Abstract
Background: The study aimed to describe the phenomenon of leads migrated (MPLE) into the cardiovascular system (CVS). Methods: Retrospective analysis of 3847 transvenous lead extractions (TLE). Results: Over a 17-year period, 72 (1.87%) MPLEs (median dwell time 137.5 months) were extracted, which included mainly ventricular leads (56.94%). Overall, 68.06% of MPLEs had their cut proximal ends in the venous system. Most of them were pacing (95.83%) and passive fixation (98.61%) leads. Independent risk factors for MPLE included abandoned leads (OR = 8.473; p < 0.001) and leads located on both sides of the chest (2.981; p = 0.045). The higher NYHA class lowered the probability of MPLE (OR = 0.380; p < 0.001). Procedure complexity was higher in the MPLE group (procedure duration, unexpected procedure difficulties, use of additional (advanced) tools and alternative venous approach). There were no more major complications in the MPLE group, but the rate of procedural success was lower due to more frequent retention of non-removable lead fragments. Extraction of MPLEs did not influence long-term survival. Conclusions: 1. Extraction of leads with MPLE is rare among other TLE procedures (1.9%), 2. risk factors include abandoned leads and presence of leads on both sides of the chest but a higher NYHA class lowers the probability of MPLE, 3. complexity of MPLE extraction is higher regarding procedure duration, unexpected procedure difficulties, use of advanced tools and techniques but rates of major complications are comparable, and 4. extraction of MPLEs did not influence long-term survival.
1. Introduction
Incorrect fixation of the retained cut leads, lead fractures due to ligature that is too tight and improper subclavian vein puncture with secondary crush syndrome can make the proximal end of the lead slip into the CVS and move further [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15].
The migration of the cut proximal lead end (MPLE) into the subclavian or anonymous vein [3,7,10,11,12,13], or even superior vena cava [1] ends up with loops, which in turn pass through the tricuspid valve to the right ventricle, triggering tricuspid valve dysfunction [1] and ventricular arrhythmias [6,16]. Sometimes, free MPLEs float via the right heart cavities into the pulmonary artery [1,2,3,5,6,7,8], causing pulmonary embolism [1,12]. For these reasons, MPLEs in the CVS become a potential source of serious secondary consequences and a class 1 indication (lead with an ending in the CVS, which may pose an immediate threat to the patient if left in place, life threatening arrhythmias secondary to retained lead or lead fragment) or class 2b indication (lead which may pose a potential future threat to the patient if left in place) for lead extraction according to the guidelines of the Heart Rhythm Society (HRS) [17,18]. This phenomenon has been described in numerous case reports [4,5,6,7,8,9,10,11,12,13,14,15], case series [3] and few publications [1,2,16] and mentioned in the guidelines [19]. Having a large, computerized database of extraction procedures, we decided to perform a deeper analysis of this phenomenon.
1.1. Aim of the Study
The aim of our study was to analyse migration of the cut proximal lead ends (MPLEs) into the cardiovascular system (CVS) in the last 17 years and to determine its frequency, type and age of migrated leads, location of MPLEs, risk factors for lead migration dependent on the patient and the CIED system, predictors of major complications or increased procedure complexity and finally, to describe complexity and complications of migrant lead extraction, and its influence on long-term outcomes.
1.2. What Is New?
Spontaneous conductor fractures and insulation breaks of the intracardiac leads near their venous entry or incorrect fixation of the retained cut leads sometimes cause the proximal end of the fractured lead to slide into the veins, with potential subsequent looping in the heart and secondary complications. So far, this topic has not been extensively studied. As it is a relatively uncommon finding, it has been described in a vast number of case studies only. To the best of our knowledge, this is the first comprehensive description of the phenomenon, its risk factors, and management of the cut proximal lead ends in the cardiovascular system.
2. Methods
2.1. Study Population
All transvenous lead extraction procedures (TLE) performed between March 2006 and March 2023 at three high-volume centres were reviewed. Patient clinical characteristics, CIED system and history of pacing, data on targeted leads, TLE complexity, efficacy and outcomes were retrospectively analysed using our computerized database. The study population consisted of 3847 patients, aged 5–99 years, the mean age was 66.02 years, and 38.01% were women.
2.2. Lead Extraction Procedure
Indications for lead extraction, effectiveness and complications of the procedure were defined according to the recent recommendations (2009 and 2017 HRS consensus and 2018 EHRA expert consensus statement) [17,18,19]. The TLE was expressed as the rate of procedural success and clinical success [17,18,19]. The complications of TLE were also defined as major complications that were life threatening, resulted in significant or permanent health disability or death, or required surgical intervention [17,18,19].
2.2.1. Procedure Complexity
Procedure complexity was expressed as whole lead extraction time (sheath-to-sheath time) and average time of single lead extraction (sheath-to-sheath/number of removed leads), and use of second line tools and advanced tools [20,21]. The third (new) complexity marker was The Complex Indicator of the Difficulty of the TLE (CID TLE) which included global sheath-to-sheath time for extraction of all leads >20 min (2 points), average duration of single lead extraction (sheath-to-sheath time) >12 min (2 points) and use of metal sheaths or Evolution/TightRail, alternative approach or lasso-catheters or basket catheters (one point for each). The sum of points was the value of CID-TLE [20].
2.2.2. Unexpected Technical Problems during TLE
They covered all situations that increased procedure complexity but were not complications. They included blockage in the lead venous entry/subclavian region preventing advancement of a polypropylene catheter into the subclavian vein, Byrd dilator collapse/fracture, lead-on-lead adhesion, necessity of using an alternative approach, loss of fractured lead fragment when the main part of the lead was dissected and removed but both free ends were retained, the mobile lead fragment which floated usually into the pulmonary circulation, and displacement of functional leads [21].
