The Role of Trans-Oesophageal Echocardiography in the Interventional Cardiology of Adult Congenital Heart Diseases
Abstract
:1. Introduction
2. Atrial Septal Defect Closure
3. Ventricular Septal Defect Closure
4. Leak Closure of Atrial Switch Operation Baffles
5. Fontan Conduit Fenestration Management
6. Ruptured Sinus of Valsalva Closure
- -
- Type I, between the right coronary sinus and the distal right ventricle outflow tract (RVOT);
- -
- Type II, between the right coronary sinus and the proximal RVOT;
- -
- Type III, between the right coronary sinus and either the inlet of the right ventricle (IIIb or IIIv) or the right atrium (IIIa);
- -
- Type IV, between the no-coronary sinus and the right atrium.
7. Miscellaneous
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ben, L.; Zhang, Y.; Wang, Y.; Xing, W.; Cai, J.; Han, Y. Efficacy and Safety of Transthoracic Versus Transesophageal Echocardiography for Monitoring Closure of Atrial or Ventricular Septal Defects: A Systematic Review and Meta-Analysis. Echocardiography 2024, 41, e15955. [Google Scholar] [CrossRef] [PubMed]
- Binder, M.S.; Binder, I.E.; Foerst, J.R. Limitations of Percutaneous Closure of a Complex Secundum Atrial Septal Defect. CASE 2022, 6, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.C.; Gelman, J.S.; Harper, R.W. Echocardiologist’s role in the deployment of the Amplatzer septal occlude device in adults. J. Am. Soc. Echocardiogr. 2001, 14, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Crawford, G.B.; Brindis, R.G.; Krucoff, M.W.; Mansalis, B.P.; Carroll, J.D. Percutaneous atrial septal occluder devices and cardiac erosion: A review of the literature. Catheter. Cardiovasc. Interv. 2012, 80, 157–167. [Google Scholar] [CrossRef]
- Takaya, Y.; Akagi, T.; Nakagawa, K.; Nakayama, R.; Miki, T.; Toh, N.; Ito, H. Morphological assessments of deficient posterior-inferior rim for transcatheter closure of atrial septal defect. Catheter. Cardiovasc. Interv. 2021, 97, 135–141. [Google Scholar] [CrossRef]
- McElhinney, D.B.; Quartermain, M.D.; Kenny, D.; Alboliras, E.; Amin, Z. Relative Risk Factors for Cardiac Erosion Following Transcatheter Closure of Atrial Septal Defects: A Case-Control Study. Circulation 2016, 133, 1738–1746. [Google Scholar] [CrossRef]
- Jung, S.Y.; Choi, J.Y. Transcatheter closure of atrial septal defect: Principles and available devices. J. Thorac. Dis. 2018, 10 (Suppl. S24), S2909–S2922. [Google Scholar] [CrossRef]
