Outcomes in Atrial Fibrillation Patients with Different Clinical Phenotypes: Insights from the French Population
Abstract
1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Follow-Up and Outcomes
2.3. Statistical Analysis
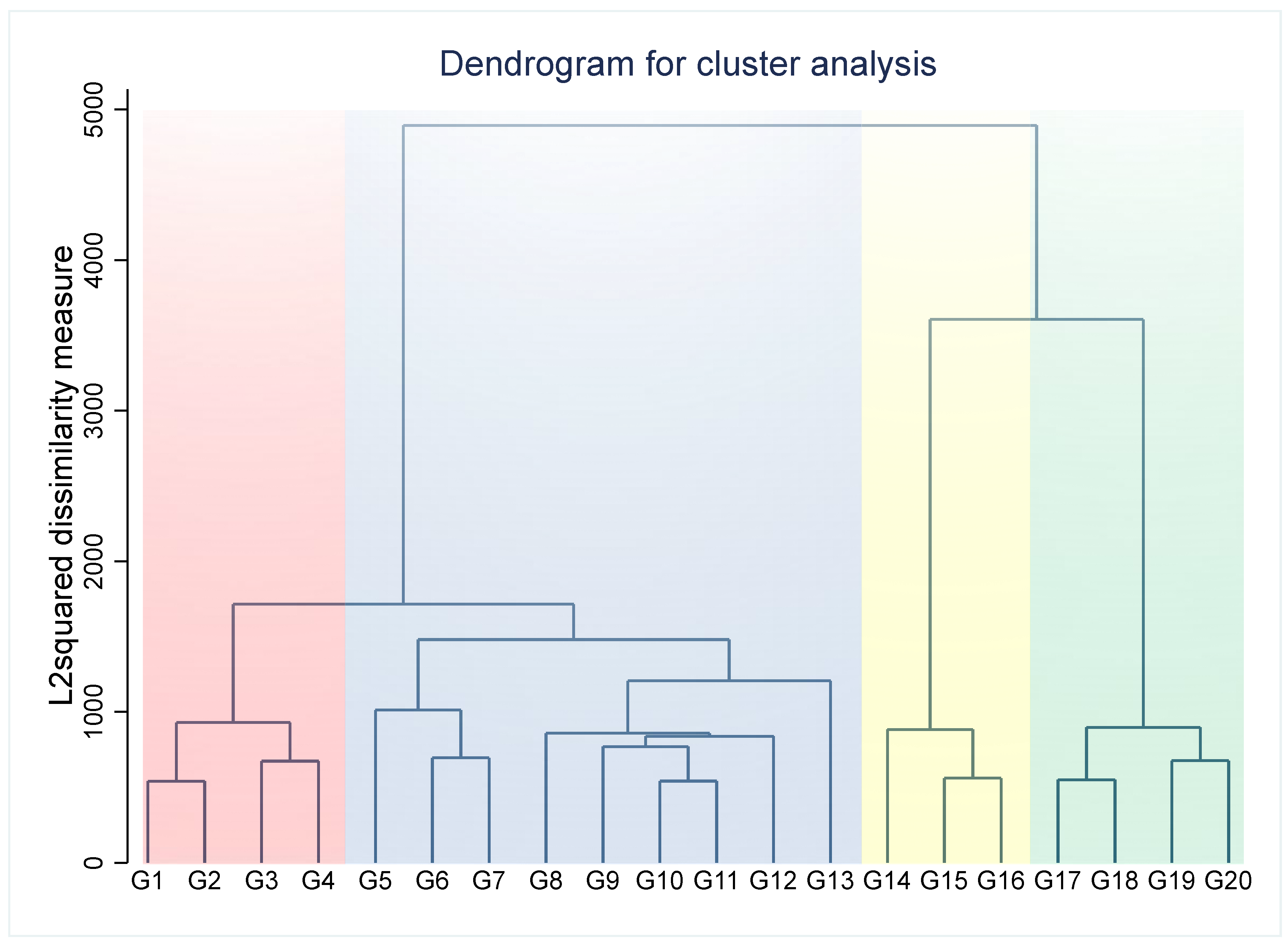
3. Results
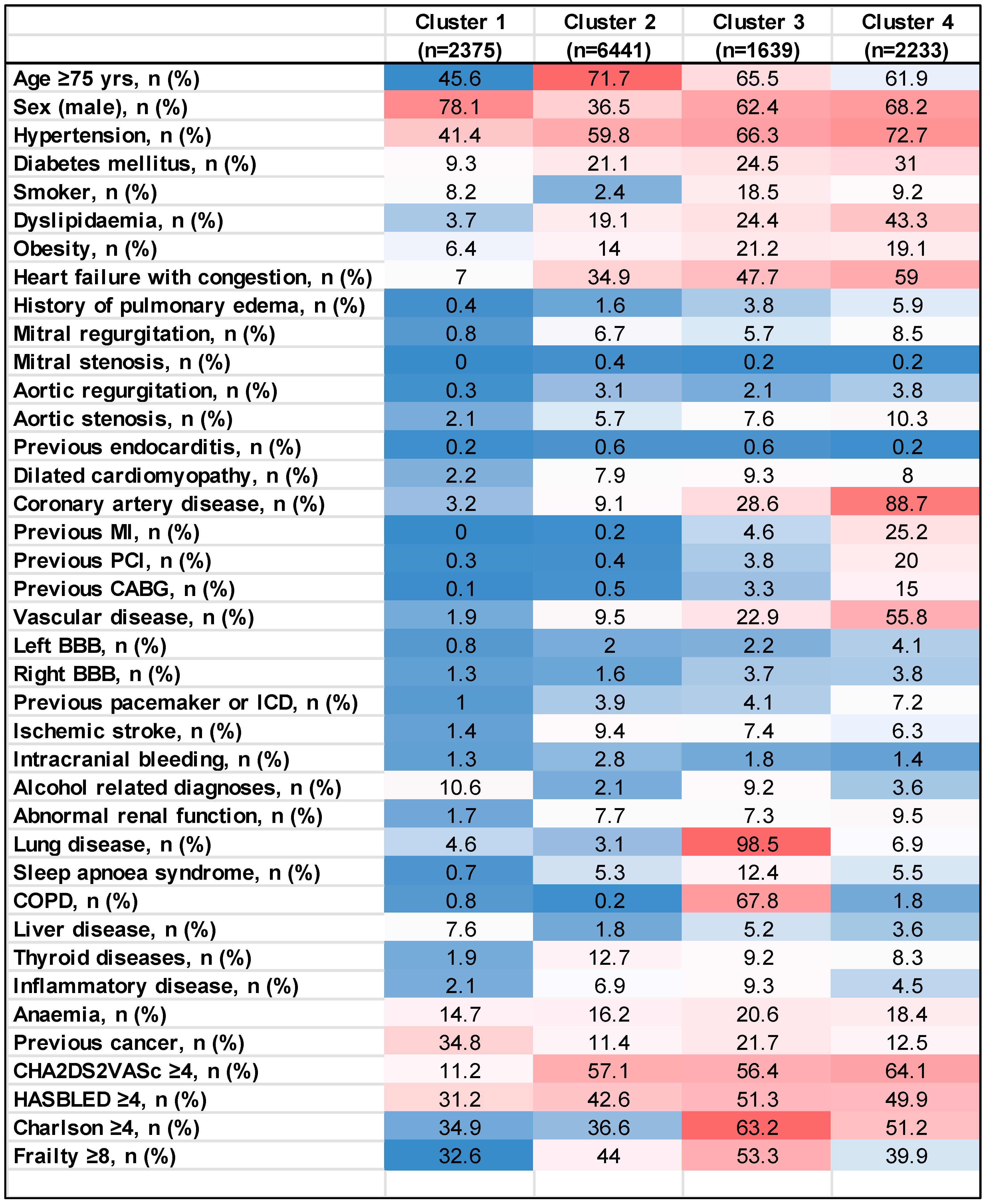
3.1. Baseline Characteristics
3.1.1. Cluster 1 (n = 2375)
3.1.2. Cluster 2 (n = 6441)
3.1.3. Cluster 3 (n = 1639)
3.1.4. Cluster 4 (n = 2233)
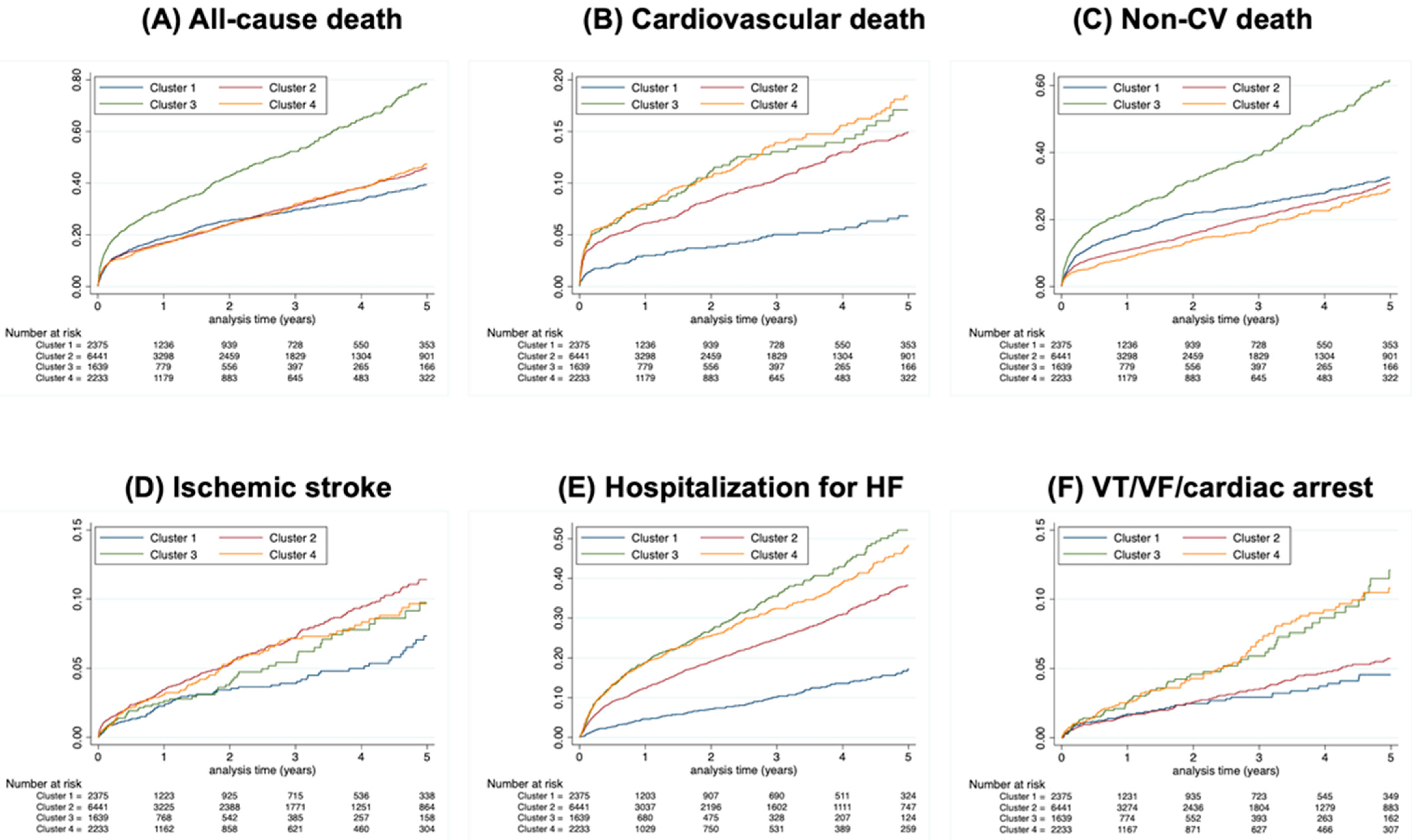
3.2. Major Adverse Cardiovascular Events
3.2.1. All-Cause Death
3.2.2. Cardiovascular Death
3.2.3. Non-Cardiovascular Death
3.2.4. Ischemic Stroke
3.2.5. Hospitalization for HF
3.2.6. VT/VF/CA
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): Developed by the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC), with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Endorsed by the European Stroke Organisation (ESO). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, J.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Proietti, M.; Vitolo, M.; Harrison, S.L.; Lane, D.A.; Fauchier, L.; Marin, F.; Nabauer, M.; Potpara, T.S.; Dan, G.A.; Boriani, G.; et al. Impact of clinical phenotypes on management and outcomes in European atrial fibrillation patients: A report from the ESC-EHRA EURObservational Research Programme in AF (EORP-AF) General Long-Term Registry. BMC Med. 2021, 19, 256. [Google Scholar] [CrossRef] [PubMed]
- Inohara, T.; Shrader, P.; Pieper, K.; Blanco, R.G.; Thomas, L.; Singer, D.E.; Freeman, J.V.; Allen, L.A.; Fonarow, G.C.; Gersh, B.; et al. Atrial Fibrillation Clinical Phenotypes: A Cluster Analysis. Circulation 2018, 136. [Google Scholar] [CrossRef]
- Vitolo, M.; Proietti, M.; Shantsila, A.; Boriani, G.; Lip, G.Y.H. Clinical Phenotype Classification of Atrial Fibrillation Patients Using Cluster Analysis and Associations with Trial-Adjudicated Outcomes. Biomedicines 2021, 9, 843. [Google Scholar] [CrossRef] [PubMed]
- Bisson, A.; Bodin, A.; Fauchier, G.; Herbert, J.; Angoulvant, D.; Ducluzeau, P.H.; Lip, G.Y.H.; Fauchier, L. Sex, age, type of diabetes and incidence of atrial fibrillation in patients with diabetes mellitus: A nationwide analysis. Cardiovasc. Diabetol. 2021, 20, 24. [Google Scholar] [CrossRef] [PubMed]
- Fauchier, L.; Clementy, N.; Pelade, C.; Collignon, C.; Nicolle, E.; Lip, G.Y. Patients with Ischemic Stroke and Incident Atrial Fibrillation: A Nationwide Cohort Study. Stroke 2015, 46, 2432–2437. [Google Scholar] [CrossRef] [PubMed]