2.2.3. Procedure Information
We utilized a stepwise approach in all patients. Standard stylets or locking stylets (Liberator Locking Stylet, Cook Medical Inc., Bloomington, IN, USA) were used, the latter ones for extraction of the oldest leads with a high estimated risk of fracture. We usually started with non-powered mechanical telescoping polypropylene sheaths (Byrd Dilator Sheaths, Cook Medical Inc., USA) of all lengths and sizes. The second-line tools included powered mechanical sheath systems (Evolution Mechanical Dilator Sheath, Cook Medical, Bloomington, IN, USA; TightRail Rotating Dilator Sheath, Phillips, Colorado Springs, CO, USA) or metal sheaths if the obstacle was encountered in the extracted lead venous entry region. A combined approach, using two or more different (jugular, subclavian, femoral) access sites, was selected when conventional methods were presumed ineffective (proximal lead ends in the cardiovascular space or in case of lead fracture during extraction) [1,2,3,11,12,13,15].
2.2.4. Leads with Proximal Ends Migrated into the Cardiovascular System—Definitions
Leads with their cut or spontaneously broken proximal ends migrated into the cardiovascular system, for various reasons and via multiple mechanisms, were defined as fractured leads in the lead implant vein when their proximal ends slipped into the CVS and migrated from the subclavian vein into the superior vena cava, right atrium, right ventricle into the pulmonary artery or rarely another vein while the distal ends (tips of the lead) remained where the lead had been implanted [1,2,3,7,8,12,13,15].
2.2.5. Extraction of Leads with Their Proximal Ends Migrated into the Cardiovascular System
