Prevalence and Impact of Concomitant Atrial Fibrillation in Patients Undergoing Percutaneous Coronary Intervention for Acute Myocardial Infarction
Abstract
:1. Introduction
2. Methods
2.1. Data Collection
2.2. Statistical Methods
3. Results
3.1. Cohort Characteristics
3.2. Patient Outcomes
3.3. Predictors of Major Adverse Cardiac and Cerebrovascular Events
3.4. Associations of AF with Coronary Artery Disease Distribution Pattern
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lip, G.Y.H.; Tse, H.-F. Management of Atrial Fibrillation. Lancet 2007, 370, 604–618. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, Y.; Barnes, M.E.; Gersh, B.J.; Cha, S.S.; Bailey, K.R.; Abhayaratna, W.P.; Seward, J.B.; Tsang, T.S.M. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006, 114, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, R.B.; Yin, X.; Gona, P.; Larson, M.G.; Beiser, A.S.; McManus, D.D.; Newton-Cheh, C.; A Lubitz, S.; Magnani, J.W.; Ellinor, P.T.; et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: A cohort study. Lancet 2015, 386, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Hylek, E.M.; Phillips, K.A.; Chang, Y.; Henault, L.E.; Selby, J.V.; Singer, D.E. Prevalence of Diagnosed Atrial Fibrillation in Adults. JAMA 2001, 285, 2370–2375. [Google Scholar] [CrossRef]
- Michniewicz, E.; Mlodawska, E.; Lopatowska, P.; Tomaszuk-Kazberuk, A.; Malyszko, J. Patients with atrial fibrillation and coronary artery disease—Double trouble. Adv. Med. Sci. 2018, 63, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Violi, F.; Soliman, E.Z.; Pignatelli, P.; Pastori, D. Atrial fibrillation and myocardial infarction: A systematic review and appraisal of pathophysiologic mechanisms. J. Am. Heart Assoc. 2016, 5, e003347. [Google Scholar] [CrossRef]
