Concurrent Resistance and Cardiorespiratory Training in Patients with Hypertrophic Cardiomyopathy: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Design
2.3. Testing Procedures
2.4. Training Protocol
3. Results
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Week | Session 1 | Session 2 |
|---|---|---|
| Week 1 | 25 min at VT1 + 5/10 bpm | 7 × (2 min pace/1 min rec) |
| Week 2 | 25 min at VT1 + 5/10 bpm | 6 × (3 min pace/1 min rec) |
| Week 3 | 30 min at VT1 + 5/10 bpm | 5 × (4 min pace/2 min rec) |
| Week 4 | 30 min at VT1 + 5/10 bpm | 4 × (5 min pace/2 min rec) |
| Week 5 | 35 min at VT1 + 5/10 bpm | 7 × (3 min pace/1 min rec) |
| Week 6 | 35 min at VT1 + 5/10 bpm | 5 × (4.5 min pace/1.5 min rec) |
| Week 7 | 40 min at VT1 + 5/10 bpm | 4 × (5 min pace/2 min rec) |
| Week 8 | 40 min at VT1 + 5/10 bpm | 6 × (4 min pace/1.5 min rec) |
| Week 9 | 45 min at VT1 + 5/10 bpm | 4 × (6 min pace/2 min rec) |
| Week 10 | 45 min at VT1 + 5/10 bpm | 5 × (5 min pace/2 min rec) |
| Week 11 | 50 min at VT1 + 5/10 bpm | 5 × (5 min pace/2 min rec) |
| Week 12 | 50 min at VT1 + 5/10 bpm | 5 × (4 min pace/1.5 min rec) |
References
- Gersh, B.J.; Maron, B.J.; Bonow, R.O.; Dearani, J.A.; Fifer, M.A.; Link, M.S.; Naidu, S.S.; Nishimura, R.A.; Ommen, S.R.; Rakowski, H.; et al. ACCF/AHA Guideline 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: Executive Summary: A Report of the American College of Cardiology Foundation / American Heart Association Task Force on Practice Guidelines. Circulation 2011, 124, 2761–2796. [Google Scholar] [CrossRef] [PubMed]
- Wigle, E.; Sasson, Z.; Henderson, M.; Ruddy, T.; Fulop, J.; Rakowski, H.; Williams, W. Hypertrophic Cardiomyopathy. The Importance of the Site and the Extent of Hypertrophy. A Review. Prog. Cardiovasc. Dis. 1985, 28, 1–83. [Google Scholar] [CrossRef] [PubMed]
- Hindieh, W.; Adler, A.; Weissler-snir, A.; Fourey, D.; Harris, S.; Rakowski, H. Exercise in Patients with Hypertrophic Cardiomyopathy: A Review of Current Evidence, National Guideline Recommendations and a Proposal for a New Direction to Fitness. J. Sci. Med. Sport 2017, 20, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Cavigli, L.; Olivotto, I.; Fattirolli, F.; Mochi, N.; Favilli, S.; Mondillo, S.; Bonifazi, M.; D’Ascenzi, F. Prescribing, Dosing and Titrating Exercise in Patients with Hypertrophic Cardiomyopathy for Prevention of Comorbidities: Ready for Prime Time. Eur. J. Prev. Cardiol. 2020, 28, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Snir, A.W.; Connelly, K.A.; Goodman, J.M.; Dorian, D.; Dorian, P. Exercise in Hypertrophic Cardiomyopathy: Restrict or Rethink. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, 2101–2111. [Google Scholar] [CrossRef] [PubMed]