- Deng, B.; Chen, K.; Huang, T.; Wei, Y.; Liu, Y.; Yang, L.; Qiu, Q.; Zheng, S.; Lv, H.; Wang, P.; et al. Assessment of atrial septal defect using 2D or real-time 3D transesophageal echocardiography and outcomes following transcatheter closure. Ann. Transl. Med. 2021, 9, 1309. [Google Scholar] [CrossRef]
- Farhaj, Z.; Hongxin, L.; Wenbin, G.; Zhang, W.L.; Liang, F.; Zhang, H.Z.; Yuan, G.D.; Zou, C.W. Device closure of diverse layout of multi-hole secundum atrial septal defect: Different techniques and long-term follow-up. J. Cardiothorac. Surg. 2019, 14, 130. [Google Scholar] [CrossRef]
- Mahmoud, H.T.; Gaio, G.; Giordano, M.; Pizzuto, A.; Cuman, M.; Asklany, H.T.; Palladino, M.T.; Russo, M.G.; Santoro, G. Transcatheter closure of fenestrated atrial septal aneurysm: Feasibility and long-term results. J. Cardiovasc. Med. 2022, 23, 49–59. [Google Scholar] [CrossRef]
- Szkutnik, M.; Masura, J.; Bialkowski, J.; Gavora, P.; Banaszak, P.; Kusa, J.; Zembala, M. Transcatheter closure of double atrial septal defects with a single Amplatzer device. Catheter. Cardiovasc. Interv. 2004, 61, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Pearson, A.C.; Nagelhout, D.; Castello, R.; Gomez, C.R.; Labovitz, A.J. Atrial septal aneurysm and stroke: A transesophageal echocardiographic study. J. Am. Coll. Cardiol. 1991, 18, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Reyes, A.; Chan, S.; Lazar, E.J.; Bandlamudi, K.; Narla, V.; Ong, K. Atrial septal aneurysm: A new classification in two hundred five adults. J. Am. Soc. Echocardiogr. 1997, 10, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Takaya, Y.; Akagi, T.; Nakagawa, K.; Nakayama, R.; Miki, T.; Watanabe, N.; Toh, N.; Ito, H. Clinical Significance of Septal Malalignment for Transcatheter Closure of Atrial Septal Defect. J. Interv. Cardiol. 2020, 2020, 6090612. [Google Scholar] [CrossRef]
- Kijima, Y.; Akagi, T.; Nakagawa, K.; Promphan, W.; Toh, N.; Nakamura, K.; Sano, S.; Ito, H. Cardiac erosion after catheter closure of atrial septal defect: Septal malalignment may be a novel risk factor for erosion. J. Cardiol. Cases. 2014, 9, 134–137. [Google Scholar] [CrossRef]
- Vettukattil, J.J.; Ahmed, Z.; Salmon, A.P.; Mohun, T.; Anderson, R.H. Defects in the oval fossa: Morphologic variations and impact on transcatheter closure. J. Am. Soc. Echocardiogr. 2013, 26, 192–199. [Google Scholar] [CrossRef]
- Montella, A.P.; Petrarcone, C.G.; Giordano, M.; D’Andrea, C.; Oppido, G. Intracardiac total anomalous pulmonary venous return or septum primum malposition? Complete repair in left atrial isomerism. Multimed. Man. Cardiothorac. Surg. 2023, 2023. [Google Scholar] [CrossRef]