- Schmitt, J.; Duray, G.; Gersh, B.J.; Hohnloser, S.H. Atrial fibrillation in acute myocardial infarction: A systematic review of the incidence, clinical features and prognostic implications. Eur. Heart J. 2008, 30, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Soliman, E.Z.; Safford, M.M.; Muntner, P.; Khodneva, Y.; Dawood, F.Z.; Zakai, N.A.; Thacker, E.L.; Judd, S.; Howard, V.J.; Howard, G.; et al. Atrial Fibrillation and the Risk of Myocardial Infarction. JAMA Intern. Med. 2014, 174, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Bayturan, O.; Puri, R.; Tuzcu, E.M.; Shao, M.; Wolski, K.; Schoenhagen, P.; Kapadia, S.; E Nissen, S.; Sanders, P.; Nicholls, S.J. Atrial fibrillation, progression of coronary atherosclerosis and myocardial infarction. Eur. J. Prev. Cardiol. 2016, 24, 373–381. [Google Scholar] [CrossRef]
- Lee, H.Y.; Yang, P.-S.; Kim, T.-H.; Uhm, J.-S.; Pak, H.-N.; Lee, M.-H.; Joung, B. Atrial fibrillation and the risk of myocardial infarction: A nation-wide propensity-matched study. Sci. Rep. 2017, 7, 12716. [Google Scholar] [CrossRef]
- Wong, C.-K.; White, H.D.; Wilcox, R.G.; Criger, D.A.; Califf, R.M.; Topol, E.J.; Ohman, E. New atrial fibrillation after acute myocardial infarction independently predicts death: The GUSTO-III experience. Am. Heart J. 2000, 140, 878–885. [Google Scholar] [CrossRef]
- Jabre, P.; Roger, V.L.; Murad, M.H.; Chamberlain, A.M.; Prokop, L.; Adnet, F.; Jouven, X. Mortality associated with atrial fibrillation in patients with myocardial infarction. Circulation 2011, 123, 1587–1593. [Google Scholar] [CrossRef] [PubMed]
- Konttila, K.K.; Punkka, O.; Koivula, K.; Eskola, M.J.; Martiskainen, M.; Huhtala, H.; Virtanen, V.K.; Mikkelsson, J.; Järvelä, K.; Laurikka, J.; et al. The Effect of Atrial Fibrillation on the Long-Term Mortality of Patients with Acute Coronary Syndrome: The TACOS Study. Cardiology 