Changes in Urinary NGAL, FN, and LN Excretion in Type 2 Diabetic Patients Following Anti-Diabetic Therapy with Metformin
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Biological Material
2.3. Anthropometric Determination
2.4. Laboratory Blood and Urine Analyses
2.5. Pancreatic Islet Cell Function
2.6. Immunoassay of NGAL and Non-Collagenous Matrix Glycoproteins—Fibronectin and Laminin
2.7. Statistical Analysis
3. Results
3.1. Renal Function
3.2. Urinary Excretion of NGAL, FN, and LN
3.3. Correlation Analysis of NGAL, FN, and LN with Kidney Function Parameters
4. Discussion
4.1. Urinary Excretion of NGAL in Obese T2DM Patients Before Metformin Therapy
4.2. Urinary Excretion of Non-Collagenous Matrix Glycoproteins (FN and LN) in Obese T2DM Patients Before Metformin Therapy
4.3. The Influence of 6 Months of Metformin Therapy on the Urinary Excretion of NGAL and Non-Collagenous Matrix Glycoproteins (FN and LN)
4.4. Limitations and Strengths of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Front. Physiol. 2020, 10, 1607. [Google Scholar] [CrossRef] [PubMed]
- Mohammedi, K.; Chalmers, J.; Herrington, W.; Li, Q.; Mancia, G.; Marre, M.; Poulter, N.; Rodgers, A.; Williams, B.; Perkovic, V.; et al. Associations between body mass index and the risk of renal events in patients with type 2 diabetes. Nutr. Diabetes 2018, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, Y.S.; Sun, L.; Xie, P.; Liu, F.Y.; Chen, S. A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu. Rev. Pathol. 2011, 6, 395–423. [Google Scholar] [CrossRef]
- Song, A.; Zhang, C.; Meng, X. Mechanism and application of metformin in kidney diseases: An update. Biomed. Pharmacother. 2021, 138, 111454. [Google Scholar] [CrossRef]
- Wang, H.H.; Lin, S.H.; Hung, S.Y.; Chiou, Y.Y.; Hsu, W.C.; Chang, C.M.; Liou, H.H.; Chang, M.Y.; Ho, L.C.; Wu, C.F.; et al. Renal protective effect of metformin in type 2 diabetes patients. J. Clin. Endocrinol. Metab. 2024, dgae477. [Google Scholar] [CrossRef]
- Kwon, S.; Kim, Y.C.; Park, J.Y.; Lee, J.; An, J.N.; Kim, C.T.; Oh, S.; Park, S.; Kim, D.K.; Oh, Y.K.; et al. The long-term effects of metformin on patients with type 2 diabetic kidney disease. Diabetes Care 2020, 43, 948–955. [Google Scholar] [CrossRef]
- Pan, Q.; Lu, X.; Zhao, C.; Liao, S.; Chen, X.; Guo, F.; Yang, C.; Liu, H.F. Metformin: The updated protective property in kidney disease. Aging 2020, 12, 8742–8759. [Google Scholar] [CrossRef]
- Wu, M.; Xu, H.; Liu, J.; Tan, X.; Wan, S.; Guo, M.; Long, Y.; Xu, Y. Metformin and fibrosis: A review of existing evidence and mechanisms. J. Diabetes Res. 2021, 2021, 6673525. [Google Scholar] [CrossRef]
- Agarwal, R. Pathogenesis of diabetic nephropathy. In Chronic Kidney Disease and Type 2 Diabetes; American Diabetes Association: Arlington, VA, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK571720/ (accessed on 7 October 2024).