- Mertz, V.; Cottin, Y.; Bentounes, S.A.; Pastier-Debeaumarché, J.; Didier, R.; Herbert, J.; Zeller, M.; Lip, G.Y.H.; Fauchier, L. Prognosis of Atrial Fibrillation with or without Comorbidities: Analysis of Younger Adults from a Nationwide Database. J. Clin. Med. 2022, 11, 1981. [Google Scholar] [CrossRef] [PubMed]
- Cottin, Y.; Ben Messaoud, B.M.; Monin, A.; Guilleminot, P.; Bisson, A.; Eicher, J.-C.; Bodin, A.; Herbert, J.; Juillière, Y.; Zeller, M.; et al. Temporal Relationship between Atrial Fibrillation and Heart Failure Development Analysis from a Nationwide Database. J. Clin. Med. 2021, 10, 5101. [Google Scholar] [CrossRef] [PubMed]
- Fauchier, L.; Bisson, A.; Bodin, A.; Herbert, J.; Angoulvant, D.; Danchin, N.; Cottin, Y. Outcomes in patients with acute myocardial infarction and new atrial fibrillation: A nationwide analysis. Clin. Res. Cardiol. 2021, 110, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Proietti, M.; Romiti, G.F.; Raparelli, V.; Diemberger, I.; Boriani, G.; Vecchia, L.A.D.; Bellelli, G.; Marzetti, E.; Lip, G.Y.; Cesari, M. Frailty prevalence and impact on outcomes in patients with atrial fibrillation: A systematic review and meta-analysis of 1,187,000 patients. Ageing Res. Rev. 2022, 79, 101652. [Google Scholar] [CrossRef] [PubMed]
- Shantha, G.; Pelosi, F.; Morady, F. Relationship between obstructive sleep apnoea and AF. Arrhythm. Electrophysiol. Rev. 2019, 8, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Proietti, M.; Laroche, C.; Drozd, M.; Vijgen, J.; Cozma, D.C.; Drozdz, J.; Maggioni, A.P.; Boriani, G.; Lip, G.Y. Impact of chronic obstructive pulmonary disease on prognosis in atrial fibrillation: A report from the EURObservational Research Programme Pilot Survey on Atrial Fibrillation (EORP-AF) General Registry. Am. Heart J. 2016, 181, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Terzano, C.; Romani, S.; Conti, V.; Paone, G.; Oriolo, F.; Vitarelli, A. Atrial fibrillation in the acute, hypercapnic exacerbations of COPD. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2908–2917. [Google Scholar] [PubMed]
- Goudis, C.A. Chronic obstructive pulmonary disease and atrial fibrillation: An unknown relationship. J. Cardiol. 2017, 69, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Marulanda-Londoño, E.; Chaturvedi, S. The Interplay between Obstructive Sleep Apnea and Atrial Fibrillation. Front. Neurol. 2017, 8, 668. [Google Scholar] [CrossRef] [PubMed]