2021, 146, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Madsen, J.M.; Jacobsen, M.R.; Sabbah, M.; Topal, D.G.; Jabbari, R.; Glinge, C.; Køber, L.; Torp-Pedersen, C.; Pedersen, F.; Sørensen, R.; et al. Long-term prognostic outcomes and implication of oral anticoagulants in patients with new-onset atrial fibrillation following st-segment elevation myocardial infarction. Am. Heart J. 2021, 238, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Køber, L.; Swedberg, K.; McMurray, J.J.; Pfeffer, M.A.; Velazquez, E.J.; Diaz, R.; Maggioni, A.P.; Mareev, V.; Opolski, G.; Van de Werf, F.; et al. Previously known and newly diagnosed atrial fibrillation: A major risk indicator after a myocardial infarction complicated by heart failure or left ventricular dysfunction. Eur. J. Heart Fail. 2006, 8, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Crenshaw, B.S.; Ward, S.R.; Granger, C.B.; Stebbins, A.L.; Topol, E.J.; Califf, R.M. Atrial fibrillation in the setting of acute myocardial infarction. J. Am. Coll. Cardiol. 1997, 30, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Eldar, M.; Canetti, M.; Rotstein, Z.; Boyko, V.; Gottlieb, S.; Kaplinsky, E.; Behar, S. Significance of Paroxysmal Atrial Fibrillation Complicating Acute Myocardial Infarction in the Thrombolytic Era. Circulation 1998, 97, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomstrom-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. Corrigendum to: 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 4194. [Google Scholar]
- Kinjo, K.; Sato, H.; Sato, H.; Ohnishi, Y.; Hishida, E.; Nakatani, D.; Mizuno, H.; Fukunami, M.; Koretsune, Y.; Takeda, H.; et al. Prognostic significance of atrial fibrillation/atrial flutter in patients with acute myocardial infarction treated with percutaneous coronary intervention. Am. J. Cardiol. 2003, 92, 1150–1154. [Google Scholar] [CrossRef] [PubMed]