- Roberson, D.A.; Javois, A.J.; Cui, W.; Madronero, L.F.; Cuneo, B.F.; Muangmingsuk, S. Double atrial septum with persistent inter-atrial space: Echocardiographic features of a rare atrial septal malformation. J. Am. Soc. Echocardiogr. 2006, 19, 1175–1181. [Google Scholar] [CrossRef]
- Butera, G.; Montinaro, A.; Carminati, M. The “pull-push” technique to deal with a redundant eustachian valve interfering with placement of a PFO occluder. Catheter. Cardiovasc. Interv. 2006, 68, 961–964. [Google Scholar] [CrossRef]
- Hascoet, S.; Hadeed, K.; Marchal, P.; Dulac, Y.; Alacoque, X.; Heitz, F.; Acar, P. The relation between atrial septal defect shape, diameter, and area using three-dimensional transoesophageal echocardiography and balloon sizing during percutaneous closure in children. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 747–755. [Google Scholar] [CrossRef]
- Alsaileek, A.A.; Omran, A.; Godman, M.; Najm, H.K. Echocardiographic visualization of laceration of atrial septum during balloon sizing of atrial septal defect. Eur. J. Echocardiogr. 2007, 8, 155–157. [Google Scholar] [CrossRef]
- Sivasankaran, S.; Harikrishnan, S.; Narayanan, N.; Jaganmohan, T. Laceration of atrial septum during balloon sizing of atrial septal defect. Eur. J. Echocardiogr. 2007, 8, 89–90. [Google Scholar] [CrossRef] [PubMed]
- Narimani, S.; Ayati, A.; Tayebi, A.; Jalai, A.; Amirsardari, Z.; Sahebjam, M.; Zoroufian, A. Three-dimensional transesophageal echocardiography measurements of ASD sizing parameters in comparison to balloon sizing method in percutaneous ASD closure. Echocardiography 2024, 41, e15822. [Google Scholar] [CrossRef] [PubMed]
- Bramlet, M.T.; Hoyer, M.H. Single pediatric center experience with multiple device implantation for complex secundum atrial septal defects. Catheter. Cardiovasc. Interv. 2008, 72, 531–537. [Google Scholar] [CrossRef]
- Butera, G.; Romagnoli, E.; Saliba, Z.; Chessa, M.; Sangiorgi, G.; Giamberti, A.; Cappato, R.; Bussadori, C.; Abella, R.; Pelissero, G.; et al. Percutaneous closure of multiple defects of the atrial septum: Procedural results and long-term follow-up. Catheter. Cardiovasc. Interv. 2010, 76, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Fang, F.; Qiu, X.; Xu, N.; Wang, Y.; Ouyang, W.B.; Zhang, F.W.; Hu, H.B.; Pan, X.B. Personalized Three-Dimensional Printing and Echoguided Procedure Facilitate Single Device Closure for Multiple Atrial Septal Defects. J. Interv. Cardiol. 2020, 2020, 1751025. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.; Hegde, S.; El-Said, H.; Beekman, R., 3rd; Benson, L.; Bergersen, L.; Holzer, R.; Jenkins, K.; Ringel, R.; Rome, J.; et al. Transcatheter device closure of atrial septal defects: A safety review. JACC Cardiovasc. Interv. 2013, 6, 433–442. [Google Scholar] [CrossRef]