- Bayonas-Ruiz, A.; Muñoz-Franco, F.M.; Sabater-Molina, M.; Oliva-Sandoval, M.J.; Gimeno, J.R.; Bonacasa, B. Current Therapies for Hypertrophic Cardiomyopathy: A Systematic Review and Meta-Analysis of the Literature. ESC Heart Fail. 2022, 10, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Wasserstrum, Y.; Barbarova, I.; Lotan, D.; Kuperstein, R.; Shechter, M.; Freimark, D.; Segal, G.; Klempfner, R.; Arad, M. Efficacy and Safety of Exercise Rehabilitation in Patients with Hypertrophic Cardiomyopathy. J. Cardiol. 2019, 74, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Saberi, S.; Agarwal, P.P.; Attili, A.; Concannon, M.; Dries, A.M.; Shmargad, Y.; Salisbury, H.; Kumar, S.; Herrera, J.; Myers, J.; et al. Effect of Moderate-Intensity Exercise Training on Peak Oxygen Consumption in Patients with Hypertrophic Cardiomyopathy A Randomized Clinical Trial. J. Am. Med. Assoc. 2017, 317, 1349–1357. [Google Scholar] [CrossRef] [PubMed]
- Klempfner, R.; Kamerman, T.; Schwammenthal, E.; Nahshon, A.; Hay, I.; Goldenberg, I.; Dov, F.; Arad, M. Efficacy of Exercise Training in Symptomatic Patients with Hypertrophic Cardiomyopathy: Results of a Structured Exercise Training Program in a Cardiac Rehabilitation Center. Eur. J. Prev. Cardiol. 2015, 22, 13–19. [Google Scholar] [CrossRef]
- MacNamara, J.P.; Dias, K.A.; Hearon, C.M.; Ivey, E.; Delgado, V.A.; Saland, S.; Samels, M.; Hieda, M.; Turer, A.T.; Link, M.S.; et al. Randomized Controlled Trial of Moderate-and High-Intensity Exercise Training in Patients with Hypertrophic Cardiomyopathy: Effects on Fitness and Cardiovascular Response to Exercise. J. Am. Heart Assoc. 2023, 12, e031399. [Google Scholar] [CrossRef]
- Limongelli, G.; Monda, E.; D’Aponte, A.; Caiazza, M.; Rubino, M.; Esposito, A.; Palmiero, G.; Moscarella, E.; Messina, G.; Calabro, P.; et al. Combined Effect of Mediterranean Diet and Aerobic Exercise on Weight Loss and Clinical Status in Obese Symptomatic Patients with Hypertrophic Cardiomyopathy. Heart Fail. Clin. 2021, 17, 303–313. [Google Scholar] [CrossRef]
- Coffey, V.G.; Hawley, J.A. Concurrent Exercise Training: Do Opposites Distract? J. Physiol. 2017, 595, 2883–2896. [Google Scholar] [CrossRef]
- Mahony, C.O.; Jichi, F.; Pavlou, M.; Monserrat, L.; Anastasakis, A.; Rapezzi, C.; Biagini, E.; Gimeno, J.R.; Limongelli, G.; Mckenna, W.J.; et al. A Novel Clinical Risk Prediction Model for Sudden Cardiac Death in Hypertrophic Cardiomyopathy. Eur. Heart J. 2014, 35, 2010–2020. [Google Scholar] [CrossRef]
- Bruce, R.A.; Blackmon, J.R.; Jones, J.W.; Strait, G. Exercising Testing in Adult Normal Subjects and Cardiac Patients. Pediatrics 1963, 32, 742–756. [Google Scholar] [CrossRef]
- Borg, G. Psychophysical Bases of Perceived Exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Epley, B. Poundage Chart: Boyd Epley Workout; Enterprises, B., Ed.; Body Enterprises: Lincoln, NE, USA, 1985. [Google Scholar]
