Plasma Biomarkers for Cerebral Amyloid Angiopathy and Implications for Amyloid-Related Imaging Abnormalities: A Comprehensive Review
Abstract
1. Introduction
2. AD, AATs, and ARIAs
3. Risk Factors for ARIA Development
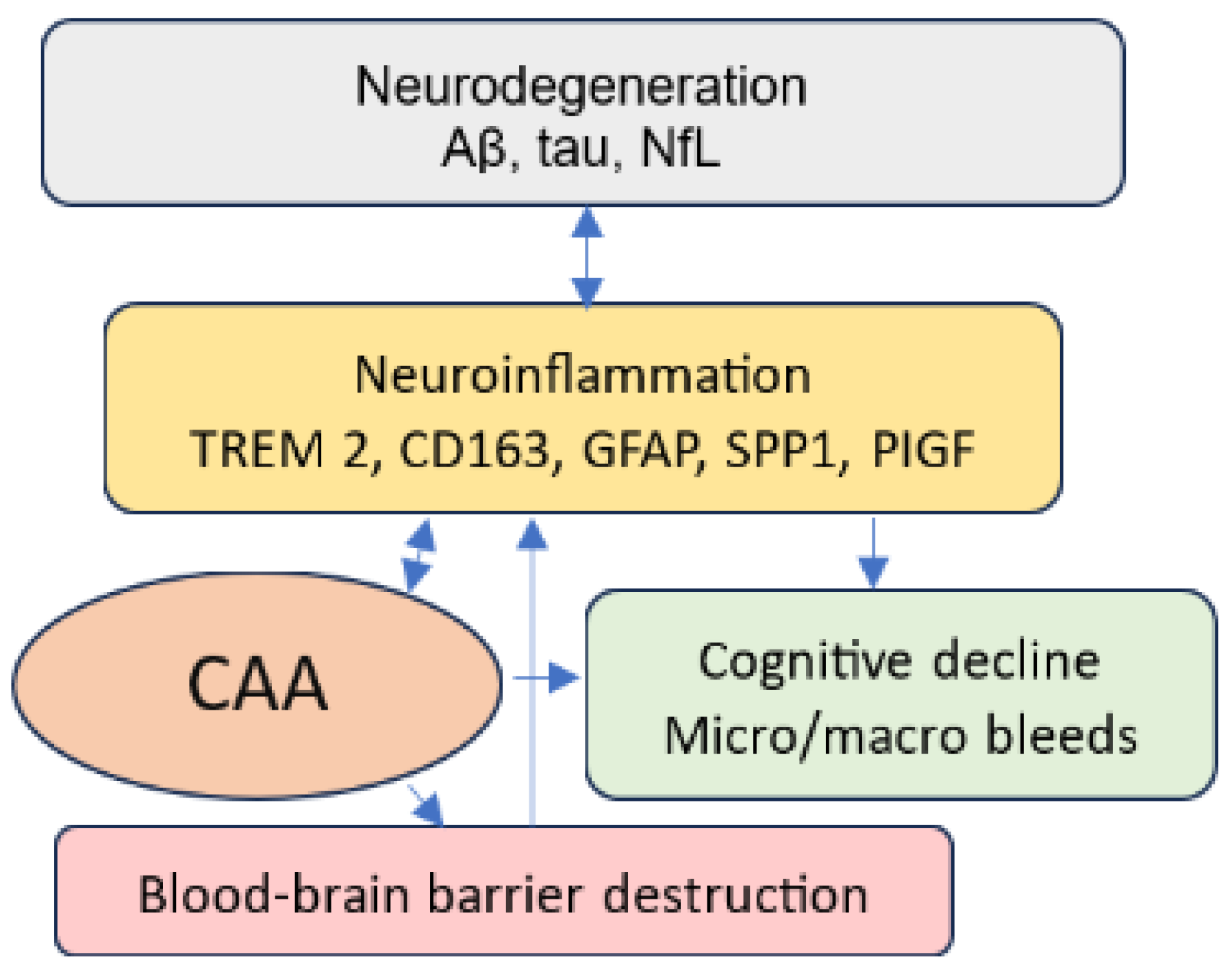
4. Diagnosis of CAA
5. Discussion
6. Limitations and Suggestions for Future Studies
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alzheimer’s Association. Alzheimer’s Facts and Figures Report. Available online: https://www.alz.org/alzheimers-dementia/facts-figures (accessed on 28 January 2025).
- National Institute on Aging. Alzheimer’s Disease Fact Sheet. Available online: https://www.nia.nih.gov/health/alzheimers-and-dementia/alzheimers-disease-fact-sheet (accessed on 28 January 2025).