- Uwaezuoke, S.N. The role of novel biomarkers in predicting diabetic nephropathy: A review. Int. J. Nephrol. Renov. Dis. 2017, 10, 221–231. [Google Scholar] [CrossRef]
- An, N.; Wu, B.T.; Yang, Y.W.; Huang, Z.H.; Feng, J.F. Re-understanding and focusing on normoalbuminuric diabetic kidney disease. Front. Endocrinol. 2022, 13, 1077929. [Google Scholar] [CrossRef] [PubMed]
- Obert, L.A.; Elmore, S.A.; Ennulat, D.; Frazier, K.S. A review of specific biomarkers of chronic renal injury and their potential application in nonclinical safety assessment studies. Toxicol. Pathol. 2021, 49, 996–1023. [Google Scholar] [CrossRef] [PubMed]
- Fiseha, T.; Tamir, Z. Urinary markers of tubular injury in early diabetic nephropathy. Int. J. Nephrol. 2016, 2016, 4647685. [Google Scholar] [CrossRef]
- Romejko, K.; Markowska, M.; Niemczyk, S. The review of current knowledge on neutrophil gelatinase-associated lipocalin (NGAL). Int. J. Mol. Sci. 2023, 24, 10470. [Google Scholar] [CrossRef] [PubMed]
- Jaberi, S.A.; Cohen, A.; D’Souza, C.; Abdulrazzaq, Y.M.; Ojha, S.; Bastaki, S.; Adeghate, E.A. Lipocalin-2: Structure, function, distribution and role in metabolic disorders. Biomed. Pharmacother. 2021, 142, 112002. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Zhu, R.; Gong, J.; He, K.; Chen, J. Neutrophil gelatinase-associated lipocalin as a potential therapeutic integrator of glycolipid metabolic and inflammatory signaling. Int. Surg. J. 2017, 4, 2381–2386. [Google Scholar]
- Wu, T.; Ding, L.; Andoh, V.; Zhang, J.; Chen, L. The mechanism of hyperglycemia-induced renal cell injury in diabetic nephropathy disease: An update. Life 2023, 13, 539. [Google Scholar] [CrossRef]
- Thomas, H.Y.; Ford Versypt, A.N. Pathophysiology of mesangial expansion in diabetic nephropathy: Mesangial structure, glomerular biomechanics, and biochemical signaling and regulation. J. Biol. Eng. 2022, 16, 19. [Google Scholar] [CrossRef]
- Bülow, R.D.; Boor, P. Extracellular matrix in kidney fibrosis: More than just a scaffold. J. Histochem. Cytochem. 2019, 67, 643–661. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; Carneiro-Freire, N. Biochemical composition of the glomerular extracellular matrix in patients with diabetic kidney disease. World J. Diabetes 2022, 13, 498–520. [Google Scholar] [CrossRef]
- Dalton, C.J.; Lemmon, C.A. Fibronectin: Molecular structure, fibrillar structure and mechanochemical signaling. Cells 2021, 10, 2443. [Google Scholar] [CrossRef] [PubMed]
- Yap, L.; Tay, H.G.; Nguyen, M.T.X.; Tjin, M.S.; Tryggvason, K. Laminins in cellular differentiation. Trends Cell Biol. 2019, 29, 987–1000. [Google Scholar] [CrossRef] [PubMed]