- Motloch, L.J.; Reda, S.; Larbig, R.; Wolff, A.; Motloch, K.A.; Wernly, B.; Granitz, C.; Lichtenauer, M.; Wolny, M.; Hoppe, U.C. Characteristics of coronary artery disease among patients with atrial fibrillation compared to patients with sinus rhythm. Hell. J. Cardiol. 2017, 58, 204–212. [Google Scholar] [CrossRef]
- Alasady, M.; Abhayaratna, W.P.; Leong, D.P.; Lim, H.S.; Abed, H.S.; Brooks, A.G.; Mattchoss, S.; Roberts-Thomson, K.C.; Worthley, M.I.; Chew, D.P.; et al. Coronary artery disease affecting the atrial branches is an independent determinant of atrial fibrillation after myocardial infarction. Heart Rhythm 2011, 8, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Obayashi, Y.; Shiomi, H.; Morimoto, T.; Tamaki, Y.; Inoko, M.; Yamamoto, K.; Takeji, Y.; Tada, T.; Nagao, K.; Yamaji, K.; et al. Newly Diagnosed Atrial Fibrillation in Acute Myocardial Infarction. J. Am. Heart Assoc. 2021, 10, e021417. [Google Scholar] [CrossRef] [PubMed]
| Concomitant Atrial Fibrillation | ||||
|---|---|---|---|---|
| N | No | Yes | p-Value | |
| Patient Demographics | ||||
| Age (Years) | 1000 | 65 ± 13 | 73 ± 11 | <0.001 |
| Gender (% Male) | 1000 | 671 (71.8%) | 49 (75.4%) | 0.571 |
| Smoking Status | 963 | 0.155 | ||
| Non- | 332 (36.7%) | 24 (40.7%) | ||
| Ex- | 300 (33.2%) | 24 (40.7%) | ||
| Current | 272 (30.1%) | 11 (18.6%) | ||
| Creatinine Clearance (ml/min) | 999 | 85 ± 38 | 67 ± 32 | <0.001 |
| Diabetes Mellitus | 999 | 0.028 | ||
| No | 642 (68.7%) | 36 (55.4%) | ||
| Diet-Controlled | 44 (4.7%) | 1 (1.5%) | ||
| Tablet-Controlled | 155 (16.6%) | 19 (29.2%) | ||
| Insulin-Dependent | 93 (10.0%) | 9 (13.8%) | ||
| Hypertension | 993 | 528 (56.9%) | 48 (73.8%) | 0.009 |
| Hypercholesterolemia | 917 | 412 (48.0%) | 29 (49.2%) | 0.893 |