- Swanson, C.J.; Zhang, Y.; Dhadda, S.; Wang, J.; Kaplow, J.; Lai, R.Y.K.; Lannfelt, L.; Bradley, H.; Rabe, M.; Koyama, A.; et al. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer’s disease with lecanemab, an anti-Aβ protofibril antibody. Alzheimers Res. Ther. 2021, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Withington, C.G.; Turner, R.S. Amyloid-Related Imaging Abnormalities With Anti-amyloid Antibodies for the Treatment of Dementia Due to Alzheimer’s Disease. Front. Neurol. 2022, 13, 862369. [Google Scholar] [CrossRef] [PubMed]
- van Dyck, C.H. Anti-Amyloid-β Monoclonal Antibodies for Alzheimer’s Disease: Pitfalls and Promise. Biol. Psychiatry 2018, 83, 311–319. [Google Scholar] [CrossRef]
- Li, Y.; Schindler, S.E.; Bollinger, J.G.; Ovod, V.; Mawuenyega, K.G.; Weiner, M.W.; Shaw, L.M.; Masters, C.L.; Fowler, C.J.; Trojanowski, J.Q.; et al. Validation of Plasma Amyloid-β 42/40 for Detecting Alzheimer Disease Amyloid Plaques. Neurology 2022, 98, e688–e699. [Google Scholar] [CrossRef]
- Brand, A.L.; Lawler, P.E.; Bollinger, J.G.; Li, Y.; Schindler, S.E.; Li, M.; Lopez, S.; Ovod, V.; Nakamura, A.; Shaw, L.M.; et al. The performance of plasma amyloid beta measurements in identifying amyloid plaques in Alzheimer’s disease: A literature review. Alzheimers Res. Ther. 2022, 14, 195. [Google Scholar] [CrossRef]
- Nakamura, T.; Kawarabayashi, T.; Seino, Y.; Hirohata, M.; Nakahata, N.; Narita, S.; Itoh, K.; Nakaji, S.; Shoji, M. Aging and APOE-ε4 are determinative factors of plasma Aβ42 levels. Ann. Clin. Transl. Neurol. 2018, 5, 1184–1191. [Google Scholar] [CrossRef]
- Vermunt, L.; Sikkes, S.A.M.; van den Hout, A.; Handels, R.; Bos, I.; van der Flier, W.M.; Kern, S.; Ousset, P.-J.; Maruff, P.; Skoog, I.; et al. Duration of preclinical, prodromal, and dementia stages of Alzheimer’s disease in relation to age, sex, and APOE genotype. Alzheimers Dement. 2019, 15, 888–898. [Google Scholar] [CrossRef]
- Honig, L.S.; Barakos, J.; Dhadda, S.; Kanekiyo, M.; Reyderman, L.; Irizarry, M.; Kramer, L.D.; Swanson, C.J.; Sabbagh, M. ARIA in patients treated with lecanemab (BAN2401) in a phase 2 study in early Alzheimer’s disease. Alzheimers Dement. 2023, 9, e12377. [Google Scholar] [CrossRef]
- Salloway, S.; Chalkias, S.; Barkhof, F.; Burkett, P.; Barakos, J.; Purcell, D.; Suhy, J.; Forrestal, F.; Tian, Y.; Umans, K.; et al. Amyloid-Related Imaging Abnormalities in 2 Phase 3 Studies Evaluating Aducanumab in Patients with Early Alzheimer Disease. JAMA Neurol. 2022, 79, 13–21. [Google Scholar] [CrossRef]
- Doran, S.J.; Sawyer, R.P. Risk factors in developing amyloid related imaging abnormalities (ARIA) and clinical implications. Front. Neurosci. 2024, 18, 1326784. [Google Scholar] [CrossRef]
- Budd Haeberlein, S.; O’Gorman, J.; Chiao, P.; Bussière, T.; von Rosenstiel, P.; Tian, Y.; Zhu, Y.; von Hehn, C.; Gheuens, S.; Skordos, L.; et al. Clinical Development of Aducanumab, an Anti-Aβ Human Monoclonal Antibody Being Investigated for the Treatment of Early Alzheimer’s Disease. J. Prev. Alzheimers Dis. 2017, 4, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.; Siemers, E.; Hake, A.; Case, M.; Hayduk, R.; Suhy, J.; Oh, J.; Barakos, J. Amyloid-related imaging abnormalities from trials of solanezumab for Alzheimer’s disease. Alzheimers Dement. 2016, 2, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.L.; Cohen, S.; van Dyck, C.H.; Brody, M.; Curtis, C.; Cho, W.; Ward, M.; Friesenhahn, M.; Rabe, C.; Brunstein, F.; et al. ABBY: A phase 2 randomized trial of crenezumab in mild to moderate Alzheimer disease. Neurology 2018, 90, e1889–e1897. [Google Scholar] [CrossRef] [PubMed]
- Doody, R.S.; Thomas, R.G.; Farlow, M.; Iwatsubo, T.; Vellas, B.; Joffe, S.; Kieburtz, K.; Raman, R.; Sun, X.; Aisen, P.S.; et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N. Engl. J. Med. 2014, 370, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, S.M.; Bacskai, B.J.; Hernandez-Guillamon, M.; Pruzin, J.; Sperling, R.; van Veluw, S.J. Cerebral amyloid angiopathy and Alzheimer disease—One peptide, two pathways. Nat. Rev. Neurol. 2020, 16, 30–42. [Google Scholar] [CrossRef]
- Landen, J.W.; Andreasen, N.; Cronenberger, C.L.; Schwartz, P.F.; Börjesson-Hanson, A.; Östlund, H.; Sattler, C.A.; Binneman, B.; Bednar, M.M. Ponezumab in mild-to-moderate Alzheimer’s disease: Randomized phase II PET-PIB study. Alzheimers Dement. 2017, 3, 393–401. [Google Scholar] [CrossRef]
- Leurent, C.; Goodman, J.A.; Zhang, Y.; He, P.; Polimeni, J.R.; Gurol, M.E.; Lindsay, M.; Frattura, L.; Sohur, U.S.; Viswanathan, A.; et al. Immunotherapy with ponezumab for probable cerebral amyloid angiopathy. Ann. Clin. Transl. Neurol. 2019, 6, 795–806. [Google Scholar] [CrossRef]
- Mintun, M.A.; Lo, A.C.; Duggan Evans, C.; Wessels, A.M.; Ardayfio, P.A.; Andersen, S.W.; Shcherbinin, S.; Sparks, J.; Sims, J.R.; Brys, M.; et al. Donanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2021, 384, 1691–1704. [Google Scholar] [CrossRef]
- Barakos, J.; Purcell, D.; Suhy, J.; Chalkias, S.; Burkett, P.; Marsica Grassi, C.; Castrillo-Viguera, C.; Rubino, I.; Vijverberg, E. Detection and Management of Amyloid-Related Imaging Abnormalities in Patients with Alzheimer’s Disease Treated with Anti-Amyloid Beta Therapy. J. Prev. Alzheimers Dis. 2022, 9, 211–220. [Google Scholar] [CrossRef]
- Chiong, W.; Tolchin, B.D.; Bonnie, R.J.; Busl, K.; Cruz-Flores, S.; Epstein, L.G.; Greene, E.P.; Illes, J.; Kirschen, M.; Larriviere, D.G.; et al. Decisions With Patients and Families Regarding Aducanumab in Alzheimer Disease, With Recommendations for Consent: AAN Position Statement. Neurology 2022, 98, 154–159. [Google Scholar] [CrossRef]
- Decourt, B.; Boumelhem, F.; Pope, E.D.; Shi, J.; Mari, Z.; Sabbagh, M.N. Critical Appraisal of Amyloid Lowering Agents in AD. Curr. Neurol. Neurosci. Rep. 2021, 21, 39. [Google Scholar] [CrossRef] [PubMed]
- Sveikata, L.; Charidimou, A.; Viswanathan, A. Vessels Sing Their ARIAs: The Role of Vascular Amyloid in the Age of Aducanumab. Stroke 2022, 53, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.A.; Chawla, S.; Nogueras-Ortiz, C.; Coresh, J.; Sharrett, A.R.; Wong, D.F.; Jack, C.R.J.; Spychalla, A.J.; Gottesman, R.F.; Kapogiannis, D. Neuronal insulin signaling and brain structure in nondemented older adults: The Atherosclerosis Risk in Communities Study. Neurobiol. Aging 2021, 97, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Eli Lilly and Company. Lilly’s Donanemab Significantly Slowed Cognitive and Functional Decline in Phase 3 Study of Early Alzheimer’s Disease. Available online: https://investor.lilly.com/news-releases/news-release-details/lillys-donanemab-significantly-slowed-cognitive-and-functional (accessed on 28 January 2025).