- Silva-Oliveira, G.; Linhares-Lacerda, L.; Mattos, T.R.F.; Sanches, C.; Coelho-Sampaio, T.; Riederer, I.; Saraiva, E.M. Laminin triggers neutrophil extracellular traps (NETs) and modulates NET release induced by Leishmania amazonensis. Biomedicines 2022, 10, 521. [Google Scholar] [CrossRef] [PubMed]
- Jura-Półtorak, A.; Olczyk, P.; Chałas-Lipka, A.; Komosińska-Vassev, K.; Kuźnik-Trocha, K.; Winsz-Szczotka, K.; Ivanova, D.; Kiselova-Kaneva, Y.; Krysik, K.; Telega, A.; et al. Urinary sulphated glycosaminoglycans excretion in obese patients with type 2 diabetes mellitus treated with metformin. Arch. Physiol. Biochem. 2022, 128, 507–513. [Google Scholar] [CrossRef]
- Al-Hazmi, S.F.; Gad, H.G.M.; Alamoudi, A.A.; Eldakhakhny, B.M.; Binmahfooz, S.K.; Alhozali, A.M. Evaluation of early biomarkers of renal dysfunction in diabetic patients. Saudi Med. J. 2020, 41, 690–697. [Google Scholar] [CrossRef]
- Bolignano, D.; Lacquaniti, A.; Coppolino, G.; Donato, V.; Fazio, M.R.; Nicocia, G.; Buemi, M. Neutrophil gelatinase-associated lipocalin as an early biomarker of nephropathy in diabetic patients. Kidney Blood Press. Res. 2009, 32, 91–98. [Google Scholar] [CrossRef]
- Kaul, A.; Behera, M.R.; Rai, M.K.; Mishra, P.; Bhaduaria, D.S.; Yadav, S.; Agarwal, V.; Karoli, R.; Prasad, N.; Gupta, A.; et al. Neutrophil gelatinase-associated lipocalin: As a predictor of early diabetic nephropathy in type 2 diabetes mellitus. Indian J. Nephrol. 2018, 28, 53–60. [Google Scholar] [CrossRef]
- Quang, T.H.; Nguyet, M.P.; Thao, D.P.; Thi, M.H.; Phuong Thi Dam, L.; Thi, H.H.; Van, A.P.; Luong, T.C.; Tuyet, M.N.T.; Duy, Q.D.; et al. Evaluation of urinary neutrophil gelatinase associated lipocalin and kidney injury molecule-1 as diagnostic markers for early nephropathy in patients with type 2 diabetes mellitus. Diabetes Metab. Syndr. Obes. 2020, 13, 2199–2207. [Google Scholar]
- Megallaa, M.H.Z.; Abdel Salam, M.S.; Saad, N.L.M.; Ahmed Altair, M.N.A.H.; Amin, N.G. NGAL (Neutrophil Gelatinase-Associated Lipocalin) as an early biomarker of nephropathy in patients with type 2 diabetes. Alex. J. Med. 2023, 59, 52–58. [Google Scholar] [CrossRef]
- de Carvalho, J.A.; Tatsch, E.; Hausen, B.S.; Bollick, Y.S.; Moretto, M.B.; Duarte, T.; Duarte, M.M.; Londero, S.W.; Premaor, M.O.; Comim, F.V.; et al. Urinary kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin as indicators of tubular damage in normoalbuminuric patients with type 2 diabetes. Clin. Biochem. 2016, 49, 232–236. [Google Scholar] [CrossRef]
- Abd el kader, O.M.E.S.; Ismail, M.I.M.; Borai, M.M.; Ragab, W.A. Serum and urine neutrophil gelatinase-associated lipocalin: As an indicator for early diabetic nephropathy in type 2 diabetes mellitus patients. Zagazig Univ. Med. J. 2020, 26, 474–482. [Google Scholar] [CrossRef]