- Parikh, R.R.; Norby, F.L.; Wang, W.; Thenappan, T.; Prins, K.W.; Hof, J.R.V.; Lutsey, P.L.; Solomon, S.D.; Shah, A.M.; Chen, L.Y. Association of Right Ventricular Afterload with Atrial Fibrillation Risk in Older Adults: The Atherosclerosis Risk in Communities Study. Chest 2022, 162, 884. [Google Scholar] [CrossRef]
- Chang, H.; Lo, L.; Lin, Y.; Chang, S.; Hu, Y.; Li, C.; Chao, T.; Chung, F.; LE Ha, T.; Singhal, R.; et al. Long-Term Outcome of Catheter Ablation in Patients with Atrial Fibrillation Originating from Nonpulmonary Vein Ectopy. J. Cardiovasc. Electrophysiol. 2013, 24, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Nalliah, C.J.; Wong, G.R.; Lee, G.; Voskoboinik, A.; Kee, K.; Goldin, J.; Watts, T.; Linz, D.; Parameswaran, R.; Sugumar, H.; et al. Impact of CPAP on the Atrial Fibrillation Substrate in Obstructive Sleep Apnea: The SLEEP-AF Study. JACC Clin. Electrophysiol. 2022, 8, 869–877. [Google Scholar] [CrossRef]
- Pastori, D.; Antonucci, E.; Milanese, A.; Menichelli, D.; Palareti, G.; Farcomeni, A.; Pignatelli, P. Clinical Phenotypes of Atrial Fibrillation and Mortality Risk—A Cluster Analysis from the Nationwide Italian START Registry. J. Pers. Med. 2022, 12, 785. [Google Scholar] [CrossRef]
- Inohara, T.; Piccini, J.P.; Mahaffey, K.W.; Kimura, T.; Katsumata, Y.; Tanimoto, K.; Inagawa, K.; Ikemura, N.; Ueda, I.; Fukuda, K.; et al. A Cluster Analysis of the Japanese Multicenter Outpatient Registry of Patients With Atrial Fibrillation. Am. J. Cardiol. 2019, 124, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Okaj, I.; Kaur, H.; Belley-Cote, E.P.; Wang, J.; Oraii, A.; Benz, A.P.; Johnson, L.S.B.; Young, J.; Wong, J.A.; et al. Sodium-glucose co-transporter inhibitors and atrial fibrillation: A systematic review and meta-analysis of randomized controlled trials. J. Am. Heart Assoc. 2021, 10, e022222. [Google Scholar] [CrossRef] [PubMed]
- Fatima, K.; Suri, A.; Rija, A.; Kalim, S.; Javaid, S.; Arif, Z.; Abedin, M.F.E.; Raza, Y.; Kalim, N.; Azam, F.; et al. The Effect of Sodium-Glucose Co-Transporter 2 Inhibitors on Stroke and Atrial Fibrillation: A Systematic Review and Meta-Analysis. Curr. Probl. Cardiol. 2023, 48, 101582. [Google Scholar] [CrossRef] [PubMed]
- Sfairopoulos, D.; Zhang, N.; Wang, Y.; Chen, Z.; Letsas, K.P.; Tse, G.; Li, G.; Lip, G.Y.H.; Liu, T. Association between sodium-glucose cotransporter-2 inhibitors and risk of sudden cardiac death or ventricular arrhythmias: A meta-analysis of randomized controlled trials. Europace 2022, 24, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, A.M.; Rivera-Caravaca, J.M.; Underhill, P.; Fauchier, L.; Lip, G.Y.H. Incident heart failure, arrhythmias and cardiovascular outcomes with sodium-glucose cotransporter 2 (SGLT2) inhibitor use in patients with diabetes: Insights from a global federated electronic medical record database. Diabetes Obes. Metab. 