- Highlights of Prescribing Information. Available online: https://us.eisai.com/-/media/files/useisai/792710051-2021-08-11 (accessed on 28 January 2025).
- Charidimou, A.; Boulouis, G.; Frosch, M.P.; Baron, J.-C.; Pasi, M.; Albucher, J.F.; Banerjee, G.; Barbato, C.; Bonneville, F.; Brandner, S.; et al. The Boston criteria version 2.0 for cerebral amyloid angiopathy: A multicentre, retrospective, MRI-neuropathology diagnostic accuracy study. Lancet Neurol. 2022, 21, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, K.A.; Rosand, J.; Karluk, D.; Greenberg, S.M. Clinical diagnosis of cerebral amyloid angiopathy: Validation of the Boston criteria. Neurology 2001, 56, 537–539. [Google Scholar] [CrossRef]
- Verbeek, M.M.; Kremer, B.P.H.; Rikkert, M.O.; Van Domburg, P.H.M.F.; Skehan, M.E.; Greenberg, S.M. Cerebrospinal fluid amyloid beta(40) is decreased in cerebral amyloid angiopathy. Ann. Neurol. 2009, 66, 245–249. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, V.; Brahmbhatt, P.; Desai, A.; Vibhute, P.; Joseph-Mathurin, N.; Bathla, G. Amyloid-related Imaging Abnormalities in Alzheimer Disease Treated with Anti-Amyloid-β Therapy. RadioGraphics 2023, 43, e230009. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Charidimou, A. Diagnosis of Cerebral Amyloid Angiopathy: Evolution of the Boston Criteria. Stroke 2018, 49, 491–497. [Google Scholar] [CrossRef]
- De Kort, A.M.; Kuiperij, H.B.; Marques, T.M.; Jäkel, L.; van den Berg, E.; Kersten, I.; van Berckel-Smit, H.E.P.; Duering, M.; Stoops, E.; Abdo, W.F.; et al. Decreased Cerebrospinal Fluid Amyloid β 38, 40, 42, and 43 Levels in Sporadic and Hereditary Cerebral Amyloid Angiopathy. Ann. Neurol. 2023, 93, 1173–1186. [Google Scholar] [CrossRef]
- Banerjee, G.; Carare, R.; Cordonnier, C.; Greenberg, S.M.; Schneider, J.A.; Smith, E.E.; van Buchem, M.; van der Grond, J.; Verbeek, M.M.; Werring, D.J. The increasing impact of cerebral amyloid angiopathy: Essential new insights for clinical practice. J. Neurol. Neurosurg. Psychiatry 2017, 88, 982–994. [Google Scholar] [CrossRef]
- Zicha, S.; Bateman, R.J.; Shaw, L.M.; Zetterberg, H.; Bannon, A.W.; Horton, W.A.; Baratta, M.; Kolb, H.C.; Dobler, I.; Mordashova, Y.; et al. Comparative analytical performance of multiple plasma Aβ42 and Aβ40 assays and their ability to predict positron emission tomography amyloid positivity. Alzheimers Dement. 2023, 19, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Xu, F.; Hoos, M.D.; Lee, H.; Benveniste, H.; Nostrand, W.E.V. Reduced Levels of Cerebrospinal Fluid/Plasma Aβ40 as an Early Biomarker for Cerebral Amyloid Angiopathy in RTg-DI Rats. Int. J. Mol. Sci. 2020, 21, 303. [Google Scholar] [CrossRef] [PubMed]
- Jefferies, W.A.; Price, K.A.; Biron, K.E.; Fenninger, F.; Pfeifer, C.G.; Dickstein, D.L. Adjusting the compass: New insights into the role of angiogenesis in Alzheimer’s disease. Alzheimers Res. Ther. 2013, 5, 64. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.M.; Yano, S.; Tabassum, S.; Mitaki, S.; Michikawa, M.; Nagai, A. Alzheimer’s Amyloid β Peptide Induces Angiogenesis in an Alzheimer’s Disease Model Mouse through Placental Growth Factor and Angiopoietin 2 Expressions. Int. J. Mol. Sci. 2023, 24, 4510. [Google Scholar] [CrossRef]
- Lai, A.Y.; McLaurin, J. Clearance of amyloid-β peptides by microglia and macrophages: The issue of what, when and where. Future Neurol. 2012, 7, 165–176. [Google Scholar] [CrossRef]
- Gu, L.; Guo, Z. Alzheimer’s Aβ42 and Aβ40 peptides form interlaced amyloid fibrils. J. Neurochem. 2013, 126, 305–311. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Xu, H. Molecular and cellular mechanisms for Alzheimer’s disease: Understanding APP metabolism. Curr. Mol. Med. 2007, 7, 687–696. [Google Scholar] [CrossRef]
- Han, B.H.; Zhou, M.L.; Vellimana, A.K.; Milner, E.; Kim, D.H.; Greenberg, J.K.; Chu, W.; Mach, R.H.; Zipfel, G.J. Resorufin analogs preferentially bind cerebrovascular amyloid: Potential use as imaging ligands for cerebral amyloid angiopathy. Mol. Neurodegener. 2011, 6, 86. [Google Scholar] [CrossRef]
- Qiu, T.; Liu, Q.; Chen, Y.-X.; Zhao, Y.-F.; Li, Y.-M. Aβ42 and Aβ40: Similarities and differences. J. Pept. Sci. 2015, 21, 522–529. [Google Scholar] [CrossRef]
- Gravina, S.A.; Ho, L.; Eckman, C.B.; Long, K.E.; Otvos, L.; Younkin, L.H.; Suzuki, N.; Younkin, S.G. Amyloid beta protein (A beta) in Alzheimer’s disease brain. Biochemical and immunocytochemical analysis with antibodies specific for forms ending at A beta 40 or A beta 42(43). J. Biol. Chem. 1995, 270, 7013–7016. [Google Scholar] [CrossRef]