- Fu, W.J.; Xiong, S.L.; Fang, Y.G.; Wen, S.; Chen, M.L.; Deng, R.T.; Zheng, L.; Wang, S.B.; Pen, L.F.; Wang, Q. Urinary tubular biomarkers in short-term type 2 diabetes mellitus patients: A cross-sectional study. Endocrine 2012, 41, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Kandeel, W.A.; Elmalt, H.A.; Abdel Samie, O.M.; Megahed, H.A.; Hegazy, G.A.; El abd, E.M.Y.; Moneam, N.A.; Masoud, M.M.; Abdel-Monem, M.A. Serum neutrophil gelatinase-associated lipocalin in obese adolescents. Bull. Natl. Res. Cent. 2018, 42, 1–8. [Google Scholar] [CrossRef]
- Kim, S.S.; Song, S.H.; Kim, I.J.; Yang, J.Y.; Lee, J.G.; Kwak, I.S.; Kim, Y.K. Clinical implication of urinary tubular markers in the early stage of nephropathy with type 2 diabetic patients. Diabetes Res. Clin. Pract. 2012, 97, 251–257. [Google Scholar] [CrossRef]
- Aslanhan, E.; Ojalvo, D.; Özsenel, E.B.; Ucak Basat, S.; Borlu, F. Association of neutrophil-gelatinase-associated lipocalin with microvascular complications in patients with type 2 diabetes: A cross-sectional study. Cardiovasc. Endocrinol. Metab. 2019, 8, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; He, X.J.; Chen, S.R.; Wang, L.; Li, E.M.; Xu, L.Y. Changes of serum and urine neutrophil gelatinase-associated lipocalin in type-2 diabetic patients with nephropathy: One year observational follow-up study. Endocrine 2009, 36, 45–51. [Google Scholar] [CrossRef]
- Yiğit Kaya, S.; Kadıoğlu, T.; Uzunlulu, M.; Oğuz, A.; İşbilen Başok, B. Urinary neutrophil gelatinase-associated lipocalin levels as an indicator of early kidney injury in patients with type 2 diabetes mellitus. Lokman Hekim. Health Sci. 2024, 4, 14–19. [Google Scholar] [CrossRef]
- Greco, M.; Chiefari, E.; Mirabelli, M.; Salatino, A.; Tocci, V.; Cianfrone, P.; Foti, D.P.; Brunetti, A. Plasma or urine neutrophil gelatinase-associated lipocalin (NGAL): Which is better at detecting chronic kidney damage in type 2 diabetes? Endocrines 2022, 3, 175–186. [Google Scholar] [CrossRef]
- Wang, Y.; Lam, K.S.; Kraegen, E.W.; Sweeney, G.; Zhang, J.; Tso, A.W.; Chow, W.S.; Wat, N.M.; Xu, J.Y.; Hoo, R.L.; et al. Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin. Chem. 2007, 53, 34–41. [Google Scholar] [CrossRef]