- Wood, T.M.; Maddalozzo, G.F.; Harter, R.A. Accuracy of Seven Equations for Predicting 1-RM Performance of Apparently Healthy, Sedentary Older Adults. Meas. Phys. Educ. Exerc. Sci. 2002, 6, 67–94. [Google Scholar] [CrossRef]
- Nájera-Ferrer, P.; Pérez-Caballero, C.; González-Badillo, J.J.; Pareja-Blanco, F. Effects of Exercise Sequence and Velocity Loss Threshold During Resistance Training on Following Endurance and Strength Performance During Concurrent Training. Int. J. Sports Physiol. Perform. 2021, 16, 811–817. [Google Scholar] [CrossRef]
- García-Pallarés, J.; Izquierdo, M. Strategies to Optimize Concurrent Training of Strength and Aerobic Fitness for Rowing and Canoeing. Sport. Med. 2011, 41, 329–343. [Google Scholar] [CrossRef]
- Grgic, J.; Schoenfeld, B.J.; Skrepnik, M.; Davies, T.B.; Mikulic, P. Effects of Rest Interval Duration in Resistance Training on Measures of Muscular Strength: A Systematic Review. Sport. Med. 2017, 48, 137–151. [Google Scholar] [CrossRef]
- Nuzzo, J.L.; Pinto, M.D.; Nosaka, K.; Steele, J. Maximal Number of Repetitions at Percentages of the One Repetition Maximum: A Meta-Regression and Moderator Analysis of Sex, Age, Training Status, and Exercise. Sport. Med. 2023, 54, 303–321. [Google Scholar] [CrossRef]
- Rodríguez-Rosell, D.; Yáñez-García, J.M.; Torres-Torrelo, J.; Mora-Custodio, R.; Marques, M.C.; González-Badillo, J.J. Effort Index as a Novel Variable for Monitoring the Level of Effort During Resistance Exercises. J. Strength Cond. Res. 2018, 32, 2139–2153. [Google Scholar] [CrossRef]
- Sánchez-Moreno, M.; Cornejo-Daza, P.J.; González-Badillo, J.J.; Pareja-Blanco, F. Effects of Velocity Loss During Body Mass Prone-Grip Pull-up Training on Strength and Endurance Performance. J. Strength Cond. Res. 2020, 34, 911–917. [Google Scholar] [CrossRef]
- Morán-Navarro, R.; García-Pallarés, J. Methodological Approach to the Cardiorespiratory Endurance Training. J. Sport Health Res. 2014, 4, 119–136. [Google Scholar]
- Wilson, J.; Marin, P.; Rhea, M.; Wilson, S.; Loenekke, J.; Anderson, J. Concurrent Training: A Meta-Analysis Examining Interference of Aerobic and Resistance Exercises. J. Strength Cond. Res. 2012, 26, 2293–2307. [Google Scholar] [CrossRef]
- Murphy, R.M.; Watt, M.J.; Febbraio, M.A. Metabolic Communication during Exercise. Nat. Metab. 2020, 2, 805–816. [Google Scholar] [CrossRef]
- Fiuza-Luces, C.; Santos-Lozano, A.; Joyner, M.; Carrera-Bastos, P.; Picazo, O.; Zugaza, J.; Izquierdo, M.; Ruilope, L.; Lucía, A. Exercise Benefits in Cardiovascular Disease: Beyond Attenuation of Traditional Risk Factors. Nat. Rev. Cardiol. 2018, 15, 731–743. [Google Scholar] [CrossRef]
- Hoffmann, C.; Weigert, C. Skeletal Muscle as an Endocrine Organ: The Role of Myokines in Exercise Adaptations. Cold Spring Harb. Perspect. Med. 2017, 7, a029793. [Google Scholar] [CrossRef]