We always attempted to remove all leads with their cut or broken proximal ends migrated into the cardiovascular system (CVS). These leads were regarded not only as abandoned non-functional leads, but also as a potential source of adverse consequences of the migrant proximal lead end (thrombosis, venous occlusion, arrhythmias) as well as adverse or potentially adverse consequences of lead looping in the heart (TV dysfunction, arrhythmias). One of these consequences is accelerated adhesion of the lead loop to the venous wall, which may significantly hinder the extraction of such leads in case of future infectious complications [1,2,7,8,11,14,16]. For this reason, we treated all such leads as “leads which may pose an immediate threat to the patient if left in place or leads which may pose a potential future threat to the patient if left in place” [17,18].
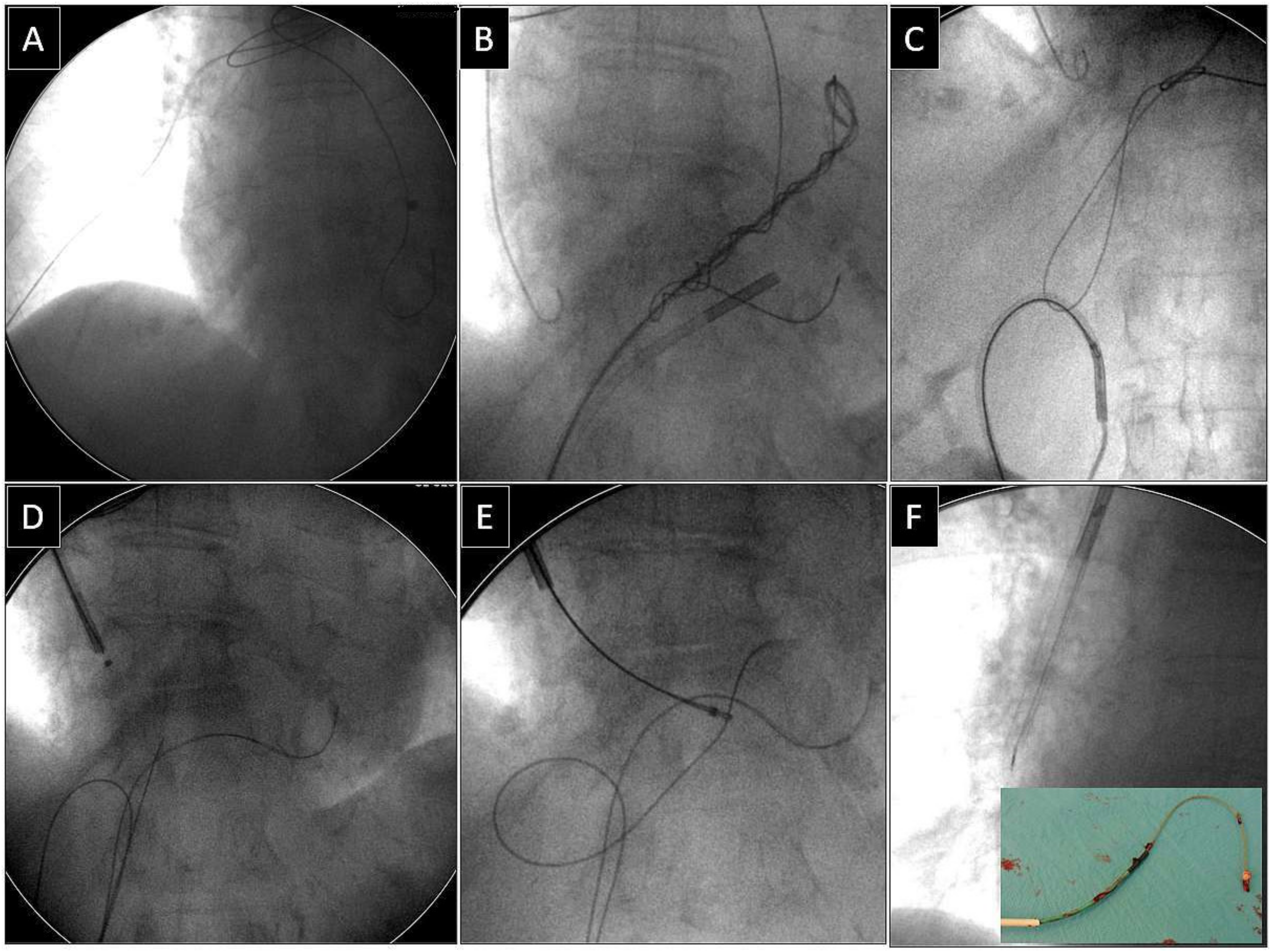
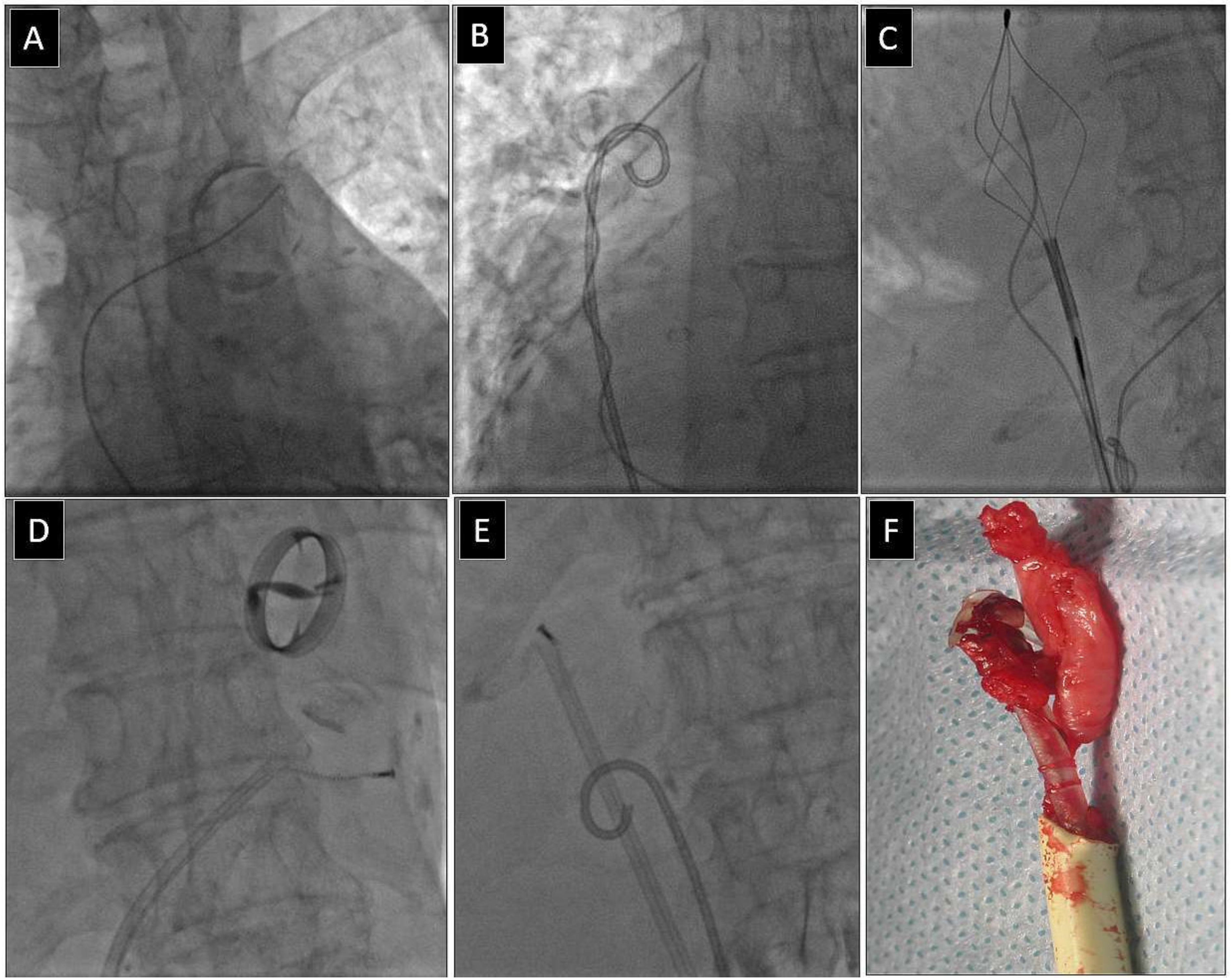
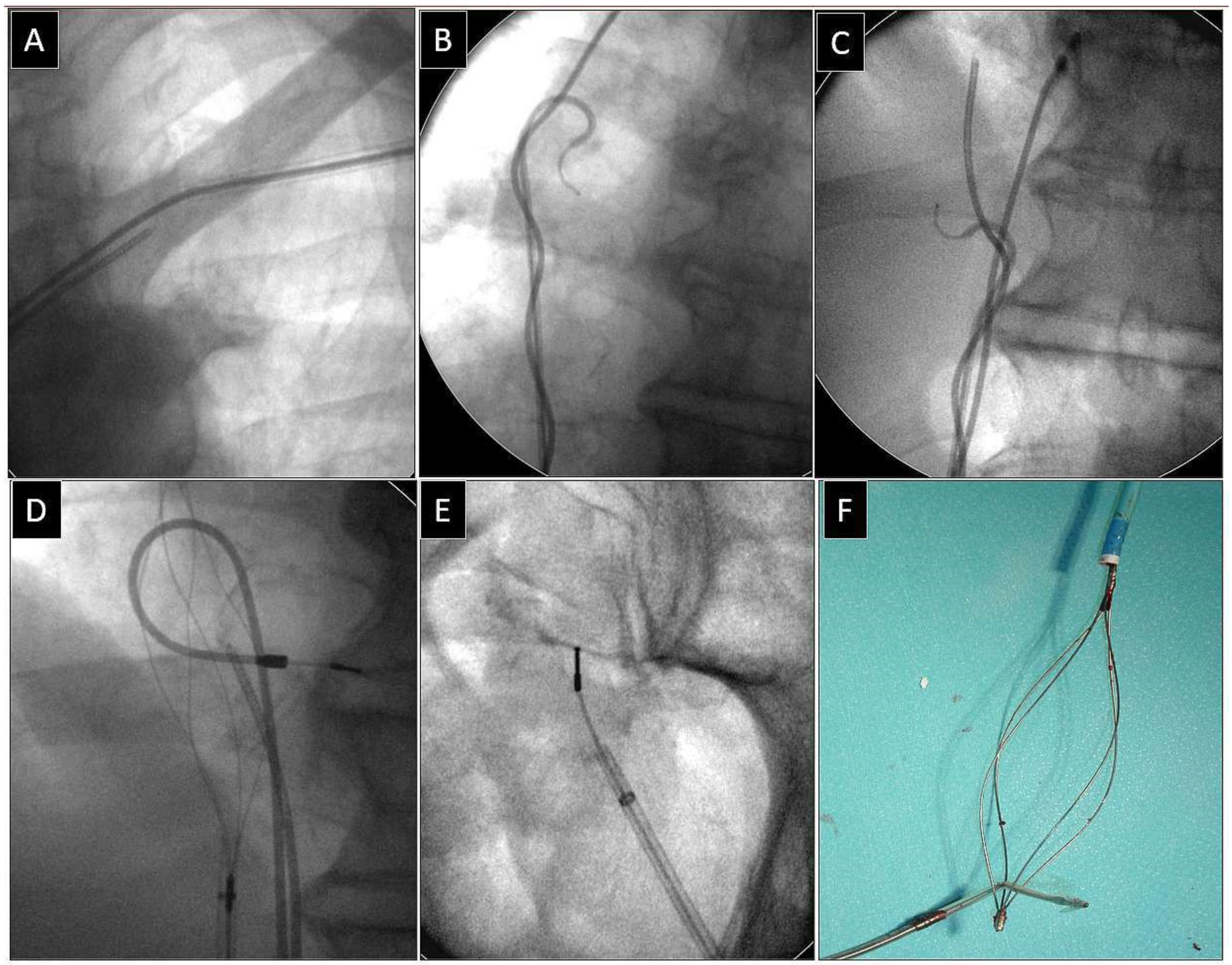
Depending on the location of the proximal end of the fractured and displaced lead, we tried to retrieve it with a lasso (Figure 1) or basket catheter (Figure 2 and Figure 3) using the femoral approach (Figure 2 and Figure 3) or, if other leads were planned for extraction, we sometimes tried to use the subclavian access re-established after extraction of another lead [1,2]. Jugular access was used less frequently (Figure 1). After firmly grasping the proximal end of the migrated lead, the lasso or basket catheter played a role of an extension (Figure 1, Figure 2 and Figure 3) of the fractured lead and we performed lead dissection until the lead was removed using polypropylene sheaths (Figure 1 and Figure 2) or 16F silicone catheters (Curved Femoral Introducer Sheath Set 16 Fr, LR-CSS16) with a bevelled end for rotational dilation [1,2,11,12]. Mechanical rotational tools were rarely used, as most of the procedures had been performed before these tools became available on the market. We rarely used the Needle’s Eye Snare© because other techniques proved to be more effective. When the proximal end of the fractured migrated lead could not be grasped, we released it from the fibrous tissue by wrapping the lead around