- Kakuda, N.; Miyasaka, T.; Iwasaki, N.; Nirasawa, T.; Wada-Kakuda, S.; Takahashi-Fujigasaki, J.; Murayama, S.; Ihara, Y.; Ikegawa, M. Distinct deposition of amyloid-β species in brains with Alzheimer’s disease pathology visualized with MALDI imaging mass spectrometry. Acta Neuropathol. Commun. 2017, 5, 73. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.L.; Papayannopoulos, I.A.; Styles, J.; Bobin, S.A.; Lin, Y.Y.; Biemann, K.; Iqbal, K. Peptide compositions of the cerebrovascular and senile plaque core amyloid deposits of Alzheimer’s disease. Arch. Biochem. Biophys. 1993, 301, 41–52. [Google Scholar] [CrossRef] [PubMed]
- DeSimone, C.V.; Graff-Radford, J.; El-Harasis, M.A.; Rabinstein, A.A.; Asirvatham, S.J.; Holmes, D.R. Cerebral Amyloid Angiopathy: Diagnosis, Clinical Implications, and Management Strategies in Atrial Fibrillation. J. Am. Coll. Cardiol. 2017, 70, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Wilcock, D.M.; Gordon, M.N.; Morgan, D. Quantification of cerebral amyloid angiopathy and parenchymal amyloid plaques with Congo red histochemical stain. Nat. Protoc. 2006, 1, 1591–1595. [Google Scholar] [CrossRef]
- Alban, S.L.; Lynch, K.M.; Ringman, J.M.; Toga, A.W.; Chui, H.C.; Sepehrband, F.; Choupan, J. Alzheimer’s Disease Neuroimaging Initiative The association between white matter hyperintensities and amyloid and tau deposition. NeuroImage Clin. 2023, 38, 103383. [Google Scholar] [CrossRef]
- Graff-Radford, J.; Arenaza-Urquijo, E.M.; Knopman, D.S.; Schwarz, C.G.; Brown, R.D.; Rabinstein, A.A.; Gunter, J.L.; Senjem, M.L.; Przybelski, S.A.; Lesnick, T.; et al. White matter hyperintensities: Relationship to amyloid and tau burden. Brain J. Neurol. 2019, 142, 2483–2491. [Google Scholar] [CrossRef]
- Smith, E.E.; Gurol, M.E.; Eng, J.A.; Engel, C.R.; Nguyen, T.N.; Rosand, J.; Greenberg, S.M. White matter lesions, cognition, and recurrent hemorrhage in lobar intracerebral hemorrhage. Neurology 2004, 63, 1606–1612. [Google Scholar] [CrossRef]
- Thanprasertsuk, S.; Martinez-Ramirez, S.; Pontes-Neto, O.M.; Ni, J.; Ayres, A.; Reed, A.; Swords, K.; Gurol, M.E.; Greenberg, S.M.; Viswanathan, A. Posterior white matter disease distribution as a predictor of amyloid angiopathy. Neurology 2014, 83, 794–800. [Google Scholar] [CrossRef]
- Walsh, P.; Sudre, C.H.; Fiford, C.M.; Ryan, N.S.; Lashley, T.; Frost, C.; Barnes, J. ADNI Investigators CSF amyloid is a consistent predictor of white matter hyperintensities across the disease course from aging to Alzheimer’s disease. Neurobiol. Aging 2020, 91, 5–14. [Google Scholar] [CrossRef]
- Antolini, L.; DiFrancesco, J.C.; Zedde, M.; Basso, G.; Arighi, A.; Shima, A.; Cagnin, A.; Caulo, M.; Carare, R.O.; Charidimou, A.; et al. Spontaneous ARIA-like Events in Cerebral Amyloid Angiopathy-Related Inflammation: A Multicenter Prospective Longitudinal Cohort Study. Neurology 2021, 97, e1809–e1822. [Google Scholar] [CrossRef]
- Grangeon, L.; Paquet, C.; Guey, S.; Zarea, A.; Martinaud, O.; Rotharmel, M.; Maltête, D.; Quillard-Muraine, M.; Nicolas, G.; Charbonnier, C.; et al. Cerebrospinal Fluid Profile of Tau, Phosphorylated Tau, Aβ42, and Aβ40 in Probable Cerebral Amyloid Angiopathy. J. Alzheimers Dis. 2022, 87, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Margraf, N.G.; Jensen-Kondering, U.; Weiler, C.; Leypoldt, F.; Maetzler, W.; Philippen, S.; Bartsch, T.; Flüh, C.; Röcken, C.; Möller, B.; et al. Cerebrospinal Fluid Biomarkers in Cerebral Amyloid Angiopathy: New Data and Quantitative Meta-Analysis. Front. Aging Neurosci. 2022, 14, 783996. [Google Scholar] [CrossRef] [PubMed]
- Sembill, J.A.; Lusse, C.; Linnerbauer, M.; Sprügel, M.I.; Mrochen, A.; Knott, M.; Engelhorn, T.; Schmidt, M.A.; Doerfler, A.; Oberstein, T.J.; et al. Cerebrospinal fluid biomarkers for cerebral amyloid angiopathy. Brain Commun. 2023, 5, fcad159. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Elhage, A.; Cho, M.; Nicoll, J.A.; Atri, A. Amyloid-related imaging abnormalities (ARIA) and their radiological, biological and clinical characteristics: A plain language summary. Neurodegener. Dis. Manag. 2024, 14, 51–62. [Google Scholar] [CrossRef]
- Ashton, N.J.; Brum, W.S.; Di Molfetta, G.; Benedet, A.L.; Arslan, B.; Jonaitis, E.; Langhough, R.E.; Cody, K.; Wilson, R.; Carlsson, C.M.; et al. Diagnostic Accuracy of a Plasma Phosphorylated Tau 217 Immunoassay for Alzheimer Disease Pathology. JAMA Neurol. 2024, 81, 255–263. [Google Scholar] [CrossRef]
- Gonzalez-Ortiz, F.; Ferreira, P.C.L.; González-Escalante, A.; Montoliu-Gaya, L.; Ortiz-Romero, P.; Kac, P.R.; Turton, M.; Kvartsberg, H.; Ashton, N.J.; Zetterberg, H.; et al. A novel ultrasensitive assay for plasma p-tau217: Performance in individuals with subjective cognitive decline and early Alzheimer’s disease. Alzheimers Dement. 2024, 20, 1239–1249. [Google Scholar] [CrossRef]
- FDA. FDA Authorizes Marketing of First Blood Test to Aid in the Evaluation of Concussion in Adults. Available online: https://www.fda.gov/news-events/press-announcements/fda-authorizes-marketing-first-blood-test-aid-evaluation-concussion-adults (accessed on 28 January 2025).