- Fumagalli, C.; Maurizi, N.; Day, S.M.; Ashley, E.A.; Michels, M.; Colan, S.D.; Jacoby, D.; Marchionni, N.; Vincent-Tompkins, J.; Ho, C.Y.; et al. Association of Obesity with Adverse Long-Term Outcomes in Hypertrophic Cardiomyopathy. JAMA Cardiol. 2020, 5, 65–72. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, M.; Cui, J.; Liu, S.; Yuan, J.; Qiao, S. Impact of Body Mass Index on Left Atrial Dimension in HOCM Patients. Open Med. 2021, 16, 207–216. [Google Scholar] [CrossRef]
- Coats, C.J.; Maron, M.S.; Abraham, T.P.; Olivotto, I.; Lee, M.M.Y.; Arad, M.; Cardim, N.; Ma, C.S.; Choudhury, L.; Düngen, H.D.; et al. Exercise Capacity in Patients with Obstructive Hypertrophic Cardiomyopathy: SEQUOIA-HCM Baseline Characteristics and Study Design. JACC Heart Fail. 2024, 12, 199–215. [Google Scholar] [CrossRef]
- Coats, C.J.; Rantell, K.; Bartnik, A.; Patel, A.; Mist, B.; McKenna, W.J.; Elliott, P.M. Cardiopulmonary Exercise Testing and Prognosis in Hypertrophic Cardiomyopathy. Circ. Heart Fail. 2015, 8, 1022–1031. [Google Scholar] [CrossRef]
- Cui, H.; Schaff, H.V.; Olson, T.P.; Geske, J.B.; Dearani, J.A.; Nishimura, R.A.; Sun, D.; Ommen, S.R. Cardiopulmonary Exercise Test in Patients with Obstructive Hypertrophic Cardiomyopathy. J. Thorac. Cardiovasc. Surg. 2022, 167, 701–710. [Google Scholar] [CrossRef]
- Magrì, D.; Limongelli, G.; Re, F.; Agostoni, P.; Zachara, E.; Correale, M.; Mastromarino, V.; Santolamazza, C.; Casenghi, M.; Pacileo, G.; et al. Cardiopulmonary Exercise Test and Sudden Cardiac Death Risk in Hypertrophic Cardiomyopathy. Heart 2016, 102, 602–609. [Google Scholar] [CrossRef]
- Magri, D.; Re, F.; Agostoni, P.; Zachara, E.; Correale, M.; Mastromarino, V.; Santolamazza, C.; Casenghi, M.; Pacileo, G.; Valente, F.; et al. Heart Failure Progression in Hypertrophic Cardiomyopathy-Possible Insights from Cardiopulmonary Exercise Testing. Circ. J. 2016, 80, 2204–2211. [Google Scholar] [CrossRef]
- Magri, D.; Agostoni, P.; Sinagra, G.; Re, F.; Correale, M.; Limongelli, G.; Zachara, E.; Mastromarino, V.; Santolamazza, C.; Casenghi, M.; et al. Clinical and Prognostic Impact of Chronotropic Incompetence in Patients with Hypertrophic Cardiomyopathy. Int. J. Cardiol. 2018, 15, 125–131. [Google Scholar] [CrossRef]
- Finocchiaro, G.; Haddad, F.; Knowles, J.; Caleshu, C.; Pavlovic, A.; Homburger, J.; Shmargad, Y.; Sinagra, G.; Magavem, E.; Wong, M.; et al. Cardiopulmonary Responses and Prognosis in Hypertrophic Cardiomyopathy: A Potential Role for Comprehensive Noninvasive Hemodynamic Assessment. JACC. Heart Fail. 2015, 3, 408–418. [Google Scholar] [CrossRef]