2023, 25, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Rådholm, K.; Figtree, G.; Perkovic, V.; Solomon, S.D.; Mahaffey, K.W.; De Zeeuw, D.; Fulcher, G.; Barrett, T.D.; Shaw, W.; Desai, M.; et al. Canagliflozin and heart failure in type 2 diabetes mellitus: Results from the CANVAS program. Circulation 2018, 138, 458–468. [Google Scholar] [CrossRef]
- Kosiborod, M.N.; Petrie, M.C.; Borlaug, B.A.; Butler, J.; Davies, M.J.; Hovingh, G.K.; Kitzman, D.W.; Møller, D.V.; Treppendahl, M.B.; Verma, S.; et al. Semaglutide in Patients with Obesity-Related Heart Failure and Type 2 Diabetes. N. Engl. J. Med. 2024, 390, 1394–1407. [Google Scholar] [CrossRef]
- Lincoff, A.M.; Brown-Frandsen, K.; Colhoun, H.M.; Deanfield, J.; Emerson, S.S.; Esbjerg, S.; Hardt-Lindberg, S.; Hovingh, G.K.; Kahn, S.E.; Kushner, R.F.; et al. Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes. N. Engl. J. Med. 2023, 389, 2221–2232. [Google Scholar] [CrossRef] [PubMed]
- Kosiborod, M.N.; Abildstrøm, S.Z.; Borlaug, B.A.; Butler, J.; Rasmussen, S.; Davies, M.; Hovingh, G.K.; Kitzman, D.W.; Lindegaard, M.L.; Møller, D.V.; et al. Semaglutide in Patients with Heart Failure with Preserved Ejection Fraction and Obesity. N. Engl. J. Med. 2023, 389, 1069–1084. [Google Scholar] [CrossRef] [PubMed]
- Bethel, M.A.; Patel, R.A.; Merrill, P.; Lokhnygina, Y.; Buse, J.B.; Mentz, R.J.; Pagidipati, N.J.; Chan, J.C.; Gustavson, S.M.; Iqbal, N.; et al. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: A meta-analysis. Lancet Diabetes Endocrinol. 2018, 6, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Parab, P.; Chaudhary, P.; Mukhtar, S.; Moradi, A.; Kodali, A.; Okoye, C.; Klein, D.; Mohamoud, I.; O Olanisa, O.; Hamid, P. Role of Glucagon-Like Peptide-1 (GLP-1) Receptor Agonists in Cardiovascular Risk Management in Patients with Type 2 Diabetes Mellitus: A Systematic Review. Cureus 2023, 15, e45487. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Chen, J.; Huang, P.; Wu, C.; Wang, Y.; Hwang, J.; Tsai, C. Sodium-Glucose Cotransporter-2 Inhibitor Prevents Stroke in Patients with Diabetes and Atrial Fibrillation. J. Am. Heart Assoc. 2023, 12, e027764. [Google Scholar] [CrossRef]