| Previous CVA | 999 | 41 (4.4%) | 8 (12.3%) | 0.011 |
| Previous Myocardial Infarction | 996 | 198 (21.3%) | 25 (38.5%) | 0.003 |
| Previous CABG | 1000 | 77 (8.2%) | 8 (12.3%) | 0.250 |
| Previous PCI | 1000 | 164 (17.5%) | 19 (29.2%) | 0.029 |
| Family History of CAD | 966 | 377 (41.8%) | 22 (34.4%) | 0.293 |
| Left Ventricular Ejection Fraction | 928 | 0.008 * | ||
| Normal | 645 (74.2%) | 35 (59.3%) | ||
| Mild Impairment | 125 (14.4%) | 10 (16.9%) | ||
| Moderate Impairment | 51 (5.9%) | 9 (15.3%) | ||
| Severe Impairment | 48 (5.5%) | 5 (8.5%) | ||
| Presentation | ||||
| Symptom Onset to PCI (Hours) | 1000 | 24 (5–81) | 36 (5–107) | 0.179 |
| Out-of-Hospital Cardiac Arrest | 1000 | 37 (4.0%) | 4 (6.2%) | 0.334 |
| NSTEMI | 1000 | 528 (56.5%) | 41 (63.1%) | 0.365 |
| Index Troponin (ng/L) | 941 | 91 (28–352) | 94 (37–719) | 0.275 |
| Peak Troponin (ng/L) | 987 | 512 (77–2512) | 800 (169–3061) | 0.155 |
| Mitral Regurgitation Severity | 1000 | <0.001 * | ||
| None | 683 (73.0%) | 29 (44.6%) | ||
| Mild | 200 (21.4%) | 22 (33.8%) | ||
| Moderate | 47 (5.0%) | 10 (15.4%) | ||
| Severe | 5 (0.5%) | 4 (6.2%) | ||
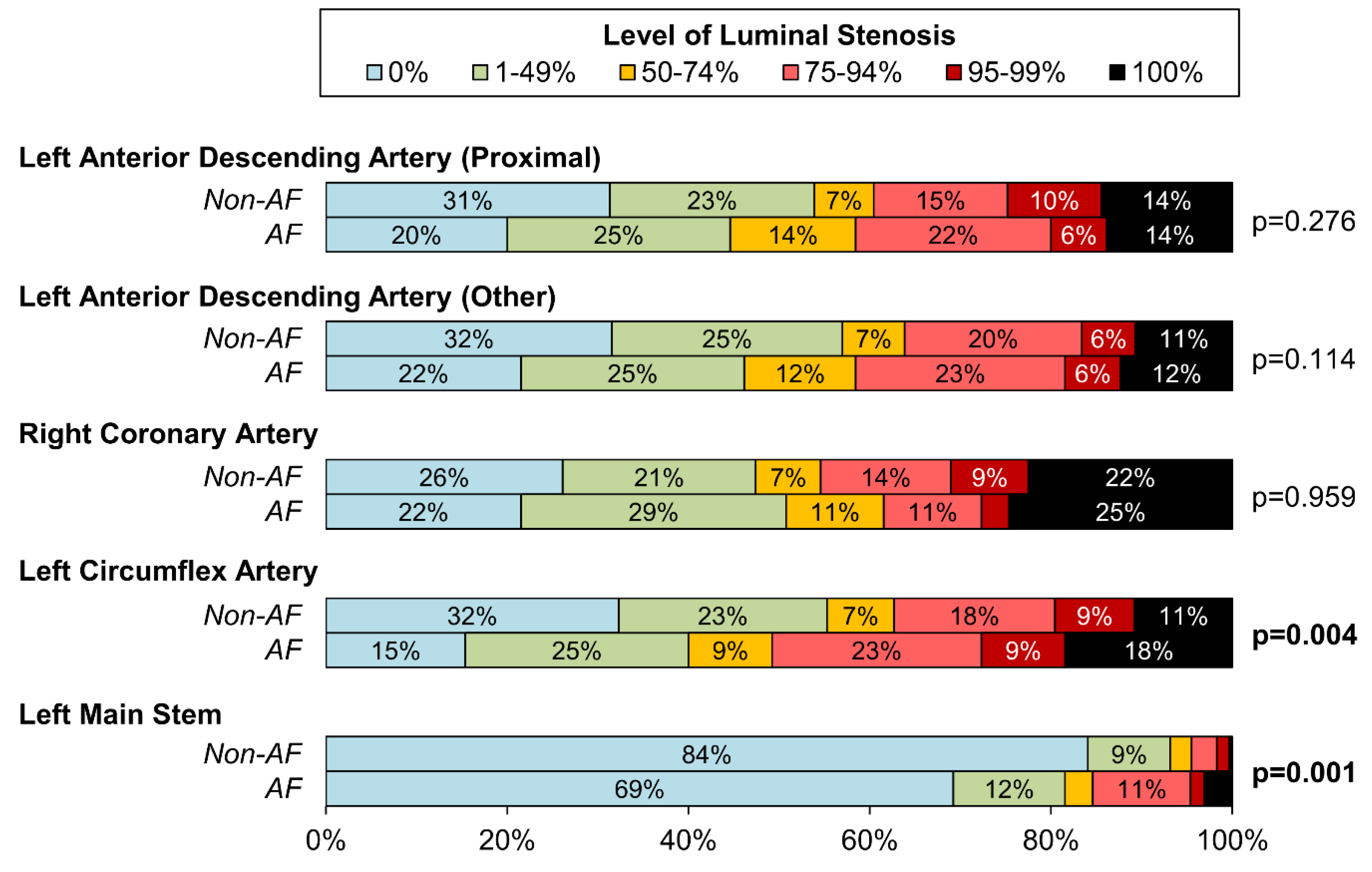
| Pattern of CAD ** | 1000 | 0.322 * | ||
| No Severe Disease | 8 (0.9%) | 2 (3.1%) | ||
| Single Vessel Disease | 590 (63.1%) | 35 (53.8%) | ||
| Double Vessel Disease | 242 (25.9%) | 19 (29.2%) | ||
| Triple Vessel Disease | 95 (10.2%) | 9 (13.8%) | ||
| Left Main Stem Involvement *** | 1000 | 64 (6.8%) | 12 (18.5%) | 0.002 |
| Concomitant Atrial Fibrillation | ||||
|---|---|---|---|---|
| N | No | Yes | p-Value | |
| Angioplasty Details | ||||
| DTB for Primary PCI (Minutes) * | 431 | 40 (28–75) | 54 (34–79) | 0.219 |
| CABG | 1000 | 31 (3.3%) | 6 (9.2%) | 0.028 |
| Complication | 1000 | 5 (0.5%) | 1 (1.5%) | 0.333 |