- Lopez, L.; Houyel, L.; Colan, S.D.; Anderson, R.H.; Béland, M.J.; Aiello, V.D.; Bailliard, F.; Cohen, M.S.; Jacobs, J.P.; Kurosawa, H.; et al. Classification of Ventricular Septal Defects for the Eleventh Iteration of the International Classification of Diseases-Striving for Consensus: A Report From the International Society for Nomenclature of Paediatric and Congenital Heart Disease. Ann. Thorac. Surg. 2018, 106, 1578–1589. [Google Scholar] [CrossRef]
- Morray, B.H. Ventricular Septal Defect Closure Devices, Techniques, and Outcomes. Interv. Cardiol. Clin. 2019, 8, 1–10. [Google Scholar] [CrossRef]
- Carminati, M.; Butera, G.; Chessa, M.; De Giovanni, J.; Fisher, G.; Gewillig, M.; Peuster, M.; Piechaud, J.F.; Santoro, G.; Sievert, H.; et al. Transcatheter closure of congenital ventricular septal defects: Results of the European Registry. Eur. Heart J. 2007, 28, 2361–2368. [Google Scholar] [CrossRef]
- Hsu, J.H.; Wu, J.R.; Dai, Z.K.; Lee, M.H. Real-time three-dimensional echocardiography provides novel and useful anatomic insights of perimembranous ventricular septal aneurysm. Int. J. Cardiol. 2007, 118, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Bant, A.; Dominguez, C.; Glazier, E.; Saririan, M.; Kaufman, J.; Breburda, C.S. Three-dimensional echocardiographic assessment of a serpiginous ventricular septal defect. J. Am. Soc. Echocardiogr. 2008, 21, e5–e6. [Google Scholar] [CrossRef] [PubMed]
- Sugita, T.; Ueda, Y.; Matsumoto, M.; Ogino, H.; Sakakibara, Y.; Matsuyama, K. Repeated procedure after radical surgery for tetralogy of Fallot. Ann. Thorac. Surg. 2000, 70, 1507–1510. [Google Scholar] [CrossRef] [PubMed]
- Egbe, A.C.; Poterucha, J.T.; Rihal, C.S.; Taggart, N.W.; Cetta, F.; Cabalka, A.K.; Pollak, P.M.; Reeder, G.S.; Hagler, D.J. Transcatheter closure of postmyocardial infarction, iatrogenic, and postoperative ventricular septal defects: The Mayo Clinic experience. Catheter. Cardiovasc. Interv. 2015, 86, 1264–1270. [Google Scholar] [CrossRef]
- Halpern, D.G.; Perk, G.; Ruiz, C.; Marino, N.; Kronzon, I. Percutaneous closure of a post-myocardial infarction ventricular septal defect guided by real-time three-dimensional echocardiography. Eur. J. Echocardiogr. 2009, 10, 569–571. [Google Scholar] [CrossRef]
- Kafes, H.; Ozeke, O.; Demirkan, B.; Acar, B.; Aysenur Ekizler, F.; Karabulut, O.; Can Konte, H.; Golbasi, Z.; Tufekcioglu, O.; Lutfi Kisacik, H. Flail Tricuspid Leaflet During the Percutaneous Closure of Post-Myocardial Infarction Ventricular Septal Defect. CASE 2017, 1, 207–209. [Google Scholar] [CrossRef]