- Abdelhak, A.; Foschi, M.; Abu-Rumeileh, S.; Yue, J.K.; D’Anna, L.; Huss, A.; Oeckl, P.; Ludolph, A.C.; Kuhle, J.; Petzold, A.; et al. Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nat. Rev. Neurol. 2022, 18, 158–172. [Google Scholar] [CrossRef]
- Beyer, L.; Stocker, H.; Rujescu, D.; Holleczek, B.; Stockmann, J.; Nabers, A.; Brenner, H.; Gerwert, K. Amyloid-beta misfolding and GFAP predict risk of clinical Alzheimer’s disease diagnosis within 17 years. Alzheimers Dement. 2023, 19, 1020–1028. [Google Scholar] [CrossRef]
- Oeckl, P.; Anderl-Straub, S.; Von Arnim, C.A.F.; Baldeiras, I.; Diehl-Schmid, J.; Grimmer, T.; Halbgebauer, S.; Kort, A.M.; Lima, M.; Marques, T.M.; et al. Serum GFAP differentiates Alzheimer’s disease from frontotemporal dementia and predicts MCI-to-dementia conversion. J. Neurol. Neurosurg. Psychiatry 2022, 93, 659–667. [Google Scholar] [CrossRef]
- Kim, K.Y.; Shin, K.Y.; Chang, K.-A. GFAP as a Potential Biomarker for Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Cells 2023, 12, 1309. [Google Scholar] [CrossRef]
- Baiardi, S.; Quadalti, C.; Mammana, A.; Dellavalle, S.; Zenesini, C.; Sambati, L.; Pantieri, R.; Polischi, B.; Romano, L.; Suffritti, M.; et al. Diagnostic value of plasma p-tau181, NfL, and GFAP in a clinical setting cohort of prevalent neurodegenerative dementias. Alzheimers Res. Ther. 2022, 14, 153. [Google Scholar] [CrossRef] [PubMed]
- Benedet, A.L.; Milà-Alomà, M.; Vrillon, A.; Ashton, N.J.; Pascoal, T.A.; Lussier, F.; Karikari, T.K.; Hourregue, C.; Cognat, E.; Dumurgier, J.; et al. Differences Between Plasma and Cerebrospinal Fluid Glial Fibrillary Acidic Protein Levels Across the Alzheimer Disease Continuum. JAMA Neurol. 2021, 78, 1471–1483. [Google Scholar] [CrossRef] [PubMed]
- Rasing, I.; Voigt, S.; Koemans, E.A.; de Kort, A.M.; van Harten, T.W.; van Etten, E.S.; van Zwet, E.W.; Stoops, E.; Francois, C.; Kuiperij, H.B.; et al. Serum and cerebrospinal fluid neurofilament light chain and glial fibrillary acid protein levels in early and advanced stages of cerebral amyloid Angiopathy. Alzheimers Res. Ther. 2024, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Coppens, S.; Lehmann, S.; Hopley, C.; Hirtz, C. Neurofilament-Light, a Promising Biomarker: Analytical, Metrological and Clinical Challenges. Int. J. Mol. Sci. 2023, 24, 11624. [Google Scholar] [CrossRef]
- Mielke, M.M.; Syrjanen, J.A.; Blennow, K.; Zetterberg, H.; Vemuri, P.; Skoog, I.; Machulda, M.M.; Kremers, W.K.; Knopman, D.S.; Jack, C.; et al. Plasma and CSF neurofilament light: Relation to longitudinal neuroimaging and cognitive measures. Neurology 2019, 93, e252–e260. [Google Scholar] [CrossRef]
- Wang, X.; Shi, Z.; Qiu, Y.; Sun, D.; Zhou, H. Peripheral GFAP and NfL as early biomarkers for dementia: Longitudinal insights from the UK Biobank. BMC Med. 2024, 22, 192. [Google Scholar] [CrossRef]
- TauRx. Neurofilament Light Chain (NfL). Available online: https://taurx.com/medical-professionals/neurofilament-light-chain-nfl (accessed on 28 January 2025).
- Lund, S.A.; Giachelli, C.M.; Scatena, M. The role of osteopontin in inflammatory processes. J. Cell Commun. Signal. 2009, 3, 311–322. [Google Scholar] [CrossRef]
- Jakovac, H.; Grubić Kezele, T.; Šućurović, S.; Mulac-Jeričević, B.; Radošević-Stašić, B. Osteopontin-metallothionein I/II interactions in experimental autoimmune encephalomyelitis. Neuroscience 2017, 350, 133–145. [Google Scholar] [CrossRef]
- Ghare, S.; Gardener, H.; Ariko, T.; Gutierrez, J.; Wright, C.B.; Goldberg, R.B.; Elkind, M.S.V.; Cooper, G.E.; Shields, C.B.; Barve, S.; et al. Osteopontin Is Associated with Dementia in the Presence of Cerebral Small Vessel Disease. Cerebrovasc. Dis. 2024, 53, 495–500. [Google Scholar] [CrossRef]
- Rosmus, D.-D.; Lange, C.; Ludwig, F.; Ajami, B.; Wieghofer, P. The Role of Osteopontin in Microglia Biology: Current Concepts and Future Perspectives. Biomedicines 2022, 10, 840. [Google Scholar] [CrossRef]
- Lopes, K.D.P.; Yu, L.; Shen, X.; Qiu, Y.; Tasaki, S.; Iatrou, A.; Beeri, M.S.; Seyfried, N.T.; Menon, V.; Wang, Y.; et al. Associations of cortical SPP1 and ITGAX with cognition and common neuropathologies in older adults. Alzheimers Dement. 2024, 20, 525–537. [Google Scholar] [CrossRef]
- Hainsworth, A.H.; Elahi, F.M.; Corriveau, R.A. An introduction to therapeutic approaches to vascular cognitive impairment. Cereb. Circ.-Cogn. Behav. 2021, 2, 100033. [Google Scholar] [CrossRef] [PubMed]
- Harris, E. Blood Biomarker Helps Distinguish Vascular Dementia. JAMA 2023, 329, 969. [Google Scholar] [CrossRef] [PubMed]