a pig-tail catheter (“spaghetti twisting technique”) [1,2,7,8,13] (Figure 2 and Figure 3) or by using the loop made of a guidewire and a lasso catheter or basket catheter placed over the removed lead and pulled (Figure 1) [1,2,5,11,12,15], if the “spaghetti twisting technique” was entirely ineffective.
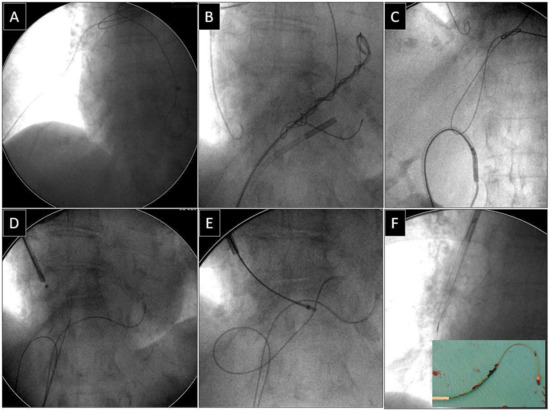
Figure 1.
Extraction of a fractured old model unipolar passive fixation ventricular lead with its proximal end in the branch of the right pulmonary artery. The lead without a graspable proximal end (A). A “spaghetti twisting technique” was used to catch the lead with a pig-tail catheter. After simple winding and pulling on the lead the end of the lead was partially withdrawn from the pulmonary artery (B). The technique of pulling down the lead with the loop had to be used (via the femoral approach) (C,D). The proximal end of the lead was grasped with a jugular lasso catheter (E). Conventional lead dissection with polypropylene sheaths proved to be successful (F). Removed lead on the table (F). An example of using a combined approach (jugular and femoral).
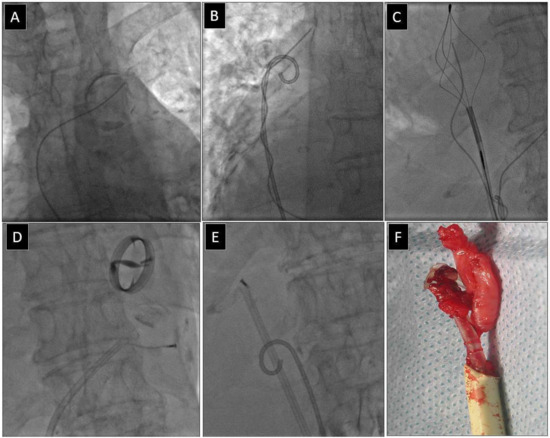
Figure 2.
Extraction of a fractured old model unipolar passive fixation ventricular lead with its proximal end in the anonymous vein. The lead without a graspable proximal end (A). Again, a “spaghetti twisting technique” was used with a pig-tail catheter. After winding and pulling on the lead, the proximal end of the fractured lead was pulled to the right atrium. (B). The end of the fractured lead was grasped with a basket catheter inside the right atrium (C). Conventional lead dissection with a polypropylene sheath (D,E). The clamped basket catheter extends the fractured lead and allows usual lead dissection in the intraventricular section. Extracted lead on the table (F). An example of using a femoral approach for fractured lead extraction.
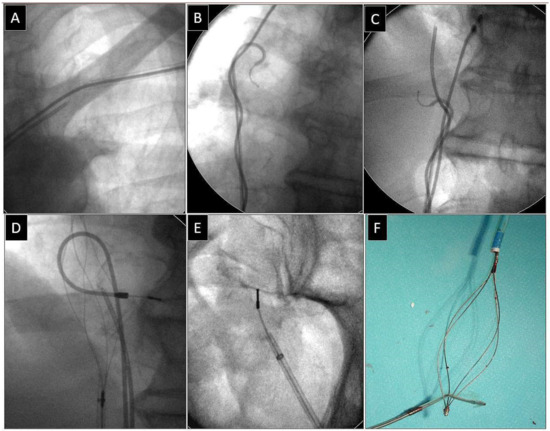
Figure 3.