- van den Bosch, A.E.; Ten Harkel, D.J.; McGhie, J.S.; Roos-Hesselink, J.W.; Simoons, M.L.; Bogers, A.J.; Meijboom, F.J. Feasibility and accuracy of real-time 3-dimensional echocardiographic assessment of ventricular septal defects. J. Am. Soc. Echocardiogr. 2006, 19, 7–13. [Google Scholar] [CrossRef]
- Baldasare, M.D.; Polyakov, M.; Laub, G.W.; Costic, J.T.; McCormick, D.J.; Goldberg, S. Percutaneous repair of post-myocardial infarction ventricular septal defect: Current approaches and future perspectives. Tex. Heart Inst. J. 2014, 41, 613–619. [Google Scholar] [CrossRef]
- Mahimarangaiah, J.; Subramanian, A.; Kikkeri Hemannasetty, S.; Chandra, S.; Karur, S.; Mandikal Kodandaramasastry, U.; Cholenahally Nanjappa, M. Transcatheter closure of perimembranous ventricular septal defects with ductal occluders. Cardiol. Young 2015, 25, 918–926. [Google Scholar] [CrossRef]
- Tanidir, I.C.; Baspinar, O.; Saygi, M.; Kervancioglu, M.; Guzeltas, A.; Odemis, E. Use of Lifetech Konar-MF, a device for both perimembranous and muscular ventricular septal defects: A multicentre study. Int. J. Cardiol. 2020, 310, 43–50. [Google Scholar] [CrossRef]
- Pérez de Prado, A.; Zunzunegui Martínez, J.L.; Carbonell de Blas, R.; Rodríguez García, M.Á.; Benito González, T.; Fernández Vázquez, F. Effect of Incomplete Closure of a Gerbode Defect With the Nit-Occlud Le VSD Coil: Severe Hemolysis Requiring a Second Procedure. Rev. Esp. Cardiol. 2017, 70, 872–873. [Google Scholar] [CrossRef] [PubMed]
- Mulvaney, S.; Grech, V. Haemolysis after Amplatzer device closure of ventricular septal defect. Images Paediatr. Cardiol. 2007, 9, 4–5. [Google Scholar] [PubMed]
- Mustard, W.T.; Keith, J.D.; Trusler, G.A.; Fowler, R.; Kidd, L. The surgical management of transposition of the great vessels. J. Thorac. Cardiovasc. Surg. 1964, 48, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Balzer, D.T.; Johnson, M.; Sharkey, A.M.; Kort, H. Transcatheter occlusion of baffle leaks following atrial switch procedures for transposition of the great vessels (d-TGV). Catheter. Cardiovasc. Interv. 2004, 61, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Porras, D.; Mitsouras, D.; Steigner, M.; Giannopoulos, A.A.; Kelil, T.; Marshall, A.C.; Menard, M.T. Transcatheter Mustard Revision Using Endovascular Graft Prostheses. Ann. Thorac. Surg. 2017, 103, e509–e512. [Google Scholar] [CrossRef]
- Giannakoulas, G.; Thanopoulos, V. Three-dimensional transesophageal echocardiography for guiding percutaneous fontan fenestration closure. Echocardiography 2014, 31, E230–E231. [Google Scholar] [CrossRef]