- Masri, A.; Pierson, L.M.; Smedira, N.G.; Agarwal, S.; Lytle, B.W.; Naji, P.; Thamilarasan, M.; Lever, H.M.; Cho, L.S.; Desai, M.Y. Predictors of Long-Term Outcomes in Patients with Hypertrophic Cardiomyopathy Undergoing Cardiopulmonary Stress Testing and Echocardiography. Am. Heart J. 2015, 169, 684–692.e1. [Google Scholar] [CrossRef]
- Hackney, A. Stress and the Neuroendocrine System: The Role of Exercise as a Stressor and Modifier of Stress. Expert Rev. Endocrinol. Metab. 2006, 1, 783–792. [Google Scholar] [CrossRef]
- Hooper, D.R.; Kraemer, W.J.; Focht, B.C.; Volek, J.S.; DuPont, W.H.; Caldwell, L.K.; Maresh, C.M. Endocrinological Roles for Testosterone in Resistance Exercise Responses and Adaptations. Sport. Med. 2017, 47, 1709–1720. [Google Scholar] [CrossRef]
- Ratamess, N.A.; Kraemer, W.J.; Volek, J.S.; Maresh, C.M.; Vanheest, J.L.; Sharman, M.J.; Rubin, M.R.; French, D.N.; Vescovi, J.D.; Silvestre, R.; et al. Androgen Receptor Content Following Heavy Resistance Exercise in Men. J. Steroid Biochem. Mol. Biol. 2005, 93, 35–42. [Google Scholar] [CrossRef]
- Novac, N.; Heinzel, T. Nuclear Receptors: Overview and Classification. Curr. Drug Targets Inflamm. Allergy 2004, 3, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Fink, J.; Kikuchi, N.; Nakazato, K. Effects of Rest Intervals and Training Loads on Metabolic Stress and Muscle Hypertrophy. Clin. Physiol. Funct. Imaging 2018, 38, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Gharahdaghi, N.; Phillips, B.E.; Szewczyk, N.J.; Smith, K.; Wilkinson, D.J.; Atherton, P.J. Links Between Testosterone, Oestrogen, and the Growth Hormone/Insulin-Like Growth Factor Axis and Resistance Exercise Muscle Adaptations. Front. Physiol. 2021, 11, 621226. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.; Zack, E.; Battaglini, C.; Viru, M.; Viru, A.; Hackney, A. Exercise and Circulating Cortisol Levels: The Intensity Threshold Effect. J. Endocrinol. Investig. 2008, 31, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Klasson, C.L.; Sadhir, S.; Pontzer, H. Daily Physical Activity Is Negatively Associated with Thyroid Hormone Levels, Inflammation, and Immune System Markers among Men and Women in the NHANES Dataset. PLoS ONE 2022, 17, e0270221. [Google Scholar] [CrossRef]
- Ahn, N.; Kim, H.S.; Kim, K. Exercise Training–Induced Changes in Metabolic Syndrome Parameters, Carotid Wall Thickness, and Thyroid Function in Middle-Aged Women with Subclinical Hypothyroidism. Pflugers Arch. Eur. J. Physiol. 2019, 471, 479–489. [Google Scholar] [CrossRef]

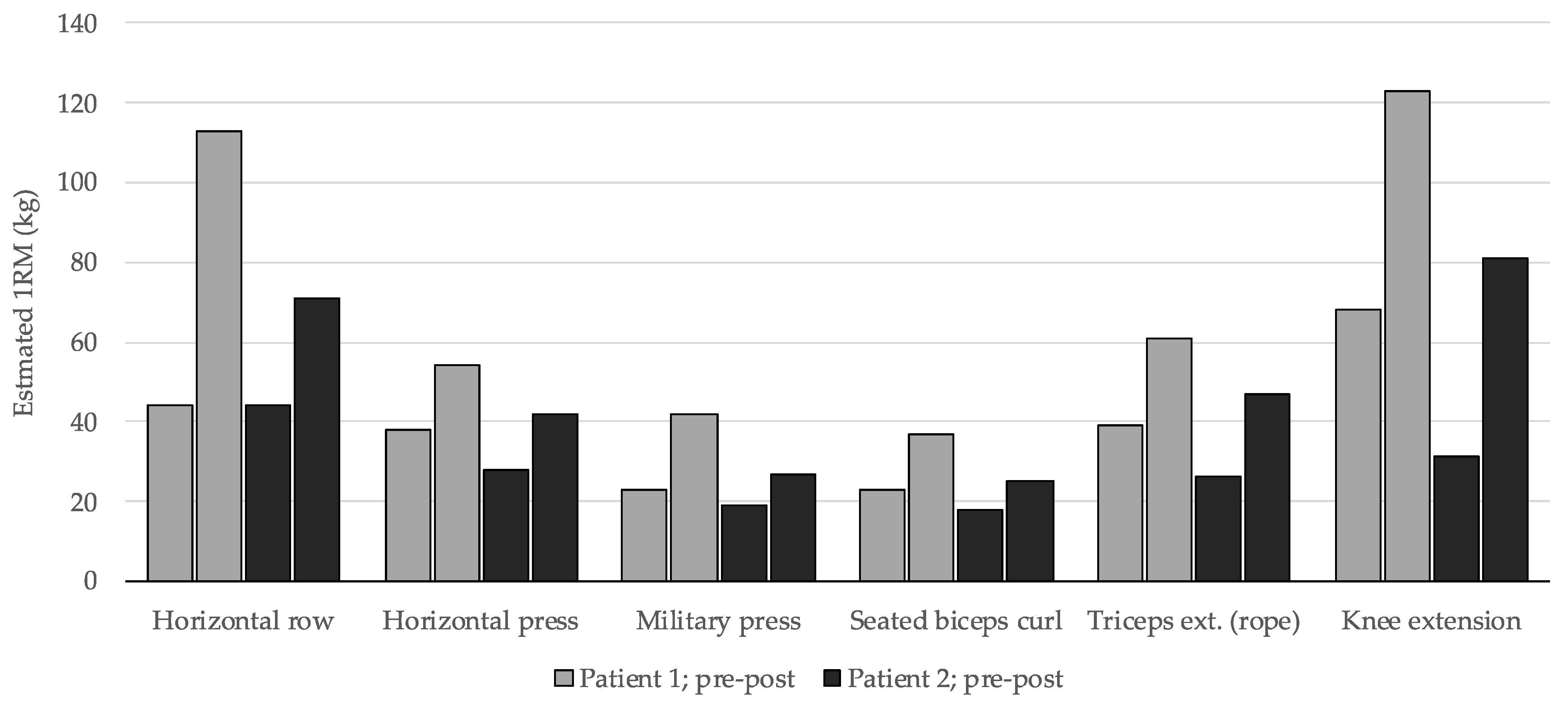
| Muscular Group | Session 1 | Session 2 |
|---|---|---|
| Back | Seated horizontal row | Prone-grip pull-downs |
| Chest | Seated horizontal press | Seated machine chest-flys |
| Shoulders | Seated military press | Lateral raises w/dumbbell |
| Biceps | Seated dumbbell curls | Standing dumbbell curls |
| Triceps | High pulley ext. (w/rope) | High pulley ext. (w/bar) |