- Newell, L.F.; Holtan, S.G. Placental growth factor: What hematologists need to know. Blood Rev. 2017, 31, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Hinman, J.D.; Elahi, F.; Chong, D.; Radabaugh, H.; Ferguson, A.; Maillard, P.; Thompson, J.F.; Rosenberg, G.A.; Sagare, A.; Moghekar, A.; et al. Placental growth factor as a sensitive biomarker for vascular cognitive impairment. Alzheimers Dement. 2023, 19, 3519–3527. [Google Scholar] [CrossRef]
- Wu, L.-Y.; Chong, J.R.; Chong, J.P.C.; Hilal, S.; Venketasubramanian, N.; Tan, B.Y.; Richards, A.M.; Chen, C.P.; Lai, M.K.P. Serum Placental Growth Factor as a Marker of Cerebrovascular Disease Burden in Alzheimer’s Disease. J. Alzheimers Dis. 2024, 97, 1289–1298. [Google Scholar] [CrossRef]
- Mammana, S.; Fagone, P.; Cavalli, E.; Basile, M.S.; Petralia, M.C.; Nicoletti, F.; Bramanti, P.; Mazzon, E. The Role of Macrophages in Neuroinflammatory and Neurodegenerative Pathways of Alzheimer’s Disease, Amyotrophic Lateral Sclerosis, and Multiple Sclerosis: Pathogenetic Cellular Effectors and Potential Therapeutic Targets. Int. J. Mol. Sci. 2018, 19, 831. [Google Scholar] [CrossRef]
- Frautschy, S.A.; Yang, F.; Irrizarry, M.; Hyman, B.; Saido, T.C.; Hsiao, K.; Cole, G.M. Microglial response to amyloid plaques in APPsw transgenic mice. Am. J. Pathol. 1998, 152, 307–317. [Google Scholar]
- Prinz, M.; Masuda, T.; Wheeler, M.A.; Quintana, F.J. Microglia and Central Nervous System-Associated Macrophages-From Origin to Disease Modulation. Annu. Rev. Immunol. 2021, 39, 251–277. [Google Scholar] [CrossRef]
- Hickman, S.; Izzy, S.; Sen, P.; Morsett, L.; El Khoury, J. Microglia in neurodegeneration. Nat. Neurosci. 2018, 21, 1359–1369. [Google Scholar] [CrossRef]
- Feng, W.; Zhang, Y.; Wang, Z.; Xu, H.; Wu, T.; Marshall, C.; Gao, J.; Xiao, M. Microglia prevent beta-amyloid plaque formation in the early stage of an Alzheimer’s disease mouse model with suppression of glymphatic clearance. Alzheimers Res. Ther. 2020, 12, 125. [Google Scholar] [CrossRef]
- Nabizadeh, F.; Seyedmirzaei, H.; Karami, S. Neuroimaging biomarkers and CSF sTREM2 levels in Alzheimer’s disease: A longitudinal study. Sci. Rep. 2024, 14, 15318. [Google Scholar] [CrossRef] [PubMed]
- Cogswell, P.M.; Barakos, J.A.; Barkhof, F.; Benzinger, T.S.; Jack, C.R.; Poussaint, T.Y.; Raji, C.A.; Ramanan, V.K.; Whitlow, C.T. Amyloid-Related Imaging Abnormalities with Emerging Alzheimer Disease Therapeutics: Detection and Reporting Recommendations for Clinical Practice. AJNR Am. J. Neuroradiol. 2022, 43, E19–E35. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Elhage, A.; Cho, M.; Apostolova, L.G.; Nicoll, J.A.R.; Atri, A. Amyloid-related imaging abnormalities (ARIA): Radiological, biological and clinical characteristics. Brain J. Neurol. 2023, 146, 4414–4424. [Google Scholar] [CrossRef] [PubMed]
- Šimončičová, E.; Gonçalves de Andrade, E.; Vecchiarelli, H.A.; Awogbindin, I.O.; Delage, C.I.; Tremblay, M.-È. Present and future of microglial pharmacology. Trends Pharmacol. Sci. 2022, 43, 669–685. [Google Scholar] [CrossRef]
- d’Errico, P.; Ziegler-Waldkirch, S.; Aires, V.; Hoffmann, P.; Mezö, C.; Erny, D.; Monasor, L.S.; Liebscher, S.; Ravi, V.M.; Joseph, K.; et al. Microglia contribute to the propagation of Aβ Into unaffected brain tissue. Nat. Neurosci. 2022, 25, 20–25. [Google Scholar] [CrossRef]
- Zgorzynska, E. TREM2 in Alzheimer’s disease: Structure, function, therapeutic prospects, and activation challenges. Mol. Cell. Neurosci. 2024, 128, 103917. [Google Scholar] [CrossRef]
- Bonomi, C.G.; Assogna, M.; Di Donna, M.G.; Bernocchi, F.; De Lucia, V.; Nuccetelli, M.; Fiorelli, D.; Loizzo, S.; Mercuri, N.B.; Koch, G.; et al. Cerebrospinal Fluid sTREM-2, GFAP, and β-S100 in Symptomatic Sporadic Alzheimer’s Disease: Microglial, Astrocytic, and APOE Contributions Along the Alzheimer’s Disease Continuum. J. Alzheimers Dis. 2023, 92, 1385–1397. [Google Scholar] [CrossRef]
- Park, S.-H.; Lee, E.-H.; Kim, H.-J.; Jo, S.; Lee, S.; Seo, S.W.; Park, H.-H.; Koh, S.-H.; Lee, J.-H. The relationship of soluble TREM2 to other biomarkers of sporadic Alzheimer’s disease. Sci. Rep. 2021, 11, 13050. [Google Scholar] [CrossRef]
- Yaghmoor, F.; Noorsaeed, A.; Alsaggaf, S.; Aljohani, W.; Scholtzova, H.; Boutajangout, A.; Wisniewski, T. The Role of TREM2 in Alzheimer’s Disease and Other Neurological Disorders. J. Alzheimers Dis. Park. 2014, 4, 160. [Google Scholar] [CrossRef]
- Zhong, R.; Xu, Y.; Williams, J.W.; Li, L. Loss of TREM2 diminishes CAA despite an overall increase of amyloid load in Tg-SwDI mice. Alzheimers Dement. 2024, 20, 7595–7612. [Google Scholar] [CrossRef]
- ALZFORUM. Is ARIA an Inflammatory Reaction to Vascular Amyloid? Available online: https://www.alzforum.org/news/conference-coverage/aria-inflammatory-reaction-vascular-amyloid (accessed on 28 January 2025).