- Jang, J.; Park, S.; Kim, S.; Kim, S.-H.; Oh, Y.-S.; Sa, Y.K.; Hwang, Y.; Jang, S.-W.; Ihm, S.-H.; Choi, Y. Clinical outcomes with the use of sodium-glucose cotransporter-2 inhibitors in patients with atrial fibrillation and type 2 diabetes mellitus: A multi-centre, real-world cohort study. Eur. J. Prev. Cardiol. 2024, 31, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Fichadiya, A.; Quinn, A.; Au, F.; Campbell, D.; Lau, D.; Ronksley, P.; Beall, R.; Campbell, D.J.T.; Wilton, S.B.; Chew, D.S. Association between sodium–glucose cotransporter-2 inhibitors and arrhythmic outcomes in patients with diabetes and pre-existing atrial fibrillation. Europace 2024, 26, euae054. [Google Scholar] [CrossRef]
- Chen, Y.; Chang, H.; Lin, Y.; Chien, K.; Hsieh, Y.; Chung, F.; Lin, C.; Lip, G.Y.H.; Chen, S. The impact of sodium-glucose co-transporter-2 inhibitors on dementia and cardiovascular events in diabetic patients with atrial fibrillation. Diabetes Metab. Res. Rev. 2024, 40, e3775. [Google Scholar] [CrossRef]
- Wei, J.; Wang, R.; Ye, H.; Wang, Y.; Wang, L.; Zhang, X. Effects of GLP-1 receptor agonists on arrhythmias and its subtypes in patients with type 2 diabetes: A systematic review and meta-analysis. Front. Endocrinol. 2022, 13, 910256. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K. Conceptual Models of Frailty: Accumulation of Deficits. Can. J. Cardiol. 2016, 32, 1046–1050. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.S.; Siddiqi, T.J.; Anker, S.D.; Bakris, G.L.; Bhatt, D.L.; Filippatos, G.; Fonarow, G.C.; Greene, S.J.; Januzzi, J.L.; Khan, M.S.; et al. Effect of SGLT2 Inhibitors on Cardiovascular Outcomes Across Various Patient Populations. J. Am. Coll. Cardiol. 2023, 81, 2377–2387. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadawi, M.A.; Aslam, F.M.; Tao, M.; Alsaiqali, M.; Almasry, I.O.; Fan, R.; Rashba, E.J.; Singh, A. Effects of GLP-1 Agonists on mortality and arrhythmias in patients with Type II diabetes. Int. J. Cardiol. Heart Vasc. 2023, 47, 101218. [Google Scholar] [CrossRef] [PubMed]
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | p | Total | |
|---|---|---|---|---|---|---|
| (n = 2375) | (n = 6441) | (n = 1639) | (n = 2233) | (n = 12,688) | ||
| Age (years), mean ± SD | 71.6 ± 13.7 | 78.9 ± 11.7 | 77.9 ± 10.4 | 76.9 ± 10.7 | <0.0001 | 77.1 ± 12.1 |
| Sex (male), n (%) | 1855 (78.1) | 2353 (36.5) | 1022 (62.4) | 1523 (68.2) | <0.0001 | 6753 (53.2) |
| Hypertension, n (%) | 984 (41.4) | 3850 (59.8) | 1086 (66.3) | 1623 (72.7) | <0.0001 | 7543 (59.5) |
| Diabetes mellitus, n (%) | 220 (9.3) | 1360 (21.1) | 401 (24.5) | 692 (31.0) | <0.0001 | 2673 (21.1) |
| Smoker, n (%) | 195 (8.2) | 155 (2.4) | 303 (18.5) | 206 (9.2) | <0.0001 | 859 (6.8) |