| Legs | Seating knee extensions | Seated horizontal leg press |
| Patient 1 | Patient 2 | |||||
|---|---|---|---|---|---|---|
| Pre | Post | Change | Pre | Post | Change | |
| Height (cm) | 170 | 170 | - | 161 | 161 | - |
| Weight (kg) | 95.7 | 97.1 | 1.4 | 67.8 | 69.9 | 2.1 |
| BMI (kg/m2) | 33.1 | 33.6 | 0.5 | 26.2 | 27 | 0.8 |
| Muscular mass (kg) | 36.3 | 37.1 | 0.8 | 25.6 | 26.8 | 1.2 |
| Body fat (%) | 32.8 | 32.2 | −0.6 | 31.9 | 31.3 | −0.6 |
| Fat mass (kg) | 31.4 | 31.2 | −0.2 | 21.6 | 21.9 | 0.3 |
| Waist circumference (cm) | 110 | 107 | −3 | 87 | 86 | −1 |
| Hip circumference (cm) | 100 | 103 | 3 | 93 | 93 | - |
| Waist-to-hip ratio | 1.10 | 1.04 | −0.06 | 0.94 | 0.93 | −0.01 |
| Patient 1 | Patient 2 | |||||
|---|---|---|---|---|---|---|
| Pre | Post | Change | Pre | Post | Change | |
| Supine heart rate | 63 | 63 | - | 68 | 60 | −8 |
| Supine systolic BP | 130 | 162 | 32 | 181 | 142 | −39 |
| Supine diastolic BP | 89 | 92 | 3 | 90 | 87 | −3 |
| Resting heart rate | 70 | 65 | −5 | 74 | 65 | −9 |
| Resting systolic BP | 124 | 151 | 27 | 148 | 163 | 15 |
| Resting diastolic BP | 88 | 94 | 6 | 84 | 95 | 11 |
| (a) Resting | ||||||
| VO2 (L/min) | 0.48 | 0.41 | −0.07 | 0.38 | 0.39 | 0.01 |
| VO2/kg (mL/kg/min) | 5 | 4 | −1 | 6 | 6 | - |
| V′E (L/min) | 16.7 | 13.9 | −2.8 | 13.2 | 11.3 | −1.9 |
| V′E/V′CO2 | 32.5 | 33.1 | 0.6 | 32.5 | 28.5 | −4.0 |
| Breathing frequency | 19 | 17 | −2 | 13 | 17 | 4 |
| (b) Ventilatory threshold 1 | ||||||
| VO2 (L/min) | 1.45 | 1.51 | 0.06 | 0.99 | 1.01 | 0.02 |
| VO2/kg (mL/kg/min) | 15 | 15 | - | 15 | 15 | - |
| VO2/kg (% max) | 60.0 | 51.7 | −8.3 | 46.9 | 41.7 | −5.2 |
| Heart rate (bpm) | 101 | 92 | −9 | 97 | 86 | −11 |
| Heart rate (% max) | 72.1 | 61.3 | −10.8 | 69.8 | 61.4 | −8.4 |
| V′E (L/min) | 35.1 | 35.3 | 0.2 | 26.4 | 24.6 | −1.8 |
| V′E/V′CO2 | 26.3 | 28.4 | 2.1 | 29.0 | 29.6 | 0.6 |
| Breathing frequency | 22 | 23 | 1 | 20 | 24 | 4 |
| (c) Ventilatory threshold 2 | ||||||
| VO2 (L/min) | 2.36 | 2.73 | 0.37 | 1.69 | 2.12 | 0.43 |
| VO2/kg (mL/kg/min) | 25 | 28 | 3 | 25 | 31 | 6 |
| VO2/kg (% max) | 100 | 96.5 | −3.5 | 78.1 | 86.1 | 8.0 |
| Heart rate (bpm) | 140 | 146 | 6 | 119 | 128 | 9 |
| Heart rate (% max) | 100 | 97.3 | −2.7 | 85.6 | 91.4 | 5.8 |
| V′E (L/min) | 68.9 | 82.4 | 13.5 | 44.1 | 64.1 | 20.0 |
| V′E/V′CO2 | 24.1 | 25.3 | 1.2 | 26.2 | 28 | 1.8 |
| Breathing frequency | 29 | 34 | 5 | 22 | 32 | 10 |
| (d) Maximum VO2 | ||||||
| VO2max (L/min) | 2.36 | 2.78 | 0.42 | 2.18 | 2.41 | 0.23 |
| VO2max (mL/kg/min) | 25 | 29 | 4 | 32 | 36 | 4 |
| VO2max (% pred.) | 95 | 112 | 17 | 109 | 121 | 12 |
| Heart rate (bpm) | 140 | 150 | 10 | 139 | 140 | 1 |
| Heart rate recovery 1′ (bpm) | 36 | 20 | −16 | 10 | 19 | 9 |
| Respiratory exchange ratio | 1.14 | 1.16 | 0.02 | 1.14 | 1.13 | 0.01 |
| V′E (L/min) | 68.4 | 87.4 | 19.0 | 71.0 | 85.3 | 14.3 |