- Rashad, N.M.; Said, N.M.; Emad, G.; Gomaa, A.F.; Kadry, H.M. Assessment of serum and urinary levels of neutrophil gelatinase-associated lipocalin in correlation with albuminuria in nondiabetic obese patients. Egypt J. Int. Med. 2019, 31, 642–651. [Google Scholar] [CrossRef]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose tissue and insulin resistance in obese. Biomed. Pharmacother. 2021, 137, 111315. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, R.; Xu, X.; Yang, M.; Xu, W.; Xiang, S.; Wang, L.; Jiang, X.; Hua, F.; Huang, X. Metabolically healthy obesity is associated with higher risk of both hyperfiltration and mildly reduced estimated glomerular filtration rate: The role of serum uric acid in a cross-sectional study. J. Transl. Med. 2023, 21, 216. [Google Scholar] [CrossRef]
- Chen, H.Y.; Lu, F.H.; Chang, C.J.; Wang, R.S.; Yang, Y.C.; Chang, Y.F.; Wu, J.S. Metabolic abnormalities, but not obesity per se, associated with chronic kidney disease in a Taiwanese population. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 418–425. [Google Scholar] [CrossRef]
- Wan, J.F.; Chen, Y.; Yao, T.H.; Wu, Y.Z.; Dai, H.Z. Impact of body mass index on adverse kidney events in diabetes mellitus patients: A systematic-review and meta-analysis. World J. Clin. Cases 2024, 12, 538–550. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, B.; Wang, B.; Zhou, H.; Yang, M.; Liu, B. Prediction factors and models for chronic kidney disease in type 2 diabetes mellitus: A review of the literature. Clin. Transl. Disc 2024, 4, e355. [Google Scholar] [CrossRef]
- Naderi, N.; Kleine, C.E.; Park, C.; Hsiung, J.T.; Soohoo, M.; Tantisattamo, E.; Streja, E.; Kalantar-Zadeh, K.; Moradi, H. Obesity paradox in advanced kidney disease: From bedside to the bench. Prog. Cardiovasc. Dis. 2018, 61, 168–181. [Google Scholar] [CrossRef]
- Aghaei, M.; Joukar, F.; Hasanipour, S.; Ranjbar, Z.A.; Naghipour, M.; Mansour-Ghanaei, F. The association between waist-to-hip ratio (WHR) with diabetes in the PERSIAN Guilan cohort study population. BMC Endocr. Disord. 2024, 24, 113. [Google Scholar] [CrossRef]
- Elsayed, E.F.; Tighiouart, H.; Weiner, D.E.; Griffith, J.; Salem, D.; Levey, A.S.; Sarnak, M.J. Waist-to-hip ratio and body mass index as risk factors for cardiovascular events in CKD. Am. J. Kidney Dis. 2008, 52, 49–57. [Google Scholar] [CrossRef]
- Benoit, S.W.; Ciccia, E.A.; Devarajan, P. Cystatin C as a biomarker of chronic kidney disease: Latest developments. Expert. Rev. Mol. Diagn. 2020, 20, 1019–1026. [Google Scholar] [CrossRef]
- Jhatta, C.; Girdhar, J.; Gupta, S.; Verma, I. To Compare the level of cystatin C in type 2 diabetes mellitus with obesity. Indian. J. Endocrinol. Metab. 2020, 24, 312–318. [Google Scholar]
- Chen, D.C.; Scherzer, R.; Ix, J.H.; Kramer, H.J.; Crews, D.C.; Nadkarni, G.; Gutierrez, O.; Bullen, A.L.; Ilori, T.; Garimella, P.S.; et al. Modification of association of cystatin c with kidney and cardiovascular outcomes by obesity. Am. J. Kidney Dis. 2024, 83, 489–496.e1. [Google Scholar] [CrossRef] [PubMed]