| Dyslipidemia, n (%) | 87 (3.7) | 1233 (19.1) | 400 (24.4) | 966 (43.3) | <0.0001 | 2686 (21.2) |
| Obesity, n (%) | 151 (6.4) | 903 (14.0) | 348 (21.2) | 426 (19.1) | <0.0001 | 1828 (14.4) |
| Heart failure with congestion, n (%) | 167 (7.0) | 2247 (34.9) | 781 (47.7) | 1317 (59.0) | <0.0001 | 4512 (35.6) |
| History of pulmonary edema, n (%) | 10 (0.4) | 101 (1.6) | 62 (3.8) | 132 (5.9) | <0.0001 | 305 (2.4) |
| Mitral regurgitation, n (%) | 18 (0.8) | 434 (6.7) | 93 (5.7) | 189 (8.5) | <0.0001 | 734 (5.8) |
| Mitral stenosis, n (%) | 1 (0.0) | 23 (0.4) | 3 (0.2) | 4 (0.2) | 0.02 | 31 (0.2) |
| Aortic regurgitation, n (%) | 8 (0.3) | 197 (3.1) | 34 (2.1) | 84 (3.8) | <0.0001 | 323 (2.5) |
| Aortic stenosis, n (%) | 49 (2.1) | 368 (5.7) | 125 (7.6) | 231 (10.3) | <0.0001 | 773 (6.1) |
| Previous endocarditis, n (%) | 4 (0.2) | 37 (0.6) | 10 (0.6) | 4 (0.2) | 0.003 | 55 (0.4) |
| Dilated cardiomyopathy, n (%) | 53 (2.2) | 512 (7.9) | 153 (9.3) | 178 (8.0) | <0.0001 | 896 (7.1) |
| Coronary artery disease, n (%) | 75 (3.2) | 583 (9.1) | 468 (28.6) | 1981 (88.7) | <0.0001 | 3107 (24.5) |
| Previous MI, n (%) | 0 (0.0) | 12 (0.2) | 76 (4.6) | 563 (25.2) | <0.0001 | 651 (5.1) |
| Previous PCI, n (%) | 7 (0.3) | 25 (0.4) | 63 (3.8) | 447 (20.0) | <0.0001 | 542 (4.3) |
| Previous CABG, n (%) | 3 (0.1) | 30 (0.5) | 54 (3.3) | 334 (15.0) | <0.0001 | 421 (3.3) |
| Vascular disease, n (%) | 46 (1.9) | 611 (9.5) | 376 (22.9) | 1245 (55.8) | <0.0001 | 2278 (18.0) |
| Left BBB, n (%) | 20 (0.8) | 126 (2.0) | 36 (2.2) | 91 (4.1) | <0.0001 | 273 (2.2) |
| Right BBB, n (%) | 31 (1.3) | 105 (1.6) | 60 (3.7) | 84 (3.8) | <0.0001 | 280 (2.2) |
| Previous pacemaker or ICD, n (%) | 24 (1.0) | 250 (3.9) | 68 (4.1) | 160 (7.2) | <0.0001 | 502 (4.0) |
| Ischemic stroke, n (%) | 34 (1.4) | 603 (9.4) | 121 (7.4) | 140 (6.3) | <0.0001 | 898 (7.1) |
| Intracranial bleeding, n (%) | 30 (1.3) | 181 (2.8) | 30 (1.8) | 31 (1.4) | <0.0001 | 272 (2.1) |
| Alcohol related diagnoses, n (%) | 252 (10.6) | 138 (2.1) | 151 (9.2) | 80 (3.6) | <0.0001 | 621 (4.9) |
| Abnormal renal function, n (%) | 40 (1.7) | 493 (7.7) | 120 (7.3) | 212 (9.5) | <0.0001 | 865 (6.8) |
| Lung disease, n (%) | 109 (4.6) | 198 (3.1) | 1614 (98.5) | 154 (6.9) | <0.0001 | 2075 (16.4) |
| Sleep apnea syndrome, n (%) | 16 (0.7) | 339 (5.3) | 203 (12.4) | 123 (5.5) | <0.0001 | 681 (5.4) |
| COPD, n (%) | 20 (0.8) | 16 (0.2) | 1112 (67.8) | 40 (1.8) | <0.0001 | 1188 (9.4) |
| Liver disease, n (%) | 181 (7.6) | 117 (1.8) | 85 (5.2) | 81 (3.6) | <0.0001 | 464 (3.7) |
