − | − | − |
| Normal Iv Conduction | + | − | + | + | + | + | + |
| Preserved Ivs Perfusion | + | + | − | + | + | + | + |
| Normal Ivs Metabolism | + | + | − | + | + | + | + |
| Normal Lv Geometry | + | − | − | − | − | − | − |
| Normal Lv Global Systolic Function | + | + | +/− | +/− | +/− | +/− | +/− |
| Respirophasic Motion | − | − | − | − | + | + | + |
| Stress Related | − | +/− | +/− | − | − | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Virgilio, E.; Basile, P.; Carella, M.C.; Monitillo, F.; Santoro, D.; Latorre, M.D.; D’Alessandro, S.; Fusini, L.; Fazzari, F.; Pontone, G.; et al. The Postoperative Paradoxical Septum (POPS): A Comprehensive Review on Physio-Pathological Mechanisms. J. Clin. Med. 2024, 13, 2309. https://doi.org/10.3390/jcm13082309
Di Virgilio E, Basile P, Carella MC, Monitillo F, Santoro D, Latorre MD, D’Alessandro S, Fusini L, Fazzari F, Pontone G, et al. The Postoperative Paradoxical Septum (POPS): A Comprehensive Review on Physio-Pathological Mechanisms. Journal of Clinical Medicine. 2024; 13(8):2309. https://doi.org/10.3390/jcm13082309
Chicago/Turabian StyleDi Virgilio, Emanuele, Paolo Basile, Maria Cristina Carella, Francesco Monitillo, Daniela Santoro, Michele Davide Latorre, Silvia D’Alessandro, Laura Fusini, Fabio Fazzari, Gianluca Pontone, and et al. 2024. "The Postoperative Paradoxical Septum (POPS): A Comprehensive Review on Physio-Pathological Mechanisms" Journal of Clinical Medicine 13, no. 8: 2309. https://doi.org/10.3390/jcm13082309