Extraction of a fractured bipolar passive fixation ventricular lead with its proximal end in the subclavian vein. Similarly, the lead without a graspable proximal end (A). Also, in this case the “spaghetti twisting technique” using a pig-tail catheter was found to be effective. After winding and pulling on the lead, the proximal end of the fractured lead was pulled to the right atrium. (B,C). The end of the fractured lead was grasped with a basket catheter in the low right atrium (D). The clamped basket catheter extended the fractured lead. Conventional counter-traction was enough to remove the lead (E,F). Extracted broken lead on the table (F). A pig-tail catheter and “spaghetti twisting technique” were used to make the proximal end accessible from a femoral approach. An example of using a femoral approach for fractured lead extraction.
We did not use sheaths equipped with laser energy. In the last 17 years, the organization of lead extraction has evolved from procedures performed in the electrophysiology laboratory using intravenous analgesia/sedation to procedures performed in the hybrid room only under general anaesthesia [22,23]. Over the last 7 years, the core extraction team has consisted of the same highly experienced extractor (now frequently serving as a proctor), experienced echocardiographer and cardiac surgeon experienced in the treatment of TLE complications [23,24].
2.3. Dataset and Statistical Methods
2.3.1. Creation of Subgroups for Future Analysis
Based on the analysis of 3847 extraction procedures, they were divided into two subgroups: 1. transvenous extraction of leads with their cut or broken proximal ends spontaneously migrated (MPLE) into the cardiovascular system (CVS) (72 procedures), 2. transvenous extraction of leads without spontaneous migration into the CVS (control group, 3775 procedures).
2.3.2. Statistical Analysis
Due to nonparametric distribution, all continuous variables are presented as the median and lower and upper quartile (Q1–Q3). The categorical variables are presented as counts and percentages. The significance of differences between the groups was determined using the nonparametric Chi2 test with Yates correction or the unpaired Mann–Whitney U test, as appropriate. Univariable and multivariable regression was used to identify the factors that influenced the probability of MPLE. Variables with p values less than 0.1 on univariable analysis were entered into the multivariable model. Survival of the patients was compared using the log rank test. A p-value less than 0.05 was considered statistically significant. Statistical analysis was performed with Statistica 13.3 (TIBCO Software Inc. Tulsa, OK, USA).
2.4. Approval of the Bioethics Committee
All patients provided written informed consent to undergo TLE and to have anonymous data from their medical records used for research purposes. The research methodology was approved by the Bioethics Committee at the Regional Chamber of Physicians in Lublin no. 288/2018/KB/VII (approval date: 27 November 2018). The study was carried out in accordance with the ethical standards of the 1964 Declaration of Helsinki.
3. Results
Removal of leads with their proximal ends spontaneously migrated into the cardiovascular system (MPLE) is relatively rare among other transvenous lead extraction procedures (1.87%). Retrospective analysis showed that most of these procedures have been performed ≥10 years earlier. (Table 1).

Table 1.
Leads with their proximal ends migrated into the cardiovascular system in the last 16 years.
When lead removal became available at our facility 17 years ago, it opened up the possibility of admitting patients who could not be admitted before. After performing all “overdue” procedures, the number of new referrals stabilized at about 0.5%.
Most of the leads spontaneously migrated into the cardiovascular system (MPLE) were ventricular leads (56.94%). Proximal ends of such leads were in the vasculature (subclavian, anonymous and vena cava) (68.06%), and leads in the right atrial location were rare (2.78%) but not in the pulmonary artery (9.72%). Most of such leads were pacing leads (95.83%) and passive fixation leads (98.61%). The median of dwell time of MPLEs was 137.5 months and ranged from 17.24 to 376.4 months (Table 2).

Table 2.
The location of tips, proximal ends, model and age of leads spontaneously migrated into the cardiovascular system.
Patients with extraction of MPLEs were younger at the first system implantation (50.50 vs. 61.00 years), less likely to have ischaemic heart disease (37.50 vs. 56.24), non-significantly more often presenting with systemic infection (29.17 vs. 21.48%) and (obviously) much more frequently with threatening/potentially threatening leads as the main indication for TLE (31.94 vs. 2.81%) as compared to those without extraction of MPLEs (Table 3).

Table 3.
Characteristics of study groups and main indications for lead extraction.
Analysis of system- and procedure-related risk factors for increased procedure complexity and major complications showed that patients with extraction of MPLEs had longer lead implant duration before TLE (144.0 vs. 86.04 months) and global lead dwell time before TLE (22.63 vs. 12.00 years), more frequent presence of abandoned leads (68.06% vs. 9.75%), presence of unnecessary (large) lead loops in the heart (54.17% vs. 3.79%), higher number of leads in the heart before TLE (2.57 vs. 1.94), more frequently multiple leads (4 and >4) in the heart (22.22% vs. 2.70%), leads on both sides of the chest (25.00% vs. 2.36%) and more procedures before lead extraction (2.82 vs. 1.84) and two or more CIED-related procedures before TLE (88.89 vs. 52.16%) (Table 4).

Table 4.
System-, history of pacing- and procedure-related risk factors for major complications and increased extraction complexity in the study groups.
Although none of the prognostic scores/calculators of increased procedure complexity included the presence of leads with MPLEs, all scores such as SAFETY score [risk of MC] [25], EROS score [risk of MC] [26], MB score [need for advanced tools] [27], LED index [predicted fluoroscopy time] [28], Advanced TLE scale [need for advanced TLE techniques] [29,30] and LECOM score [predicted procedure complexity] [20] indicated a significantly higher chance of a difficult and complicated procedure in patients with MPLEs (Table 4).