| Failure | 1000 | 20 (2.1%) | 0 (0.0%) | 0.635 |
| Rotablation | 1000 | 26 (2.8%) | 4 (6.2%) | 0.125 |
| Circulatory Support | 1000 | 3 (0.3%) | 0 (0.0%) | 1.000 |
| Discharge Medication ** | ||||
| Aspirin | 948 | 870 (98.3%) | 56 (88.9%) | <0.001 |
| Clopidogrel | 948 | 173 (19.5%) | 32 (50.8%) | <0.001 |
| Ticagrelor | 948 | 649 (73.3%) | 25 (39.7%) | <0.001 |
| Prasugrel | 948 | 48 (5.4%) | 2 (3.2%) | 0.767 |
| NOAC | 948 | 23 (2.6%) | 21 (33.3%) | <0.001 |
| Warfarin | 948 | 14 (1.6%) | 11 (17.5%) | <0.001 |
| Beta Blocker | 948 | 786 (88.8%) | 53 (84.1%) | 0.303 |
| ACE Inhibitor | 948 | 691 (78.1%) | 38 (60.3%) | 0.003 |
| Angiotensin Receptor Blocker | 948 | 93 (10.5%) | 11 (17.5%) | 0.095 |
| Aldosterone Receptor Antagonist | 948 | 75 (8.5%) | 15 (23.8%) | <0.001 |
| Diuretic | 948 | 134 (15.1%) | 30 (47.6%) | <0.001 |
| Number of Patients with Events | Kaplan–Meier Estimated Event Rate at Five Years | Hazard Ratio(95% CI) | ||||
|---|---|---|---|---|---|---|
| Outcome | Non-AF | AF | Non-AF | AF | p-Value | |
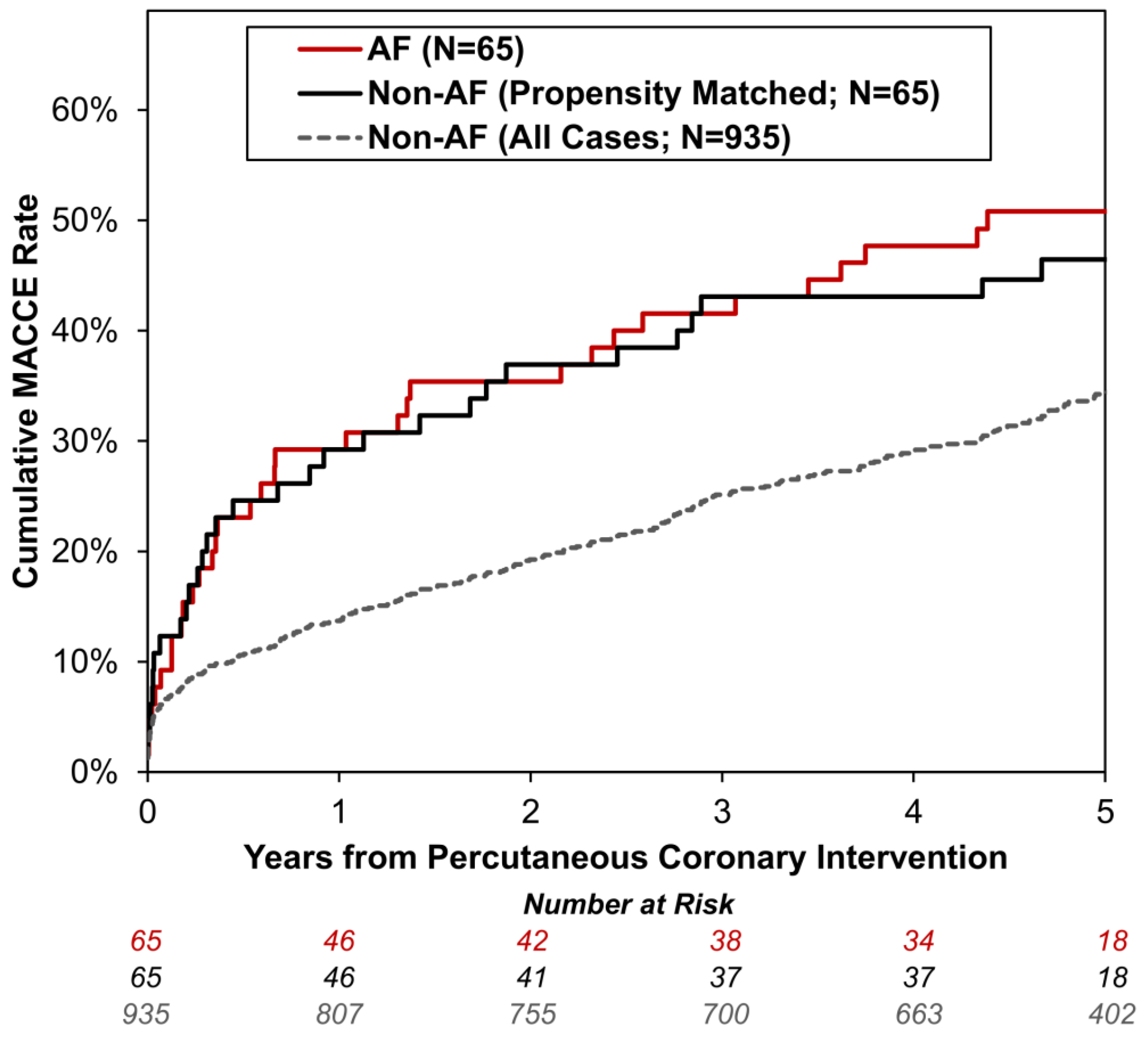
| Any MACCE | 329 | 35 | 34.2% | 50.8% | 1.86 (1.32–2.64) | <0.001 |
| Death | 228 | 30 | 23.3% | 43.7% | 2.25 (1.54–3.29) | <0.001 |
| MACCE Readmission * | 152 | 16 | 18.0% | 31.0% | 1.86 (1.11–3.11) | 0.018 |
| Myocardial Infarction Readmission ** | 93 | 10 | 10.8% | 17.5% | 1.86 (0.97–3.57) | 0.063 |
| Cerebrovascular Accident Readmission ** | 27 | 4 | 3.2% | 7.8% | 2.59 (0.91–7.41) | 0.076 |
| Major Gastrointestinal Bleed Readmission ** | 57 | 6 | 6.9% | 11.8% | 1.83 (0.79–4.24) | 0.160 |
| Univariable Models | Multivariable Model | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
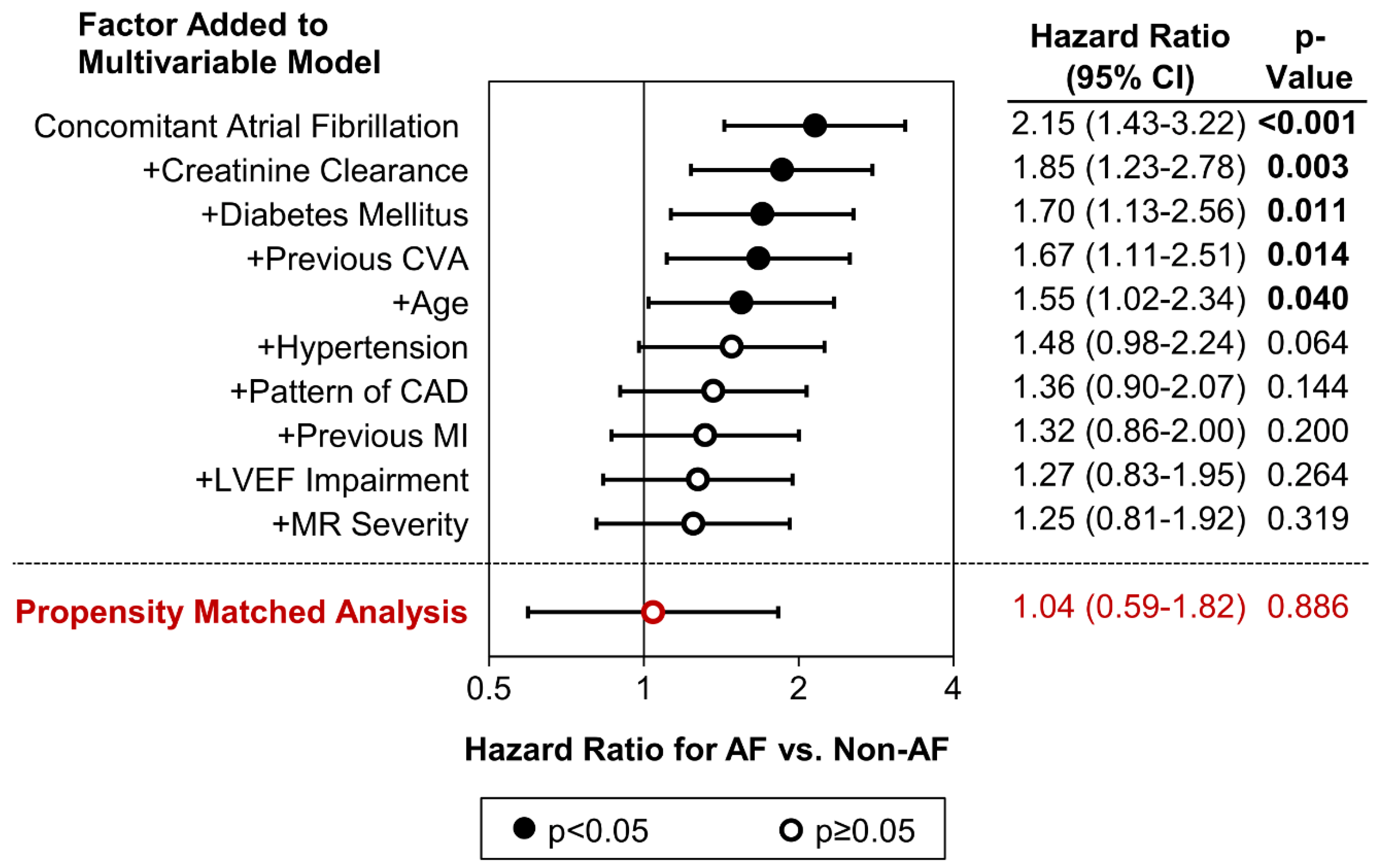
| Concomitant Atrial Fibrillation | 1.86 (1.32–2.64) | <0.001 | 1.25 (0.81–1.92) | 0.319 |
| Age (Years) * | <0.001 | <0.001 | ||