- Chen, Y.; Lu, P.; Wu, S.; Yang, J.; Liu, W.; Alzheimer’s Disease Neuroimaging Initiative; Zhang, Z.; Xu, Q. CD163-Mediated Small-Vessel Injury in Alzheimer’s Disease: An Exploration from Neuroimaging to Transcriptomics. Int. J. Mol. Sci. 2024, 25, 2293. [Google Scholar] [CrossRef]
- Hawkes, C.A.; McLaurin, J. Selective targeting of perivascular macrophages for clearance of beta-amyloid in cerebral amyloid angiopathy. Proc. Natl. Acad. Sci. USA 2009, 106, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Taylor, X.; Cisternas, P.; You, Y.; You, Y.; Xiang, S.; Marambio, Y.; Zhang, J.; Vidal, R.; Lasagna-Reeves, C.A. A1 reactive astrocytes and a loss of TREM2 are associated with an early stage of pathology in a mouse model of cerebral amyloid angiopathy. J. Neuroinflamm. 2020, 17, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Grand Moursel, L.; van der Graaf, L.M.; Bulk, M.; van Roon-Mom, W.M.C.; van der Weerd, L. Osteopontin and phospho-SMAD2/3 are associated with calcification of vessels in D-CAA, an hereditary cerebral amyloid angiopathy. Brain Pathol. 2019, 29, 793–802. [Google Scholar] [CrossRef]
- Handa, T.; Sasaki, H.; Takao, M.; Tano, M.; Uchida, Y. Proteomics-based investigation of cerebrovascular molecular mechanisms in cerebral amyloid angiopathy by the FFPE-LMD-PCT-SWATH method. Fluids Barriers CNS 2022, 19, 56. [Google Scholar] [CrossRef]
- Hondius, D.C.; Eigenhuis, K.N.; Morrema, T.H.J.; van der Schors, R.C.; van Nierop, P.; Bugiani, M.; Li, K.W.; Hoozemans, J.J.M.; Smit, A.B.; Rozemuller, A.J.M. Proteomics analysis identifies new markers associated with capillary cerebral amyloid angiopathy in Alzheimer’s disease. Acta Neuropathol. Commun. 2018, 6, 46. [Google Scholar] [CrossRef]
- Vervuurt, M.; Schrader, J.M.; de Kort, A.M.; Kersten, I.; Wessels, H.J.C.T.; Klijn, C.J.M.; Schreuder, F.H.B.M.; Kuiperij, H.B.; Gloerich, J.; Van Nostrand, W.E.; et al. Cerebrospinal fluid shotgun proteomics identifies distinct proteomic patterns in cerebral amyloid angiopathy rodent models and human patients. Acta Neuropathol. Commun. 2024, 12, 6. [Google Scholar] [CrossRef]
- Westwood, S.; Baird, A.L.; Anand, S.N.; Nevado-Holgado, A.J.; Kormilitzin, A.; Shi, L.; Hye, A.; Ashton, N.J.; Morgan, A.R.; Bos, I.; et al. Validation of Plasma Proteomic Biomarkers Relating to Brain Amyloid Burden in the EMIF-Alzheimer’s Disease Multimodal Biomarker Discovery Cohort. J. Alzheimers Dis. 2020, 74, 213–225. [Google Scholar] [CrossRef]
- Li, Y.-B.; Fu, Q.; Guo, M.; Du, Y.; Chen, Y.; Cheng, Y. MicroRNAs: Pioneering regulators in Alzheimer’s disease pathogenesis, diagnosis, and therapy. Transl. Psychiatry 2024, 14, 367. [Google Scholar] [CrossRef]
- Weldon Furr, J.; Morales-Scheihing, D.; Manwani, B.; Lee, J.; McCullough, L.D. Cerebral Amyloid Angiopathy, Alzheimer’s Disease and MicroRNA: MiRNA as Diagnostic Biomarkers and Potential Therapeutic Targets. NeuroMol. Med. 2019, 21, 369–390. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Rapp, J.; Rainone, S.; Goupil, C.; Dorval, V.; Smith, P.Y.; Saint-Pierre, M.; Vallée, M.; Planel, E.; Droit, A.; Calon, F.; et al. microRNA-132/212 deficiency enhances Aβ production and senile plaque deposition in Alzheimer’s disease triple transgenic mice. Sci. Rep. 2016, 6, 30953. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Li, Y.; Su, B. Identification of Circulating miR-125b as a Potential Biomarker of Alzheimer’s Disease in APP/PS1 Transgenic Mouse. J. Alzheimers Dis. 2017, 59, 1449–1458. [Google Scholar] [CrossRef]
- Cheng, L.; Doecke, J.D.; Sharples, R.A.; Villemagne, V.L.; Fowler, C.J.; Rembach, A.; Martins, R.N.; Rowe, C.C.; Macaulay, S.L.; Masters, C.L.; et al. Prognostic serum miRNA biomarkers associated with Alzheimer’s disease shows concordance with neuropsychological and neuroimaging assessment. Mol. Psychiatry 2015, 20, 1188–1196. [Google Scholar] [CrossRef]
- Huang, X.; Yuan, T.; Tschannen, M.; Sun, Z.; Jacob, H.; Du, M.; Liang, M.; Dittmar, R.L.; Liu, Y.; Liang, M.; et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genom. 2013, 14, 319. [Google Scholar] [CrossRef]
- Soreq, H.; Wolf, Y. NeurimmiRs: microRNAs in the neuroimmune interface. Trends Mol. Med. 2011, 17, 548–555. [Google Scholar] [CrossRef]
- Walgrave, H.; Zhou, L.; De Strooper, B.; Salta, E. The promise of microRNA-based therapies in Alzheimer’s disease: Challenges and perspectives. Mol. Neurodegener. 2021, 16, 76. [Google Scholar] [CrossRef]
- Abidin, S.Z.; Mat Pauzi, N.A.; Mansor, N.I.; Mohd Isa, N.I.; Hamid, A.A. A new perspective on Alzheimer’s disease: microRNAs and circular RNAs. Front. Genet. 2023, 14, 1231486. [Google Scholar] [CrossRef]
- Hospitalization–Health, United States. Available online: https://www.cdc.gov/nchs/hus/topics/hospitalization.htm (accessed on 28 January 2025).