| V′E/V′CO2 | 24.2 | 25.4 | 1.2 | 28.6 | 29.3 | 0.7 |
| Breathing frequency | 29 | 37 | 8 | 32 | 37 | 5 |
| Max. slope (%) | 14 | 14 | - | 14 | 14 | - |
| Max. speed (km/h) | 5.3 | 5.6 | 0.3 | 5.4 | 5.6 | 0.2 |
| Test duration (min) | 8′21″ | 8′56″ | 0′35″ | 8′31″ | 9′27″ | 0′56″ |
| Patient 1 | Patient 2 | |||||
|---|---|---|---|---|---|---|
| Pre | Post | Change | Pre | Post | Change | |
| P wave (ms) | 156 | 98 | −58 | 116 | 114 | −2 |
| PQ interval (ms) | 174 | 154 | −20 | 120 | 120 | - |
| QRS complex (ms) | 110 | 90 | −20 | 98 | 98 | - |
| QT interval (ms) | 396 | 396 | - | 438 | 448 | 10 |
| QTc interval (ms) | 402 | 401 | −1 | 453 | 450 | −3 |
| Patient 1 | Patient 2 | |||||
|---|---|---|---|---|---|---|
| Pre | Post | Change | Pre | Post | Change | |
| MWT (mm) | 17 | 17 | - | 15 | 15 | - |
| LVEDD (cm) | 4.4 | 5.0 | −0.6 | 4.8 | 5.1 | 0.3 |
| LVESD (cm) | 3.0 | 2.8 | −0.2 | 3.5 | 3.2 | −0.3 |
| LVEDV 4C (mL) | 134.1 | 114.3 | −19.8 | 68.3 | 86.5 | 18.2 |
| LVESV 4C (mL) | 29.5 | 32.9 | 3.4 | 18.5 | 27.4 | 8.9 |
| Indexed LVEDV (mL/m2) | 63.7 | 55.0 | −8.7 | 39.8 | 49.7 | 9.9 |
| Indexed LVESV (mL/m2) | 14.0 | 15.8 | 1.8 | 10.8 | 15.8 | 5.0 |
| LV mass (g) | 220.5 | 276.5 | 56.0 | 171.3 | 197.1 | 27.8 |
| Indexed LV mass (g/m2) | 104.7 | 133.0 | 28.3 | 99.9 | 113.3 | 13.4 |
| FS (%) | 31.5 | 43.6 | 12.1 | 26.5 | 36.6 | 10.1 |
| PWT (mm) | 10.0 | 10.4 | 0.4 | 8.1 | 10.1 | 2.0 |
| LVEF 4C (%) | 78.0 | 71.2 | −6.8 | 72.9 | 68.3 | −4.6 |
| TAPSE (cm) | 2.10 | 2.48 | 0.38 | 2.10 | 2.42 | 0.32 |
| LA SGL (%) | 31.0 | 27.9 | −3.1 | 23.8 | 17.6 | −6.2 |
| LA diameter (cm) | 4.8 | 4.8 | - | 4.5 | 4.0 | −0.5 |
| LA volume 4C (mL) | 88.9 | 89.0 | 0.1 | 101.9 | 89.0 | −12.9 |
| Indexed LA volume (mL/m2) | 40.5 | 42.8 | 2.3 | 48.4 | 51.2 | 2.8 |
| Vmax E (cm/s) | 97.0 | 87.3 | −9.7 | 83.4 | 63.5 | −19.9 |
| Vmax A (cm/s) | 105.3 | 103.8 | −1.5 | 38.3 | 35.9 | 2.4 |
| E/A | 0.92 | 0.84 | −0.08 | 2.20 | 1.77 | 0.43 |
| Vmax medial E′ (cm/s) | 4.5 | 6.3 | 1.8 | 6.7 | 6.8 | −0.1 |
| Vmax lateral E′ (cm/s) | 6.5 | 9.1 | 2.6 | 7.3 | 5.9 | −1.4 |
| Medial E/E′ | 21.3 | 13.9 | −7.4 | 12.5 | 9.4 | −3.1 |
| Lateral E/E′ | 15.0 | 9.6 | −5.4 | 11.4 | 5.9 | −5.5 |
| Patient 1 | Patient 2 | |||||
|---|---|---|---|---|---|---|
| Pre | Post | Change | Pre | Post | Change | |
| Glucose (mg/dL) | 83 | 90 | 7 | 85 | 96 | 11 |
| Urea (mg/dL) | 33 | 37 | 4 | 38 | 44 | 6 |
| Sodium (mEq/L) | 139 | 139 | - | 138 | 138 | - |
| Potassium (mEq/L) | 4.7 | 4.1 | −0.6 | 4.0 | 3.8 | −0.2 |
| Chlorine (mEq/L) | 101 | 103 | 2 | 98 | 97 | −1 |
| Cardiac troponin T (pg/mL) | 17 | 14 | −3 | 6 | 8 | 2 |
| NT-proBNP (pg/mL) | 144 | 243 | 99 | 514 | 282 | −232 |
| Interleukin 6 | 3.2 | 2.8 | −0.4 | 1.5 | 1.5 | - |
| Testosterone (ng/mL) | 3.11 | 2.12 | −0.99 | 5.42 | 5.03 | −0.39 |