| Thyroid diseases, n (%) | 44 (1.9) | 815 (12.7) | 150 (9.2) | 185 (8.3) | <0.0001 | 1194 (9.4) |
| Inflammatory disease, n (%) | 49 (2.1) | 444 (6.9) | 153 (9.3) | 100 (4.5) | <0.0001 | 746 (5.9) |
| Anemia, n (%) | 350 (14.7) | 1041 (16.2) | 338 (20.6) | 410 (18.4) | <0.0001 | 2139 (16.9) |
| Previous cancer, n (%) | 826 (34.8) | 737 (11.4) | 356 (21.7) | 279 (12.5) | <0.0001 | 2198 (17.3) |
| CHA2DS2VASc score, mean ±SD | 2.0 ± 1.3 | 3.7 ± 1.4 | 3.7 ± 1.6 | 4.1 ± 1.7 | <0.0001 | 3.4 ± 1.6 |
| HASBLED score, mean ± SD | 2.0 ± 1.3 | 2.3 ± 1.1 | 2.6 ± 1.1 | 2.6 ± 1.2 | <0.0001 | 2.4 ± 1.2 |
| Charlson index, mean ± SD | 2.9 ± 2.9 | 3.0 ± 2.5 | 4.7 ± 2.7 | 4.1 ± 3.0 | <0.0001 | 3.4 ± 2.8 |
| Frailty index, mean ± SD | 7.0 ± 8.2 | 9.3 ± 9.2 | 10.7 ± 8.6 | 8.7 ± 8.9 | <0.0001 | 9.0 ± 9.0 |
| Cluster 1 | ARD | Cluster 2 | ARD | Cluster 3 | ARD | Cluster 4 | ARD | |
|---|---|---|---|---|---|---|---|---|
| (n = 2375) | % | (n = 6441) | % | (n = 1639) | % | (n = 2233) | % | |
| All-cause death | 520 (10.4) | Ref | 1459 (11.3) | 0.9 | 589 (20.7) | 10.3 | 522 (11.2) | 0.8 |
| Cardiovascular death | 88 (1.8) | Ref | 496 (3.8) | 2.0 | 146 (5.1) | 3.3 | 220 (4.7) | 2.9 |
| Non-cardiovascular death | 432 (8.6) | Ref | 963 (7.4) | −1.2 | 443 (15.5) | 6.9 | 302 (6.5) | −2.1 |
| Ischemic stroke | 79 (1.6) | Ref | 329 (2.6) | 1.0 | 58 (2.1) | 0.5 | 105 (2.3) | 0.7 |
| Hospitalization for HF | 180 (3.8) | Ref | 1037 (9.0) | 5.2 | 314 (12.9) | 9.1 | 470 (11.9) | 8.1 |
| VT/VF/CA | 55 (1.1) | Ref | 162 (1.3) | 0.2 | 67 (2.4) | 1.3 | 99 (2.2) | 1.1 |
| Total | 1354 | 4446 | 1617 | 1718 | ||||
| Univariate | Multivariable (Model 1) | Multivariable (Model 2) | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| All-cause death | ||||||
| Cluster 2 (vs. Cluster 1) | 1.07 (0.97–1.18) | 0.19 | 0.88 (0.79–0.98) | 0.02 | 0.71 (0.64–0.79) | <0.0001 |
| Cluster 3 (vs. Cluster 1) | 1.86 (1.65–2.09) | <0.0001 | 1.55 (1.37–1.74) | <0.0001 | 1.24 (1.09–1.41) | 0.001 |
| Cluster 4 (vs. Cluster 1) | 1.07 (0.95–1.21) | 0.26 | 0.93 (0.82–1.05) | 0.23 | 0.64 (0.56–0.73) | <0.0001 |
| Cardiovascular death | ||||||
| Cluster 2 (vs. Cluster 1) | 2.14 (1.70–2.68) | <0.0001 | 1.70 (1.34–2.15) | <0.0001 | 1.24 (0.98–1.58) | 0.07 |
| Cluster 3 (vs. Cluster 1) | 2.67 (2.05–3.48) | <0.0001 | 2.17 (1.66–2.83) | <0.0001 | 1.56 (1.19–2.06) | 0.002 |
| Cluster 4 (vs. Cluster 1) | 2.66 (2.08–3.41) | <0.0001 | 2.26 (1.76–2.90) | <0.0001 | 1.34 (1.03–1.75) | 0.03 |
| Non-cardiovascular death | ||||||
| Cluster 2 (vs. Cluster 1) | 0.85 (0.76–0.95) | 0.006 | 0.71 (0.63–0.81) | <0.0001 | 0.60 (0.53–0.68) | <0.0001 |