| <50 | - | - | - | - |
| 50–59 | 1.24 (0.78–1.95) | 0.361 | 0.88 (0.51–1.52) | 0.646 |
| 60–69 | 1.48 (0.95–2.29) | 0.081 | 0.83 (0.47–1.44) | 0.497 |
| 70–79 | 2.19 (1.43–3.35) | <0.001 | 0.96 (0.53–1.72) | 0.886 |
| 80+ | 4.36 (2.85–6.67) | <0.001 | 1.91 (1.04–3.49) | 0.037 |
| Gender (Female) | 1.27 (1.02–1.58) | 0.033 | - | NS |
| Smoking Status | 0.002 | NS | ||
| Non- | - | - | - | - |
| Ex- | 1.22 (0.96–1.55) | 0.111 | - | - |
| Current | 0.74 (0.56–0.97) | 0.031 | - | - |
| Creatinine Clearance (per 10 mL/min) | 0.86 (0.84–0.89) | <0.001 | 0.94 (0.90–0.99) | 0.011 |
| Diabetes Mellitus | <0.001 | <0.001 | ||
| No | - | - | - | - |
| Diet-Controlled | 1.23 (0.75–2.01) | 0.421 | 1.64 (0.95–2.84) | 0.076 |
| Tablet-Controlled | 1.33 (1.01–1.76) | 0.040 | 1.12 (0.79–1.57) | 0.532 |
| Insulin-Dependent | 3.11 (2.37–4.09) | <0.001 | 2.42 (1.69–3.45) | <0.001 |
| Hypertension | 2.10 (1.67–2.64) | <0.001 | 1.72 (1.27–2.33) | <0.001 |
| Hypercholesterolemia | 1.00 (0.80–1.24) | 0.979 | - | NS |
| Previous CVA | 2.70 (1.89–3.84) | <0.001 | 2.46 (1.61–3.76) | <0.001 |
| Previous Myocardial Infarction | 2.06 (1.65–2.56) | <0.001 | 1.50 (1.13–1.98) | 0.004 |
| Previous CABG | 1.86 (1.37–2.52) | <0.001 | - | NS |
| Previous PCI | 1.48 (1.16–1.89) | 0.001 | - | NS |
| Family History of CAD | 0.84 (0.68–1.05) | 0.120 | - | NS |
| Left Ventricular Ejection Fraction | <0.001 | 0.009 | ||
| Normal | - | - | - | - |
| Mild Impairment | 1.48 (1.10–2.00) | 0.011 | 1.42 (1.00–2.01) | 0.053 |
| Moderate Impairment | 2.38 (1.65–3.44) | <0.001 | 1.70 (1.09–2.63) | 0.019 |
| Severe Impairment | 2.98 (2.08–4.29) | <0.001 | 1.80 (1.13–2.86) | 0.013 |
| Symptom Onset to PPCI (per Day) | 1.01 (1.00–1.03) | 0.072 | - | NS |
| Out-of-Hospital Cardiac Arrest | 1.42 (0.90–2.26) | 0.135 | - | NS |
| NSTEMI | 1.16 (0.94–1.43) | 0.166 | - | NS |