Analysis of potential procedure-related risk factors for major complications and procedure complexity showed a higher number of extracted leads per patient (2 (1–3) vs. 2 (1–2)), more frequent extraction of abandoned leads (65.28% vs. 8.90%), extraction of old model UP leads (41.76% vs. 8.45%), extraction of passive fixation leads (97.22% vs. 57.22%), extraction of leads with abnormal loops in the heart (52.78% vs. 3.87%) in the MPLE group. Similarly, implant duration was significantly longer: longest lead dwell time (137.5 vs. 84.00 months) and longer average targeted lead implant duration per patient (9.96 vs. 6.86 years).
The comparative analysis showed that in patients with MPLEs, extraction complexity was higher than in the control group because the procedure duration expressed as global lead dissection time (55.00 vs. 9.00 min) and time of single lead extraction were significantly longer (50.00 vs. 4.50 min). Similarly, unexpected procedure difficulties (technical problems) were more frequent (25.00% vs. 5.72%) in patients with MPLE extraction. The use of additional (advanced) tools was more frequent during such procedures in comparison with TLE in the control group: lasso catheters/snares/(52.78% vs. 2.94%), basket catheters (45.83% vs. 0.58%), loops to releasing the end of the lead (70.83% vs. 0.11%), pig-tail catheters (11.11% vs. 0.42%) and alternative approach (79.17% vs. 1.85%). It is worth mentioning that there is another phenomenon that occurs during lead extraction: extracted lead fracture. For this reason, these types of tools were used in the control group, too, but rarely. The complexity of the extraction procedure is well reflected in the CID-TLE which is also called the “retrospective TLE combined difficulty score” (including dilatation time, use of second line tools, advanced tools and advanced techniques): (3.85 vs. 0.49 points) (Table 5).

Table 5.
Procedure complexity, complications, and long-term outcome in the study groups.
The two study groups did not differ in the rate of major complications (2.78% vs. 2.01%), but they did in the rate of clinical success (91.67% vs. 98.09%) and procedural success (87.50% vs. 95.39%). This can be easily explained by the more frequent retention of a non-removable fragment of the lead (12.50% vs. 4.00%), which is mainly due to the global implant duration in patients with MPLEs. Mortality after the extraction procedure was similar in the two groups. (Table 5).
Multivariable regression analysis showed that abandoned leads (OR = 8.473; p < 0.001) and leads on both sides of the chest (2.981; p = 0.045) were independent risk factors for MPLE. Patients with a higher NYHA FC class had a lower probability of MPLE (OR = 0.380; p < 0.001) (Table 6).

Table 6.
Factors affecting migration of the proximal lead end into the cardiovascular system. Results of univariable and multivariable regression analysis.
4. Discussion
This study describes a rare and increasingly rare phenomenon of the cut or broken proximal end of the lead migrated into the cardiovascular system. The complication is causally related to implantation errors (too parasternal puncture and crush syndrome, and too strong clamping of the ligature fixing the lead), cutting the connector of the abandoned lead and leaving such leads in the infected generator pocket.
A review of the literature, i.e., multiple case studies [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19], two smaller studies [1,2] and case series reports [3,4] including a total of 76 cases [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15] shows that MPLE slightly more often affects ventricular leads (55.5%) and significantly more often pacing leads than ICD leads (single cases only). Of the 76 MPLEs, the proximal end of the fractured lead was located in the venous system (55.3%), pulmonary artery (25.0%) and right ventricle (14.4%), and less often in the right atrium (5.2%). The indications for TLE in these 76 cases were systemic infection or local pocket infection 35 (46.0%), non-infectious indications in 41/76 cases (53.9%) including prophylactic indications 19/76 (25.0%), pacing disturbances 16/76 (21.0%) and interactions with the ICD system 2/76 (2.6%), and ventricular arrhythmias 4/76 (5.3%). In three cases (3.9%), the MPLEs were left in place because they were entirely asymptomatic [6,8].
It is obvious, however, that in the case of the extraction of leads with a shorter stay in the patient’s body, the procedure is likely to be of low complexity, the risk of major complications is negligible, and selected patients even have a chance of being discharged on the same day [30].
The present study showed that extraction of leads with their proximal ends migrated into the CVS was relatively rare among other TLEs (2.5%). The rate of new referrals stabilized at about 0.5%.
Most of the MPLEs were ventricular leads (56,9%) and their proximal ends were in the venous system (68.1%) or in the RV (18.0%), pulmonary artery (9.7%) and in the RA (2.6%). Most of them were pacing leads (95.8%), and passive fixation leads (98.6%), and their dwell time ranged from 17 to 376 months, with a median of 137.5 months.
The patients with MPLEs were younger at the first system implantation and less likely to develop IHD but (obviously) more often, the main indication for TLE was a threatening/potentially threatening lead in comparison with the control group.
The patients with MPLEs had longer implant duration, more frequently abandoned leads, unnecessary (large) lead loops in the heart, more leads in the heart, leads on both sides of the chest and more CIED-related procedures before lead extraction. Lead abandonment, including leads on both sides of the chest significantly increased the risk of MPLE. On the other hand, heart failure expressed as a higher NYHA FC class decreased the probability of this complication.
Although none of the prognostic scores/calculators of increased procedure complexity included the presence of MPLE, all scores indicated a significantly higher chance of a difficult and complicated procedure in such patients.
The level of TLE complexity was higher in the MPLE group than in the control group regarding procedure duration, unexpected technical problems, use of additional (advanced) tools and alternative approach.
There were no more major complications in the MPLE group, but the rate of clinical success and procedural success was lower because of the more frequent retention of a non-removable lead fragment.
Extraction of migrant leads with their cut proximal ends in the heart and vasculature should be included in the TLE training program.
5. Conclusions
- Removal of leads with their cut or broken proximal ends migrated into the heart and vasculature was rare among other extraction procedures (1.87%).
- Lead abandonment and leads located on both sides of the chest significantly increased the risk of MPLE. Higher NYHA FC class decreased the probability of this complication.
- Procedure complexity in patients with MPLEs is higher in regards to procedure duration, unexpected procedure difficulties, use of advanced tools and techniques but there are no more major complications.