| Basal cortisol (mcg/dL) | 6.9 | 7.3 | 0.4 | 9.2 | 11.6 | 2.4 |
| Basal GH (ng/mL) | 0.03 | 1.71 | 1.68 | 0.50 | 0.16 | −0.34 |
| TSH (uIU/mL) | 2.95 | 3.78 | 0.83 | 4.43 | 7.29 | 2.86 |
| Author, Year | Duration, Sessions | Cardiorespiratory Training | Resistance Training | Additional Measures |
|---|---|---|---|---|
| Klempfner, 2015 [9] | 2 sessions/week (41 h in total) no more details on duration/schedule | 50 to 85% of HRR and 13 to 15 points of RPE, progression not explained | Not included | Holter, physical examination and echocardiography |
| Saberi, 2017 [8] | 16 weeks 4–7 sessions/week | 60 to 70% of HRR and 12 to 14 points of RPE, progression explained | Not included | Electrocardiogram, echocardiography, blood tests, CMR, genetic testing |
| Wasserstrum, 2019 [7] | 3 to 4 months 2 sessions/week | 60 to 70% of HRR and 13 points of RPE, progression not explained | Not included | Electrocardiogram, echocardiography |
| Limongelli, 2021 [11] | 18 months 3 sessions/week | 60 to 80% of VO2max, progression not explained | Exercises poorly defined. Intensity at 65% of 1RM but neither 1RM estimation nor volume, rest, etc. explained | Holter, physical exam, CMR, blood tests, echo-electro cardiography |
| MacNamara, 2023 [10] | 5 months 3–5 sessions/week | Two groups with different %HR based on peak HR and MSS from CPET; progression well-explained | Not included | Echocardiography, body composition, blood tests |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayonas-Ruiz, A.; Muñoz-Franco, F.M.; Sabater-Molina, M.; Martínez-González-Moro, I.; Gimeno-Blanes, J.R.; Bonacasa, B. Concurrent Resistance and Cardiorespiratory Training in Patients with Hypertrophic Cardiomyopathy: A Pilot Study. J. Clin. Med. 2024, 13, 2324. https://doi.org/10.3390/jcm13082324
Bayonas-Ruiz A, Muñoz-Franco FM, Sabater-Molina M, Martínez-González-Moro I, Gimeno-Blanes JR, Bonacasa B. Concurrent Resistance and Cardiorespiratory Training in Patients with Hypertrophic Cardiomyopathy: A Pilot Study. Journal of Clinical Medicine. 2024; 13(8):2324. https://doi.org/10.3390/jcm13082324
Chicago/Turabian StyleBayonas-Ruiz, Adrián, Francisca M. Muñoz-Franco, María Sabater-Molina, Ignacio Martínez-González-Moro, Juan Ramon Gimeno-Blanes, and Bárbara Bonacasa. 2024. "Concurrent Resistance and Cardiorespiratory Training in Patients with Hypertrophic Cardiomyopathy: A Pilot Study" Journal of Clinical Medicine 13, no. 8: 2324. https://doi.org/10.3390/jcm13082324
APA StyleBayonas-Ruiz, A., Muñoz-Franco, F. M., Sabater-Molina, M., Martínez-González-Moro, I., Gimeno-Blanes, J. R., & Bonacasa, B. (2024). Concurrent Resistance and Cardiorespiratory Training in Patients with Hypertrophic Cardiomyopathy: A Pilot Study. Journal of Clinical Medicine, 13(8), 2324. https://doi.org/10.3390/jcm13082324