- Muino, L.; Suys, B.; De Wolf, D. Closure of a lateral baffle leak in a Fontan patient with an amplatzer PDA II AS plug. Acta Cardiol. 2012, 67, 599–602. [Google Scholar] [CrossRef]
- Uberoi, R.; Roberts, P.; Barnes, N.; Wilson, N. Transcatheter closure of total cavopulmonary connection lateral tunnel dehiscence and fenestration using a custom-made, self-expanding stent graft. Cardiovasc. Interv. Radiol. 2009, 32, 385–387. [Google Scholar] [CrossRef]
- Manuri, L.; Calaciura, R.E.; De Zorzi, A.; Oreto, L.; Raponi, M.; Lehner, A.; Haas, N.; Agati, S. Atrial flow regulator for failing Fontan circulation: An initial European experience. Interact. Cardiovasc. Thorac. Surg. 2018, 27, 761–764. [Google Scholar] [CrossRef]
- Sakakibara, S.; Konno, S. Congenital aneurysm of the sinus of Valsalva associated with ventricular septal defect. Anat. Aspects. Am. Heart J. 1968, 75, 595–603. [Google Scholar] [CrossRef]
- Xin-Jin, L.; Xuan, L.; Bo, P.; Hong-Wei, G.; Wei, W.; Shou-Jun, L.; Sheng-Shou, H. Modified Sakakibara classification system for ruptured sinus of Valsalva aneurysm. J. Thorac. Cardiovasc. Surg. 2013, 146, 874–878. [Google Scholar] [CrossRef]
- Liu, S.; Xu, X.; Chen, F.; Zhao, Z.; Zhang, Y.; Wang, C.; Xiang, J.; Wu, G.; Chen, X.; Zhao, X.; et al. Angiographic features of ruptured sinus of Valsalva aneurysm: New classification. J. Cardiol. 2014, 64, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Sasaki, N.; Matsuura, Y.; Uemura, R. Surgical correction of aneurysm of the sinus of Valsalva: A report of forty-five consecutive patients including eight with total replacement of the aortic valve. Am. J. Cardiol. 1969, 23, 180–191. [Google Scholar] [CrossRef]
- Mahimarangaiah, J.; Chandra, S.; Subramanian, A.; Srinivasa, K.H.; Usha, M.K.; Manjunath, C.N. Transcatheter closure of ruptured sinus of Valsalva: Different techniques and mid-term follow-up. Catheter. Cardiovasc. Interv. 2016, 87, 516–522. [Google Scholar] [CrossRef]
- Galeczka, M.; Glowacki, J.; Yashchuk, N.; Ditkivskyy, I.; Rojczyk, D.; Knop, M.; Smerdzinski, S.; Cherpak, B.; Szkutnik, M.; Bialkowski, J.; et al. Medium- and long-term follow-up of transcatheter closure of ruptured sinus of Valsalva aneurysm in Central Europe population. J. Cardiol. 2019, 74, 381–387. [Google Scholar] [CrossRef]