- Nerlich, A.; Schleicher, E. Immunohistochemical localization of extracellular matrix components in human diabetic glomerular lesions. Am. J. Pathol. 1991, 139, 889–899. [Google Scholar] [PubMed]
- Van Vliet, A.; Baelde, H.J.; Vleming, L.J.; de Heer, E.; Bruijn, J.A. Distribution of fibronectin isoforms in human renal disease. J. Pathol. 2001, 193, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Dixon, A.J.; Burns, J.; Dunnill, M.S.; McGee, J.O. Distribution of fibronectin in normal and diseased human kidneys. J. Clin. Pathol. 1980, 33, 1021–1028. [Google Scholar] [CrossRef]
- Vleming, L.J.; Baelde, J.J.; Westendorp, R.G.; Daha, M.R.; van Es, L.A.; Bruijn, J.A. The glomerular deposition of PAS positive material correlates with renal function in human kidney diseases. Clin. Nephrol. 1997, 47, 158–167. [Google Scholar]
- Nahman, N.S. Mesangial cell responses to high glucose levels and the development of diabetic glomerulosclerosis. Nefrologia 1994, 14, 358–390. [Google Scholar]
- Ozata, M.; Kurt, I.; Azal, O.; Bolu, E.; Corakci, A.; Beyhan, Z.; Karaca, L.; Gündogăn, M.A. Can we use plasma fibronectin levels as a marker for early diabetic nephropathy. Endocr. J. 1995, 42, 301–305. [Google Scholar] [CrossRef][Green Version]
- De Giorgio, L.A.; Bartolomei, G.; Gironi, A.; Caselli, P.; Seghieri, G. Increased plasma fibronectin concentration in diabetic patients with microalbuminuria. Diabetes Care 1988, 11, 527–530. [Google Scholar] [CrossRef]
- Skrha, J.; Vackova, I.; Kvasnicka, J.; Stibor, V.; Stolba, P.; Richter, H.; Horman, H. Plasma free N-terminal fibronectin 30-kDa domain as a marker of endothelial dysfunction in type 1 diabetes mellitus. Eur. J. Clin. Investig. 1990, 20, 171–176. [Google Scholar] [CrossRef]
- Solerte, S.B.; Piovella, F.; Viola, C.; Carnevale, G.P.; Gamba, G.; Fiovaranti, M.; Ferrari, E. Plasma fibronectin, Von Willebrand factor antigen, and blood rheology: Association with diabetic microvascular disease. Acta Diabetol. Lat. 1985, 22, 239–246. [Google Scholar] [CrossRef]
- Wautier, J.L.; Paton, C.; Wauher, M.P.; Pintigny, D.; Abadie, E.; Passa, P.; Caen, P. Increased adhesion of erythrocytes to endothelial cells in diabetes mellitus and its relation to vascular complications. N. Engl. J. Med. 1981, 305, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M. Increased urinary fibronectin excretion in type II diabetic patients with microalbuminuria. Nihon Jinzo Gakkai Shi 1995, 37, 336–342. [Google Scholar] [PubMed]
- Fagerudd, J.A.; Groop, P.H.; Honkanen, E.; Teppo, A.M.; Grönhagen-Riska, C. Urinary excretion of TGF-beta 1, PDGF-BB and fibronectin in insulin-dependent diabetes mellitus patients. Kidney Int. Suppl. 1997, 63, S195–S197. [Google Scholar] [PubMed]