- Extraction of leads migrated to the heart and vasculature had no effect on long-term survival.
6. Study Limitations
This study has some limitations. It describes the experience of the same first operator serving usually as a proctor at three facilities. Data were collected prospectively but analysed retrospectively. All procedures were performed using all kinds of mechanical systems but not laser powered sheaths. An important limitation of the present study is the selection of patients. It would be ideal to assess the incidence of a migrant lead using a very large database of all implantations and patient histories collected for 10–20 years. Our research is limited to a specific population, i.e., patients referred to our facility as the reference TLE centre (15–10 years ago), and from other high- and mid- volume centres. This population does not reflect all patients with implanted CIEDs. We do not know anything about patients with migrant leads and their fate who were not referred for TLE.
A certain minor limitation of the present study is the fact that some of the case studies mentioned in the discussion section were previously described based on our material by investigators from other national centres. The study analyses a very large population of patients undergoing TLE with an implant duration longer than in many recent studies because our centre for many years was an unofficial reference centre and we were receiving the most difficult patients in the country. This explains the rate of major complications and a lower rate of radiographic success. Our experiences should be of interest to all those who will face extractions of old passive-fixation leads and management of less frequent lead-related permanent pacing complications.
Author Contributions
A.K. supervision, original draft editing, operator, W.J.—methodology, statistical study, writing—review, R.P. co-operator, data curation, P.S. co-operator, data curation, J.K. co-operator, data curation, M.C. co-operator, data curation, S.S. data curation, Ł.T.—co-operator, data curation, D.N.—investigation, data curation, corresponding author. All authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors have read and agreed to the published version of the manuscript.
Funding
The study is supported by the Medical University of Lublin, Poland.
Institutional Review Board Statement
The study was performed according to the principles expressed in the Declaration of Helsinki and approved by the Bioethics Committee at the Regional Chamber of Physicians in Lublin (no. 288/2018/KB/VII). Approval date: 27 November 2018.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available upon request to authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kutarski, A.; Malecka, B.; Zabek, A.; Pietura, R. Broken leads with proximal endings in the cardiovascular system: Serious consequences and extraction difficulties. Cardiol. J. 2013, 20, 161–169. [Google Scholar] [CrossRef]
- Polewczyk, M.; Jacheć, W.; Polewczyk, A.M.; Polewczyk, A.; Czajkowski, M.; Kutarski, A. Leads dislodged into the pulmonary vascular bed in patients with cardiac implantable electronic devices. Adv. Interv. Cardiol. 2016, 4, 348–354. [Google Scholar] [CrossRef]
- Paskudzka, D.; Kołodzińska, A.; Stolarz, P.; Łyżwiński, Ł.; Grabowski, M.; Kutarski, A.; Opolski, G. Management of the late endocardial lead dislocation into the pulmonary trunk. Hear. Beat J. 2018, 3, 72–76. [Google Scholar] [CrossRef]
- Storm, C.; van Mechelen, R. A Severed Pacemaker Lead Entrapped in a Hepatic Vein. Pacing Clin. Electrophysiol. 1993, 16, 1349–1353. [Google Scholar] [CrossRef]
- Erkan, H.; Varol, O.; Karadeniz, A.; Erkan, M. Embolisation of permanent pacemaker lead to pulmonary artery: A 15-year follow up. Kardiol. Pol. 2014, 72, 759. [Google Scholar] [CrossRef]
- Stein, A.; Mazzitelli, D.; Kolb, C. Very-late proarrhythmia of a migrant pacemaker lead. J. Electrocardiol. 2011, 44, 232–234. [Google Scholar] [CrossRef]
- Ruparelia, N.; Newton, J.; Ormerod, O.J.; Bhindi, R. Percutaneous retrieval of an embolized pacemaker lead from the pulmonary artery. Int. J. Cardiol. 2011, 149, e106–e107. [Google Scholar] [CrossRef]
- Małecka, B.; Kutarski, A.; Zabek, A.; Maziarz, A.; Pytkowski, M. Percutaneous removal of endocardial implantable cardioverter-defibrillator lead displaced to the right pulmonary artery. Cardiol. J. 2010, 17, 293–298. [Google Scholar]
- Sochman, J.; Peregrin, J.H.; Bytesnik, J. percutaneous extraction of a fractured permanent pacemaker lead with no free end. Pacing Clin. Electrophysiol. 2005, 28, 1000–1001. [Google Scholar] [CrossRef]
- Lickfett, L.; Jung, W.; Pizzulli, L.; Wolpert, C.; Luderitz, B. Percutaneous extraction of an abandoned coiled pacing lead. Pacing Clin. Electrophysiol. 1999, 22, 1100–1102. [Google Scholar] [CrossRef]
- Kutarski, A.; Chudzik, M.; Tomaszewski, A.; Pietura, R.; Oszczygiel, A.; Czajkowski, M.; Wranicz, J.K. Difficult dual-stage transcutaneous multiple lead extraction with loss of external silicone tube of broken lead. Cardiol. J. 2013, 20, 94–99. [Google Scholar] [CrossRef][Green Version]
- Kutarski, A.; Chudzik, M.; Oszczygieł, A.; Wranicz, J.K. Extraction of abandoned, potentially dangerous lead with uncovered proximal ending: A case report and method description. Cardiol. J. 2012, 19, 192–196. [Google Scholar] [CrossRef]