- Faccini, A.; Butera, G. Tricuspid regurgitation as a complication of Edwards Sapien XT valve implantation in pulmonary position a problem to deal with. Catheter. Cardiovasc. Interv. 2018, 91, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.H.; Duong, P.; Jivanji, S.G.M.; Jones, M.; Kabir, S.; Butera, G.; Qureshi, S.A.; Rosenthal, E. Transcatheter Correction of Superior Sinus Venosus Atrial Septal Defects as an Alternative to Surgical Treatment. J. Am. Coll. Cardiol. 2020, 75, 1266–1278. [Google Scholar] [CrossRef] [PubMed]
- Hai-Bo HNaqvi, S.Y.; Kai, Y.; Xiang-Bin, P. Transcatheter closure for baffle leak after Takeuchi repair of anomalous left coronary artery from the pulmonary artery: A case report. Eur. Heart J. Case Rep. 2018, 2, yty028. [Google Scholar]
- Naqvi, S.Y.; Joynt, M.; Prasad, S.; Ling, F. Severe baffle leak after Takeuchi repair successfully treated with coronary bypass and percutaneous baffle closure: A case report. Eur. Heart J. Case Rep. 2021, 5, ytab074. [Google Scholar] [CrossRef]
- Latcu, D.G.; Rinaldi, J.P.; Yaici, K.; Saoudi, N. The use of transesophageal echocardiography to guide the trans-septal puncture. Practical guide, tips and tricks. Ann. Cardiol. Angeiol. 2015, 64, 14–20. [Google Scholar] [CrossRef]
- Nam, H.H.; Herz, C.; Lasso, A.; Drouin, S.; Posada, A.; Morray, B.; O’Byrne, M.L.; Paniagua, B.; Joffe, D.; Mackensen, B.; et al. Simulation of Transcatheter Atrial and Ventricular Septal Defect Device Closure Within Three-Dimensional Echocardiography-Derived Heart Models on Screen and in Virtual Reality. J. Am. Soc. Echocardiogr. 2020, 33, 641–644.e2. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, R.A.; Haider, A.; Kim, H.S.; Jeong, D.; Lee, S.W. Transformative Noise Reduction: Leveraging a Transformer-Based Deep Network for Medical Image Denoising. Mathematics 2024, 12, 2313. [Google Scholar] [CrossRef]
- Abdulrazzaq, M.M.; Ramaha, N.T.A.; Hameed, A.A.; Salman, M.; Yon, D.K.; Fitriyani, N.L.; Syafrudin, M.; Lee, S.W. Consequential Advancements of Self-Supervised Learning (SSL) in Deep Learning Contexts. Mathematics 2024, 12, 758. [Google Scholar] [CrossRef]

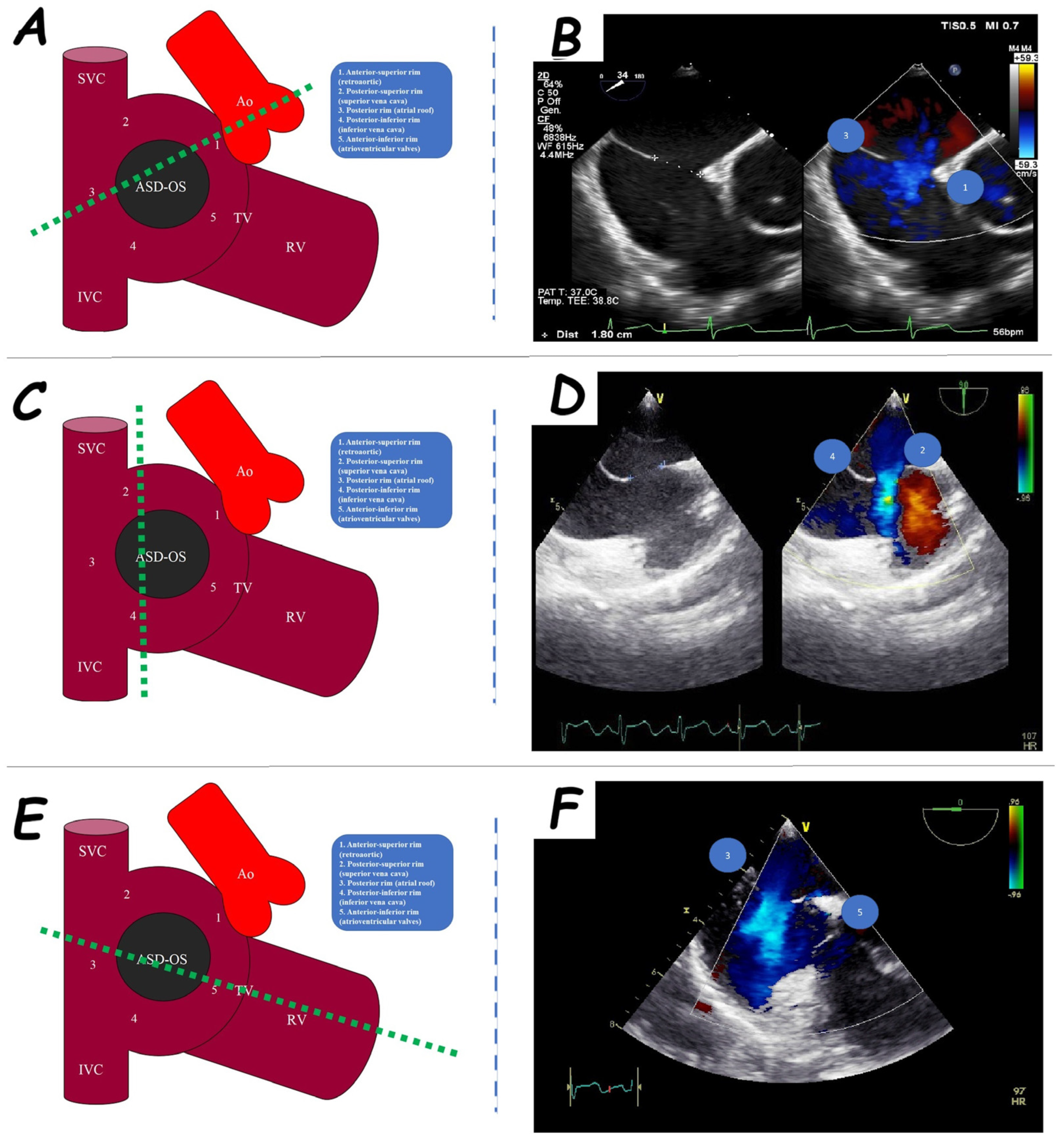


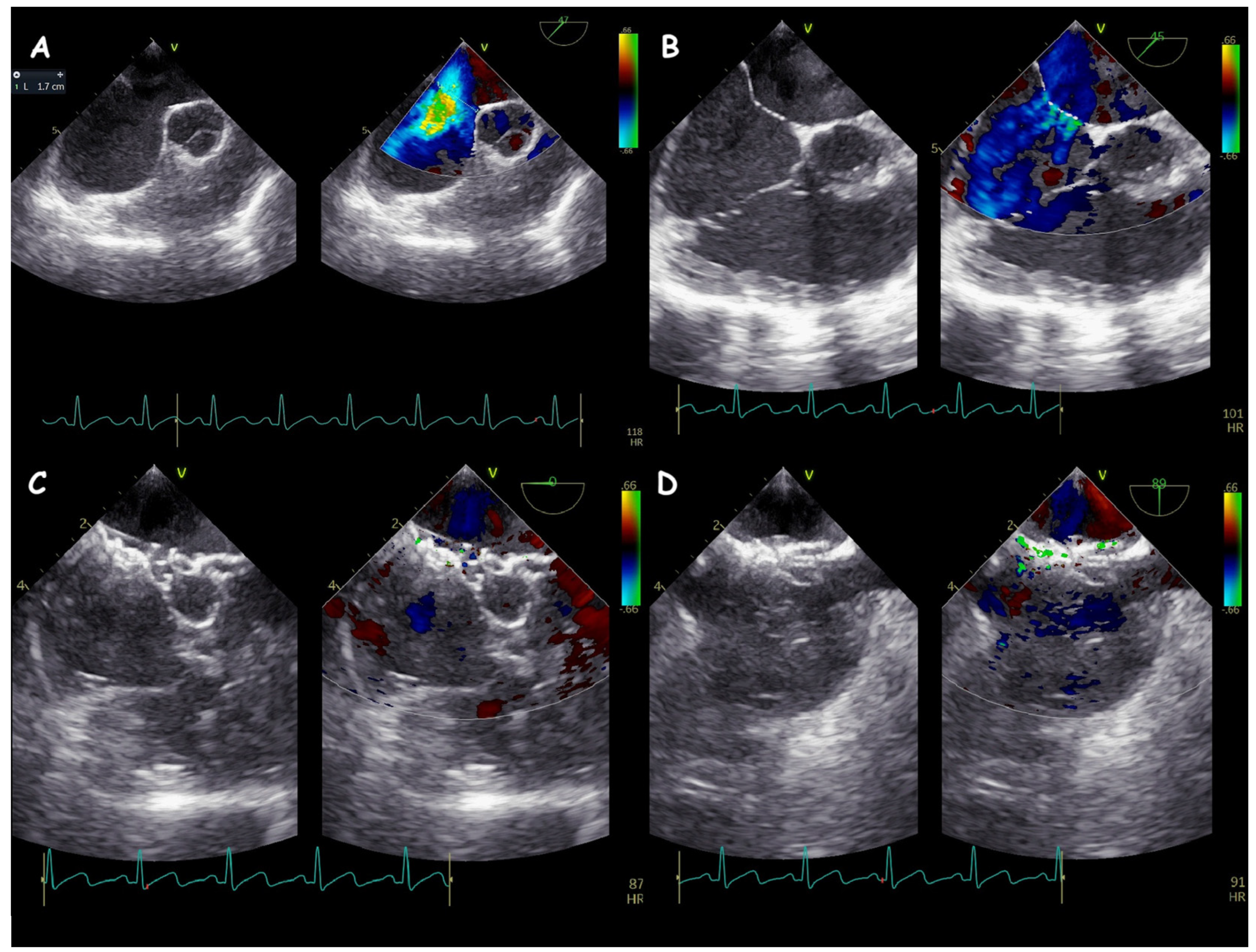


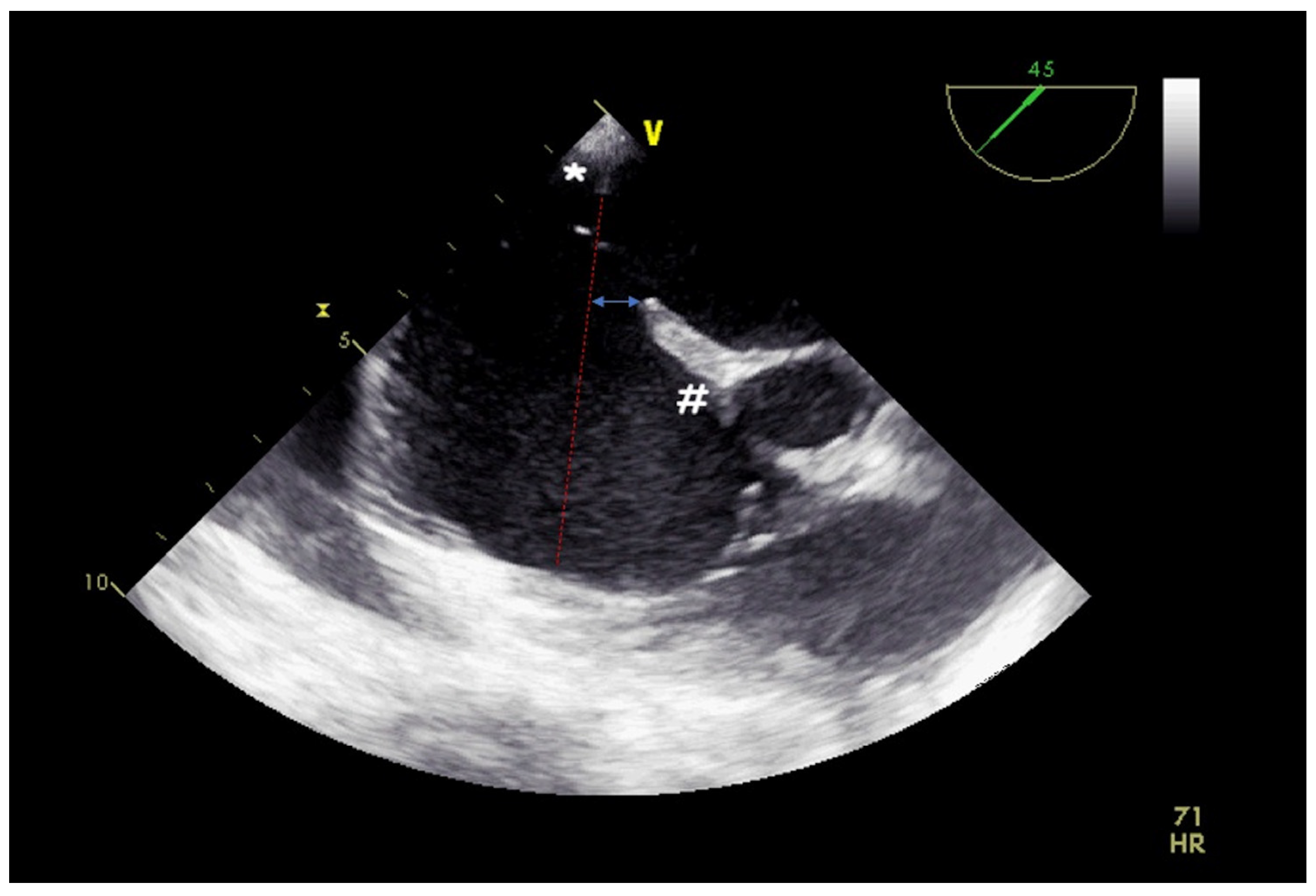
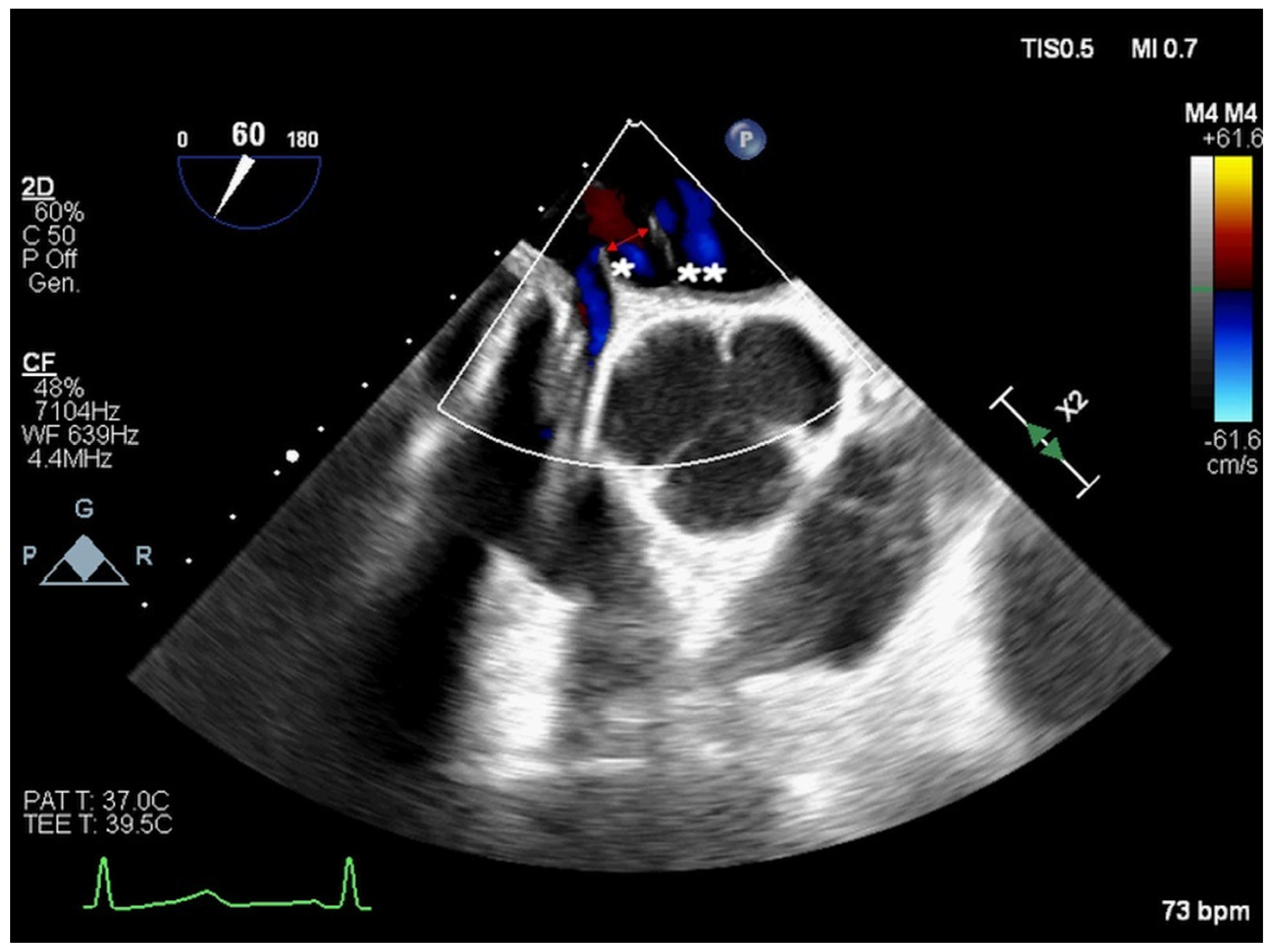
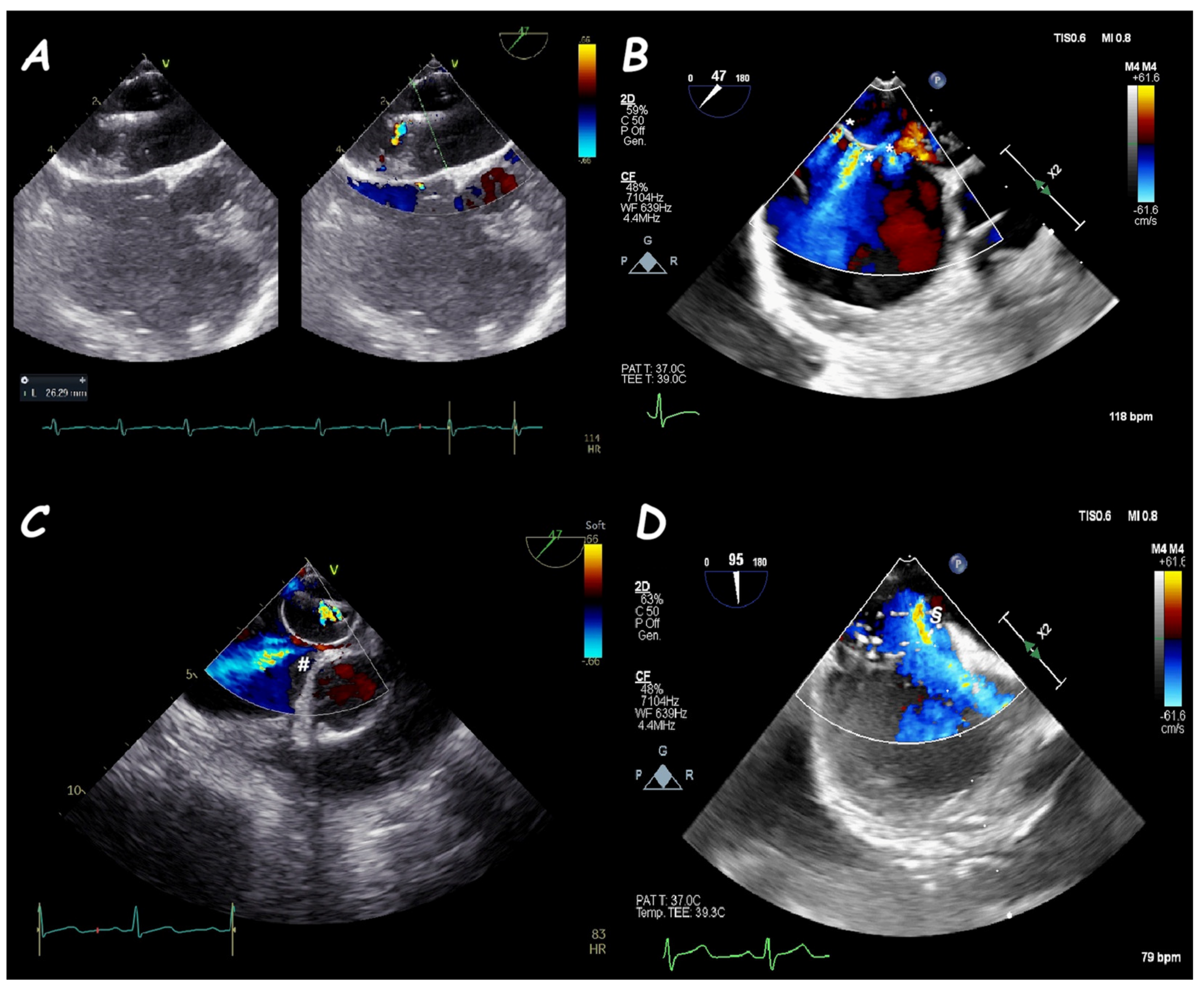



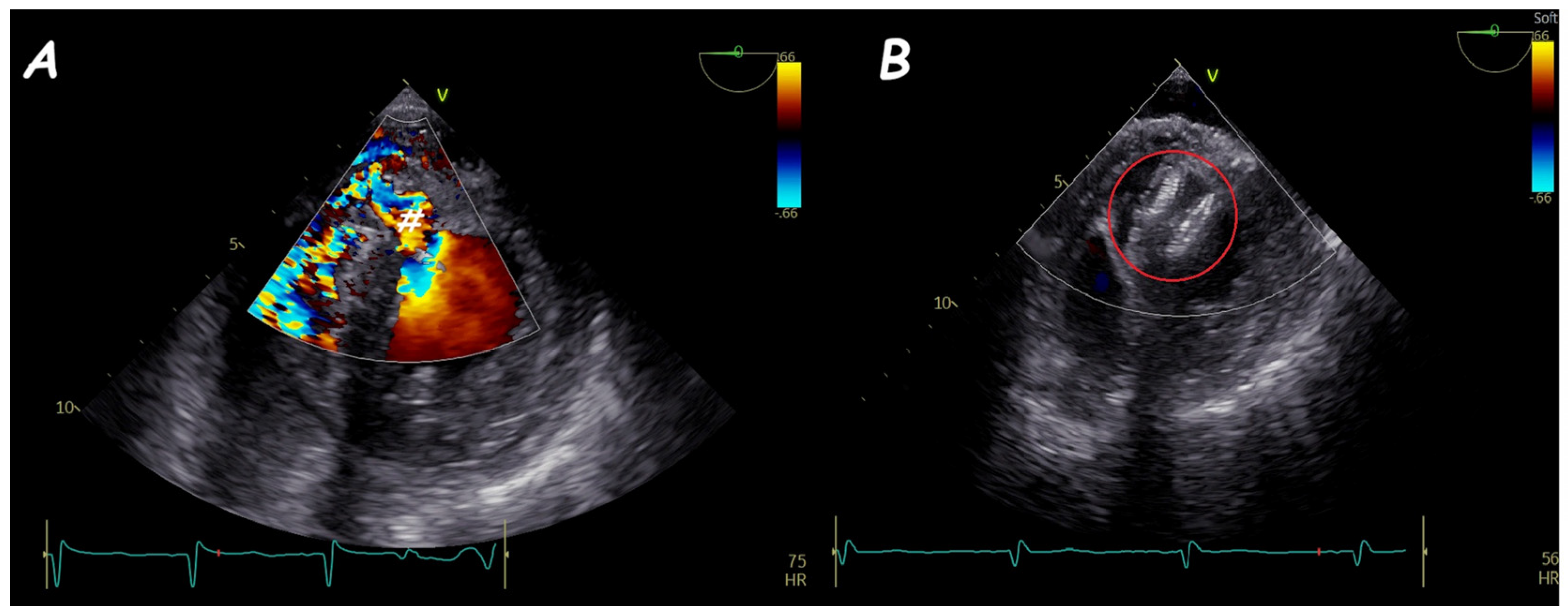
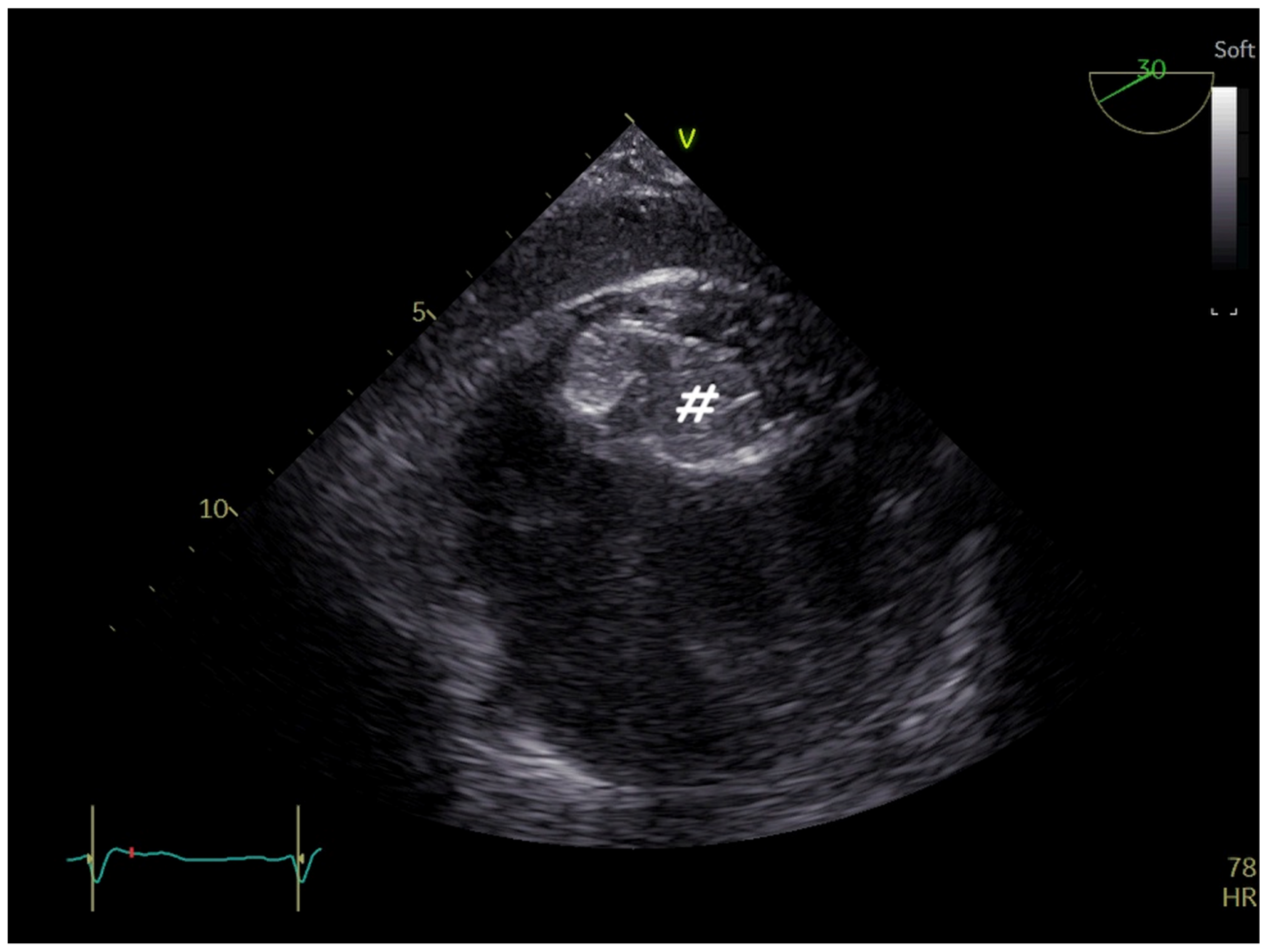
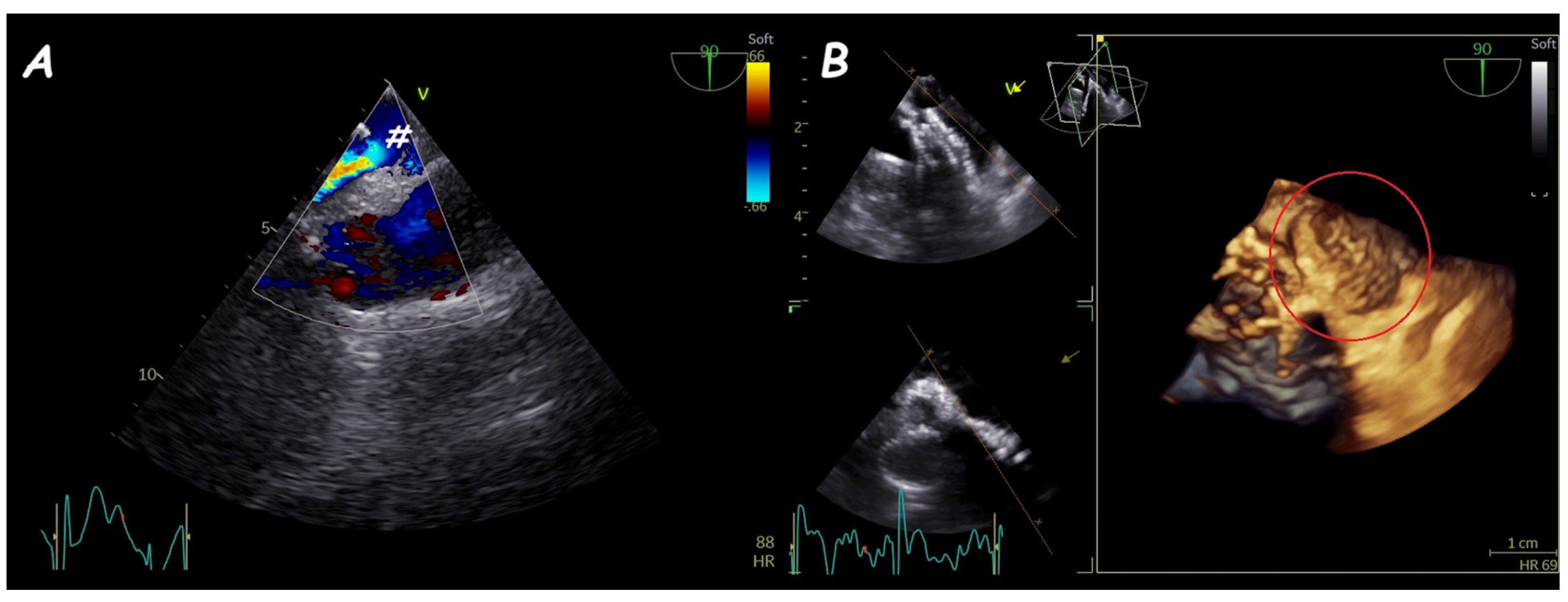


| ASD Closure: Pre-Procedural Assessment | |
|---|---|
| ASD shape | elliptical, round or irregular shapes (star-, reniform- or other irregular shapes), internal seedings |
| ASD size | maximum ASD diameter |
| ASD shunt direction | left-to-right, bidirectional, or right-to-left |
| ASD periorificial rims | adequate (>5 mm), poor (<5 mm), or absent |