- Orih, M.C.; Obiorah, C.C. Usefulness of urinary fibronectin and laminin as biomarkers of glomerular injury in diabetics in Port Harcourt, Nigeria. J. Diabetes Endocrinol. 2020, 11, 30–37. [Google Scholar]
- Kuboki, K.; Tada, H.; Shin, K.; Oshima, Y.; Isogai, S. Relationship between urinary excretion of fibronectin degradation products and proteinuria in diabetic patients, and their suppression after continuous subcutaneous heparin infusion. Diabetes Res. Clin. Pract. 1993, 21, 61–66. [Google Scholar] [CrossRef]
- Muh Anshar, J.; Kurniawan, L.B.; Nurahmi; Zainuddin, A.A.; Umar, H.; Arif, M. Analysis of Urinary Fibronectin Levels in Patients with Type 2 Diabetes Mellitus and Non-Diabetes Mellitus. FITOFARMAKA J. Ilm. Farm. 2023, 13, 61–69. [Google Scholar]
- Banu, N.; Hara, H.; Okamura, M.; Egusa, G.; Yamakido, M. Urinary excretion of type IV collagen and laminin in the evaluation of nephropathy in NIDDM: Comparison with urinary albumin and markers of tubular dysfunction and/or damage. Diabetes Res. Clin. Pract. 1995, 29, 57–67. [Google Scholar] [CrossRef]
- Hayashi, Y.; Makino, H.; Ota, Z. Serum and urinary concentrations of type IV collagen and laminin as a marker of microangiopathy in diabetes. Diabet. Med. 1992, 9, 366–370. [Google Scholar] [CrossRef]
- Miyake, H.; Nagashima, K.; Yagi, H.; Onigata, K. Urinary laminin P1 as an index of glycemic control in children with insulin-dependent diabetes mellitus. Diabet. Res. 1992, 23, 131–138. [Google Scholar]
- Eilenberg, W.; Stojkovic, S.; Piechota-Polanczyk, A.; Kaider, A.; Kozakowski, N.; Weninger, W.J.; Nanobachvili, J.; Wojta, J.; Huk, I.; Demyanets, S.; et al. Neutrophil gelatinase associated lipocalin (NGAL) is elevated in type 2 diabetics with carotid artery stenosis and reduced under metformin treatment. Cardiovasc. Diabetol. 2017, 16, 98. [Google Scholar] [CrossRef]
- Wyczalkowska-Tomasik, A.; Bartlomiejczyk, I.; Gornicka, B.; Paczek, L. Strong association between fibronectin accumulation and lowered cathepsin B activity in glomeruli of diabetic rats. J. Physiol. Pharmacol. 2012, 63, 525–530. [Google Scholar] [PubMed]
- Wang, Y.; Jin, M.; Cheng, C.K.; Li, Q. Tubular injury in diabetic kidney disease: Molecular mechanisms and potential therapeutic perspectives. Front. Endocrinol. 2023, 14, 1238927. [Google Scholar] [CrossRef] [PubMed]
- Mengstie, M.A.; Chekol Abebe, E.; Behaile Teklemariam, A.; Tilahun Mulu, A.; Agidew, M.M.; Teshome Azezew, M.; Zewde, E.A.; Agegnehu Teshome, A. Endogenous advanced glycation end products in the pathogenesis of chronic diabetic complications. Front. Mol. Biosci. 2022, 9, 1002710. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fernandez, N.; Jacobs-Cachá, C.; Mora-Gutiérrez, J.M.; Vergara, A.; Orbe, J.; Soler, M.J. Matrix metalloproteinases in diabetic kidney disease. J. Clin. Med. 2020, 9, 472. [Google Scholar] [CrossRef]