| Index Troponin (per 1000 ng/L) | 1.03 (0.95–1.12) | 0.479 | - | NS |
| Peak Troponin (per 1000 ng/L) | 1.00 (0.96–1.04) | 0.920 | - | NS |
| Mitral Regurgitation Severity | <0.001 | 0.042 | ||
| None | - | - | - | - |
| Mild | 1.23 (0.96–1.57) | 0.100 | 0.78 (0.57–1.06) | 0.112 |
| Moderate/Severe ** | 2.14 (1.53–3.00) | <0.001 | 1.35 (0.88–2.09) | 0.174 |
| Pattern of CAD | <0.001 | 0.007 | ||
| None/Single Vessel ** | - | - | - | - |
| Double Vessel | 1.21 (0.95–1.54) | 0.122 | 0.85 (0.63–1.15) | 0.285 |
| Triple Vessel | 2.52 (1.90–3.35) | <0.001 | 1.60 (1.12–2.28) | 0.010 |
| Left Main Stem Involvement | 1.70 (1.22–2.38) | 0.002 | - | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakeel, I.; Sharma, H.; Hodson, J.; Iqbal, H.; Tashfeen, R.; Ludman, P.F.; Steeds, R.P.; Townend, J.N.; Doshi, S.N.; Nadir, M.A. Prevalence and Impact of Concomitant Atrial Fibrillation in Patients Undergoing Percutaneous Coronary Intervention for Acute Myocardial Infarction. J. Clin. Med. 2024, 13, 2318. https://doi.org/10.3390/jcm13082318
Shakeel I, Sharma H, Hodson J, Iqbal H, Tashfeen R, Ludman PF, Steeds RP, Townend JN, Doshi SN, Nadir MA. Prevalence and Impact of Concomitant Atrial Fibrillation in Patients Undergoing Percutaneous Coronary Intervention for Acute Myocardial Infarction. Journal of Clinical Medicine. 2024; 13(8):2318. https://doi.org/10.3390/jcm13082318
Chicago/Turabian StyleShakeel, Iqra, Harish Sharma, James Hodson, Hamna Iqbal, Rashna Tashfeen, Peter F. Ludman, Richard P. Steeds, Jonathan N. Townend, Sagar N. Doshi, and M. Adnan Nadir. 2024. "Prevalence and Impact of Concomitant Atrial Fibrillation in Patients Undergoing Percutaneous Coronary Intervention for Acute Myocardial Infarction" Journal of Clinical Medicine 13, no. 8: 2318. https://doi.org/10.3390/jcm13082318