- 2023 National Population Projections Tables: Main Series. Available online: https://www.census.gov/data/tables/2023/demo/popproj/2023-summary-tables.html (accessed on 28 January 2025).
| Stage 1 | Stage 2 | Stage 3 | Stage 4 | Stage 5 |
|---|---|---|---|---|
| Preclinical AD | Mild cognitive impairment due to AD | Dementia due to AD: Mild | Dementia due to AD: Moderate | Dementia due to AD: Severe |
| No clinical symptoms but possible biological changes in the brain (e.g., abnormally increased accumulation of amyloid β and tau) | Subtle symptoms (e.g., problems with memory, language, and thinking) that may not interfere with daily activities | Symptoms interfering with some daily activities | Symptoms interfering with many daily activities | Symptoms interfering with most daily activities |
| Aβ42 (pg/mL) a | Aβ40 (pg/mL) a | Aβ40/42 a | |
|---|---|---|---|
| CAA | 355 ± 146 (n = 17) | 2912 ± 977 (n = 17) | 9.43 ± 4.95 (n = 17) |
| AD | 433 ± 135 (n = 72) d | 3713 ± 1081 (n = 72) c | 8.95 ± 2.57 (n = 72) b |
| Controls | 838 ± 253 (n = 58) e | 4003 ± 1185 (n = 48) c | 4.91 ± 1.13 (n = 48) e |
| Authors | Study Objective | Methods and Type of Study | Results |
|---|---|---|---|
| Banerjee G et al. (2020) [34] | To compare amyloid markers (Aβ38, Aβ40, Aβ42, sAβPP α, and sAβPPβ) and other markers in neurodegenerative disease (t-tau, p-tau, NFL, sTREM2, and neurogranin) in the CSF of patients with AD and CAA and control subjects (CSs), comprising 10 patients with CAA and 5 CS participants from the Biomarkers and Outcomes in Cerebral Amyloid Angiopathy study, making use of 20 samples from patients with AD and 5 samples from age-matched CS participants from the Specialist Cognitive Disorders Service at the National Hospital of Neurology and Neurosurgery: University College London Hospitals (UCLH) NHS Trust, London, UK | Exploratory hypothesis-generating study. Aβ38, Aβ40, and Aβ42 were measured using a Meso Scale Discovery V-PLEX Aβ peptide panel 1(6E10) kit. sAβPPα and sAβPPβ measured using a Meso-Scale Discovery sAβPPα/sAβPPβ Kit. Tau markers measured using a sandwich ELISA | The results of unadjusted analyses showed lower levels of all amyloid components measured (Aβ38, Aβ40, Aβ42, sAβPP α, and sAβPPβ) and higher NFL levels in participants with CAA. There were no differences in CSF t-tau, p-tau, sTREM2, or neurogranin profiles. |
| Rasing I et al. (2024) [68] | To investigate whether NFL and GFAP levels in serum and CSF were abnormal in CAA conditions in 187 participants (28 presymptomatic D-CAA mutation carriers, 29 symptomatic D-CAA carriers, 59 individuals with sporadic CAA, and 33 controls) | Cross-sectional study. NFL and GFAP levels were measured using Simoa™ and Simoa™ GFAP Discovery Kit. | Increased CSF GFAP levels were found in the early presymptomatic stage of CAA. Increased serum and CSF NFL and GFAP levels were found in either sporadic or more severe hereditary symptomatic stages of CAA. |
| Taylor X et al. (2020) [102] | To examine the association between TREM2 and early-stage CAA in a mouse model | Cross-sectional. Outcomes were measured based on mouse (9-month-old Tg-FDD and wild-type C57/BL6 mice) brain cells. | Decreased levels of TREM2 were found in early-stage CAA |
| Zhong R et al. (2024) [97] | To determine the impact of TREM2 deficiency on CAA and parenchymal Aβdeposition in the brain using Tg-SwDI mice | Cross-sectional. Outcomes were measured via immunohistochemical, immunofluorescent, and histochemical staining of mouse brain tissue. | There was a robust increase in amyloid load in the cortex, hippocampus, and thalamus following the loss of TREM 2. There was an association between loss of TREM2 and markedly reduced CAA levels in thalamus. |
| Moursel LG et al. (2019) [103] | To examine the association between osteoponin and vascular calcification (severe form of CAA) in 8 people with a hereditary form of CAA | Cross-sectional. Outcome was measured via immunohistochemistry of huma brain tissues | There was a correlation between the vascular accumulation of collagen 1 and osteoponin and CAA severity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sin, M.-K.; Dage, J.L.; Nho, K.; Dowling, N.M.; Seyfried, N.T.; Bennett, D.A.; Levey, A.I.; Ahmed, A. Plasma Biomarkers for Cerebral Amyloid Angiopathy and Implications for Amyloid-Related Imaging Abnormalities: A Comprehensive Review. J. Clin. Med. 2025, 14, 1070. https://doi.org/10.3390/jcm14041070
Sin M-K, Dage JL, Nho K, Dowling NM, Seyfried NT, Bennett DA, Levey AI, Ahmed A. Plasma Biomarkers for Cerebral Amyloid Angiopathy and Implications for Amyloid-Related Imaging Abnormalities: A Comprehensive Review. Journal of Clinical Medicine. 2025; 14(4):1070. https://doi.org/10.3390/jcm14041070
Chicago/Turabian StyleSin, Mo-Kyung, Jeffrey L. Dage, Kwangsik Nho, N. Maritza Dowling, Nicholas T. Seyfried, David A. Bennett, Allan I. Levey, and Ali Ahmed. 2025. "Plasma Biomarkers for Cerebral Amyloid Angiopathy and Implications for Amyloid-Related Imaging Abnormalities: A Comprehensive Review" Journal of Clinical Medicine 14, no. 4: 1070. https://doi.org/10.3390/jcm14041070
APA StyleSin, M.-K., Dage, J. L., Nho, K., Dowling, N. M., Seyfried, N. T., Bennett, D. A., Levey, A. I., & Ahmed, A. (2025). Plasma Biomarkers for Cerebral Amyloid Angiopathy and Implications for Amyloid-Related Imaging Abnormalities: A Comprehensive Review. Journal of Clinical Medicine, 14(4), 1070. https://doi.org/10.3390/jcm14041070