| Cluster 3 (vs. Cluster 1) | 1.69 (1.48–1.93) | <0.0001 | 1.43 (1.25–1.63) | <0.0001 | 1.20 (1.04–1.38) | 0.01 |
| Cluster 4 (vs. Cluster 1) | 0.75 (0.65–0.87) | <0.0001 | 0.65 (0.56–0.76) | <0.0001 | 0.48 (0.41–0.57) | <0.0001 |
| Ischemic stroke | ||||||
| Cluster 2 (vs. Cluster 1) | 1.61 (1.26–2.06) | <0.0001 | 1.41 (1.09–1.83) | 0.009 | 0.99 (0.76–1.29) | 0.93 |
| Cluster 3 (vs. Cluster 1) | 1.25 (0.89–1.75) | 0.20 | 1.09 (0.78–1.54) | 0.61 | 0.78 (0.55–1.11) | 0.17 |
| Cluster 4 (vs. Cluster 1) | 1.44 (1.07–1.92) | 0.02 | 1.29 (0.96–1.73) | 0.09 | 0.79 (0.57–1.08) | 0.14 |
| Hospitalization for HF | ||||||
| Cluster 2 (vs. Cluster 1) | 2.35 (2.01–2.76) | <0.0001 | 0.99 (0.76–1.29) | <0.0001 | 1.48 (1.26–1.75) | <0.0001 |
| Cluster 3 (vs. Cluster 1) | 3.24 (2.70–3.90) | <0.0001 | 2.69 (2.23–3.24) | <0.0001 | 2.07 (1.71–2.50) | <0.0001 |
| Cluster 4 (vs. Cluster 1) | 3.09 (2.60–3.67) | <0.0001 | 2.68 (2.26–3.19) | <0.0001 | 1.76 (1.46–2.11) | <0.0001 |
| VT/VF/CA | ||||||
| Cluster 2 (vs. Cluster 1) | 1.14 (0.84–1.55) | 0.39 | 1.29 (0.94–1.78) | 0.11 | 0.94 (0.68–1.31) | 0.72 |
| Cluster 3 (vs. Cluster 1) | 2.09 (1.46–2.99) | <0.0001 | 2.16 (1.50–3.09) | <0.0001 | 1.74 (1.20–2.53) | 0.004 |
| Cluster 4 (vs. Cluster 1) | 1.97 (1.41–2.73) | <0.0001 | 1.98 (1.42–2.76) | <0.0001 | 1.56 (1.09–2.23) | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fawzy, A.M.; Bisson, A.; Lochon, L.; Lenormand, T.; Lip, G.Y.H.; Fauchier, L. Outcomes in Atrial Fibrillation Patients with Different Clinical Phenotypes: Insights from the French Population. J. Clin. Med. 2025, 14, 1044. https://doi.org/10.3390/jcm14041044
Fawzy AM, Bisson A, Lochon L, Lenormand T, Lip GYH, Fauchier L. Outcomes in Atrial Fibrillation Patients with Different Clinical Phenotypes: Insights from the French Population. Journal of Clinical Medicine. 2025; 14(4):1044. https://doi.org/10.3390/jcm14041044
Chicago/Turabian StyleFawzy, Ameenathul M., Arnaud Bisson, Lisa Lochon, Thibault Lenormand, Gregory Y. H. Lip, and Laurent Fauchier. 2025. "Outcomes in Atrial Fibrillation Patients with Different Clinical Phenotypes: Insights from the French Population" Journal of Clinical Medicine 14, no. 4: 1044. https://doi.org/10.3390/jcm14041044
APA StyleFawzy, A. M., Bisson, A., Lochon, L., Lenormand, T., Lip, G. Y. H., & Fauchier, L. (2025). Outcomes in Atrial Fibrillation Patients with Different Clinical Phenotypes: Insights from the French Population. Journal of Clinical Medicine, 14(4), 1044. https://doi.org/10.3390/jcm14041044