| Accessory ASDs | numbers, size, and distance (if present) |
| Atrial septum aneurysm | base width and an aneurysm excursion into the right or left atrium (if present) |
| Misaligned ASD | distance of separation between the septum primum surface and the septum secundum one (if present) |
| Double atrial septum | separation between the left atrial and right atrial rims of the defects (if present) |
| Redundant Eustachian valve | length and excursion (if present) |
| Chiari network | width and extension (if present) |
| VSD Closure: Pre-Procedural Assessment | |
|---|---|
| VSD topography | peri-membranous or muscular VSD (inlet, outlet, or trabecular VSD; doubly committed VSD; malalignment VSD; Gerbode VSD) |
| VSD shunt direction | left-to-right, bidirectional, or right-to-left |
| VSD shape | elliptical, circular, or irregular shapes |
| VSD size | maximum ASD diameter |
| VSD periorificial rims | adequate (>2 mm), poor (<2 mm), or absent |
| Accessory VSDs | numbers, size, and distance (if present) |
| VSD aneurysmal pouch | base width and aneurysmal depth |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giordano, M.; Scognamiglio, G.; Gaio, G.; Marzullo, R.; Palma, M.; Barracano, R.; Fusco, F.; Borrelli, N.; Sperlongano, S.; Cimmino, G.; et al. The Role of Trans-Oesophageal Echocardiography in the Interventional Cardiology of Adult Congenital Heart Diseases. J. Clin. Med. 2025, 14, 1049. https://doi.org/10.3390/jcm14041049
Giordano M, Scognamiglio G, Gaio G, Marzullo R, Palma M, Barracano R, Fusco F, Borrelli N, Sperlongano S, Cimmino G, et al. The Role of Trans-Oesophageal Echocardiography in the Interventional Cardiology of Adult Congenital Heart Diseases. Journal of Clinical Medicine. 2025; 14(4):1049. https://doi.org/10.3390/jcm14041049
Chicago/Turabian StyleGiordano, Mario, Giancarlo Scognamiglio, Gianpiero Gaio, Raffaella Marzullo, Michela Palma, Rosaria Barracano, Flavia Fusco, Nunzia Borrelli, Simona Sperlongano, Giovanni Cimmino, and et al. 2025. "The Role of Trans-Oesophageal Echocardiography in the Interventional Cardiology of Adult Congenital Heart Diseases" Journal of Clinical Medicine 14, no. 4: 1049. https://doi.org/10.3390/jcm14041049
APA StyleGiordano, M., Scognamiglio, G., Gaio, G., Marzullo, R., Palma, M., Barracano, R., Fusco, F., Borrelli, N., Sperlongano, S., Cimmino, G., Russo, M. G., & Sarubbi, B. (2025). The Role of Trans-Oesophageal Echocardiography in the Interventional Cardiology of Adult Congenital Heart Diseases. Journal of Clinical Medicine, 14(4), 1049. https://doi.org/10.3390/jcm14041049