- Małecki, D.; Orszulak, W.; Tomków, K.; Nowosielecka, D.; Tułecki, Ł.; Grabowski, M.; Kutarski, A.W. Transvenous lead extraction of broken and abandoned azygos vein lead—Case report. Hear. Beat J. 2019, 4, 39–41. [Google Scholar] [CrossRef]
- Sochman, J.; Bytesnik, J.; Skalsky, I.; Peregrin, J.H. Percutaneous extraction of a severed and frayed permanent pacing lead. Pacing Clin. Electrophysiol. 2004, 27, 412–414. [Google Scholar] [CrossRef]
- Kutarski, A.; Czajkowski, M.; Tomaszewski, A.; Młynaczyk, K.; Głowniak, A. Extraction of a 17-year-old pacing lead chronically dislocated into the liver vein. J. Vasc. Access 2012, 13, 130–131. [Google Scholar] [CrossRef]
- Böhm, A.; Pintér, A.; Duray, G.; Lehoczky, D.; Dudás, G.; Tomcsányi, I.; Préda, I. Complications due to abandoned noninfected pacemaker leads. Pacing Clin. Electrophysiol. 2001, 24, 1721–1724. [Google Scholar] [CrossRef]
- Wilkoff, B.L.; Love, C.J.; Byrd, C.L.; Bongiorni, M.G.; Carrillo, R.G.; Crossley, G.H.; Epstein, L.M.; Friedman, R.A.; Kennergren, C.E.; Mitkowski, P.; et al. Transvenous lead extraction: Heart Rhythm Society expert consensus on facilities, training, indications, and patient management: This document was endorsed by the American Heart Association (AHA). Hear. Rhythm. 2009, 6, 1085–1104. [Google Scholar] [CrossRef]
- Kusumoto, F.M.; Schoenfeld, M.H.; Wilkoff, B.L.; Berul, C.I.; Birgersdotter-Green, U.M.; Carrillo, R.; Cha, Y.-M.; Clancy, J.; Deharo, J.-C.; Ellenbogen, K.A.; et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm 2017, 14, e503–e551. [Google Scholar] [CrossRef]
- Bongiorni, M.G.; Burri, H.; Deharo, J.C.; Starck, C.; Kennergren, C.; Saghy, L.; Rao, A.; Tascini, C.; Lever, N.; Kutarski, A.; et al. 2018 EHRA expert consensus statement on lead extraction: Recommendations on definitions, endpoints, research trial design, and data collection requirements for clinical scientific studies and registries: Endorsed by APHRS/HRS/LAHRS. Europace 2018, 20, 1217. [Google Scholar] [CrossRef]
- Jacheć, W.; Nowosielecka, D.; Ziaja, B.; Polewczyk, A.; Kutarski, A. Prediction of increased complexity of transvenous lead extraction. LECOM (Lead Extraction COMplexity): A New Calculator for Prediction of a Difficult Procedure. Cardiol. J. 2023, 30, 15602. [Google Scholar]
- Kutarski, A.; Jacheć, W.; Nowosielecka, D.; Polewczyk, A. Unexpected difficulties complicating transvenous lead extraction and increasing procedure complexity. J. Clin. Med. 2023, 12, 2811. [Google Scholar] [CrossRef]
- Kutarski, A.; Czajkowski, M.; Pietura, R.; Obszański, B.; Polewczyk, A.; Jacheć, W.; Polewczyk, M.; Młynarczyk, K.; Grabowski, M.; Opolski, G. Effectiveness, safety, and long-term outcomes of non-powered mechanical sheaths for transvenous lead extraction. Europace 2018, 20, 1324–1333. [Google Scholar] [CrossRef]
- Tułecki, Ł.; Jacheć, W.; Polewczyk, A.; Czajkowski, M.; Targońska, S.; Tomków, K.; Karpeta, K.; Nowosielecka, D.; Kutarski, A. Assessment of the impact of organisational model of transvenous lead extraction on the effectiveness and safety of procedure: An observational study. BMJ Open 2022, 12, e062952. [Google Scholar] [CrossRef]
- Nowosielecka, D.; Jacheć, W.; Polewczyk, A.; Tułecki, Ł.; Kleinrok, A.; Kutarski, A. The role of transesophageal echocardiography in predicting technical problems and complications of transvenous lead extractions procedures. Clin. Cardiol. 2021, 44, 1233–1242. [Google Scholar] [CrossRef]
- Jacheć, W.; Polewczyk, A.; Polewczyk, M.; Tomasik, A.; Kutarski, A. Transvenous Lead Extraction SAFeTY Score for Risk Stratification and Proper Patient Selection for Removal Procedures Using Mechanical Tools. J. Clin. Med. 2020, 9, 361. [Google Scholar] [CrossRef]
- Sidhu, B.S.; Ayis, S.; Gould, J.; Elliott, M.K.; Mehta, V.; Kennergren, C.; Butter, C.; Deharo, J.-C.; Kutarski, A.; Maggioni, A.P.; et al. Risk stratification of patients undergoing transvenous lead extraction with the ELECTRa Registry Outcome Score (EROS): An ESC EHRA EORP European lead extraction ConTRolled ELECTRa registry analysis. Europace 2021, 23, 1462–1471. [Google Scholar] [CrossRef]
- Bontempi, L.; Curnis, A.; Della Bella, P.; Cerini, M.; Radinovic, A.; Inama, L.; Melillo, F.; Salghetti, F.; Marzi, A.; Gargaro, A.; et al. The MB score: A new risk stratification index to predict the need for advanced tools in lead extraction procedures. Europace 2020, 22, 613–621. [Google Scholar] [CrossRef]
- Bontempi, L.; Vassanelli, F.; Cerini, M.; Inama, L.; Salghetti, F.; Giacopelli, D.; Gargaro, A.; Raweh, A.; Curnis, A. Predicting the difficulty of a transvenous lead extraction procedure: Validation of the LED index. J. Cardiovasc. Electrophysiol. 2017, 28, 811–818. [Google Scholar] [CrossRef]
- Mazzone, P.; Tsiachris, D.; Marzi, A.; Ciconte, G.; Paglino, G.; Sora, N.; Sala, S.; Vergara, P.; Gulletta, S.; DELLA Bella, P. Predictors of advanced lead extraction based on a systematic stepwise approach: Results from a high volume center. Pacing Clin. Electrophysiol. 2013, 36, 837–844. [Google Scholar] [CrossRef]
- Gianni, C.; Elchouemi, M.; Helmy, R.; Spinetta, L.; La Fazia, V.M.; Pierucci, N.; Asfour, I.; Della Rocca, D.G.; Mohanty, S.; Bassiouny, M.A.; et al. Safety and feasibility of same-day discharge following uncomplicated transvenous lead extraction. J. Cardiovasc. Electrophysiol. 2024, 35, 278–287. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).