- Yi, H.; Huang, C.; Shi, Y.; Cao, Q.; Chen, J.; Chen, X.M.; Pollock, C.A. Metformin attenuates renal fibrosis in a mouse model of adenine-induced renal injury through inhibiting TGF-β1 signaling pathways. Front. Cell Dev. Biol. 2021, 9, 603802. [Google Scholar] [CrossRef]
- Wang, F.; Sun, H.; Zuo, B.; Shi, K.; Zhang, X.; Zhang, C.; Sun, D. Metformin attenuates renal tubulointerstitial fibrosis via upgrading autophagy in the early stage of diabetic nephropathy. Sci. Rep. 2021, 11, 16362. [Google Scholar] [CrossRef]
- Lin, C.X.; Li, Y.; Liang, S.; Tao, J.; Zhang, L.S.; Su, Y.F.; Huang, Y.X.; Zhao, Z.K.; Liu, S.Y.; Zheng, J.M. Metformin attenuates cyclosporine A-induced renal fibrosis in rats. Transplantation 2019, 103, e285–e296. [Google Scholar] [CrossRef]
- Tseng, C.H.; Shah, K.M.; Chiu, I.J.; Hsiao, L.L. The role of autophagy in type 2 diabetic kidney disease management. Cells 2023, 12, 2691. [Google Scholar] [CrossRef]
- Liang, S.; Wu, Y.S.; Li, D.Y.; Tang, J.X.; Liu, H.F. Autophagy and renal fibrosis. Aging Dis. 2022, 13, 712–731. [Google Scholar] [CrossRef]
- Lu, G.; Wu, Z.; Shang, J.; Xie, Z.; Chen, C.; Zhang, C. The effects of metformin on autophagy. Biomed. Pharmacother. 2021, 137, 111286. [Google Scholar] [CrossRef]
- Sun, T.; Liu, J.; Xie, C.; Yang, J.; Zhao, L.; Yang, J. Metformin attenuates diabetic renal injury via the AMPK-autophagy axis. Exp. Ther. Med. 2021, 21, 578. [Google Scholar] [CrossRef] [PubMed]
- Hasanvand, A. The role of AMPK-dependent pathways in cellular and molecular mechanisms of metformin: A new perspective for treatment and prevention of diseases. Inflammopharmacology 2022, 30, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Huang, C.; Shi, Y.; Cao, Q.; Zhao, Y.; Zhang, L.; Chen, J.; Pollock, C.A.; Chen, X.M. Metformin attenuates folic-acid induced renal fibrosis in mice. J. Cell Physiol. 2018, 233, 7045–7054. [Google Scholar] [CrossRef] [PubMed]
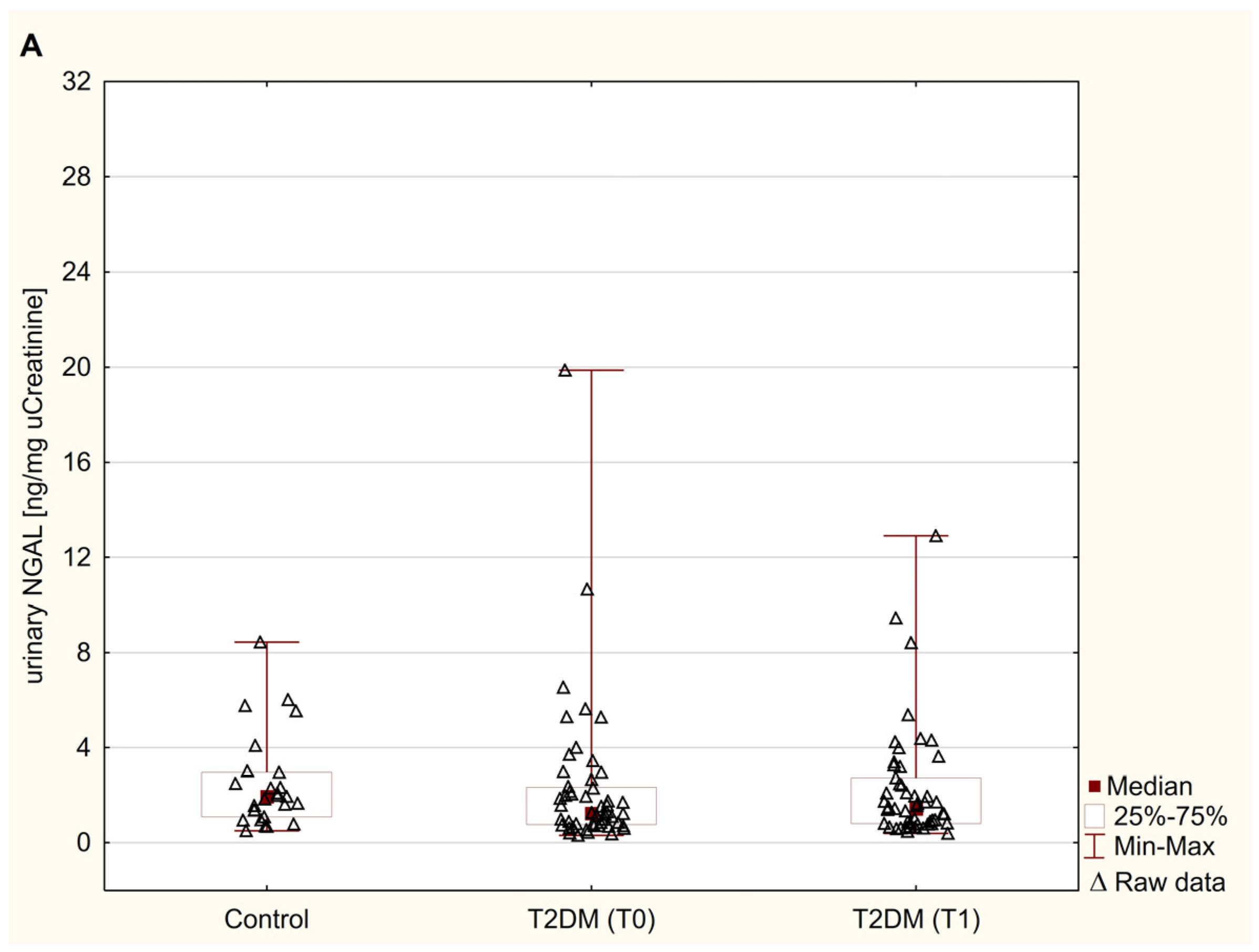
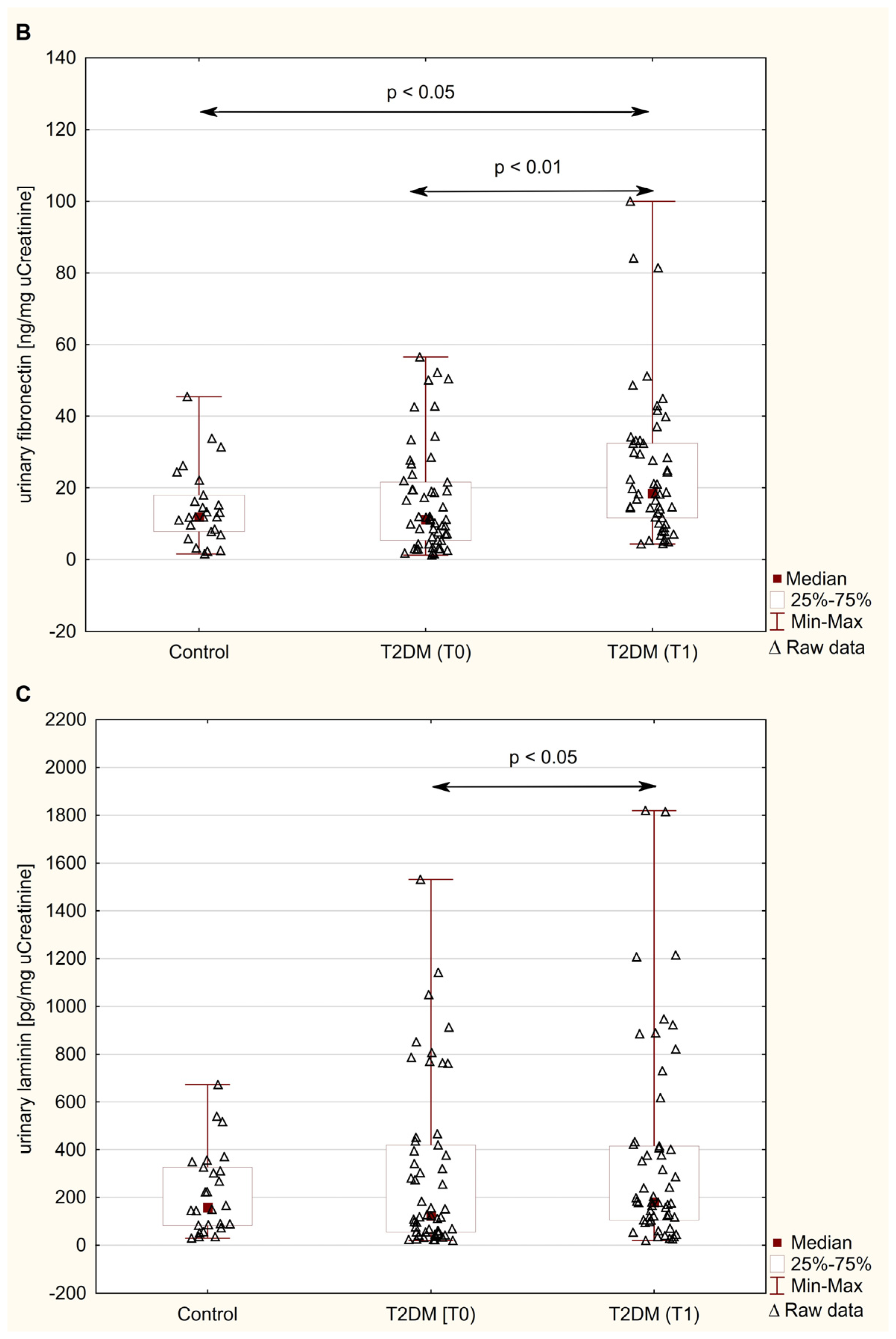
| Parameter | Control (n = 26) | Patients with T2DM (n = 53) | |||
|---|---|---|---|---|---|
| Baseline (T0) | 6 Months After Metformin Therapy (T1) | p-Values C vs. T0 | p-Values T0 vs. T1 | ||
| Gender female/male (n) | 12 F/14 M | 36 F/17 M | |||
| Age (years) | 56.00 (52.00–60.00) | 59.00 (53.00–65.00) | 0.106 | ||
| Weight (kg) | 82.50 (76.00–89.00) | 85.00 (80.00–96.00) | 84.00 (79.00–97.00) | 0.085 | 0.010 |
| Height (cm) | 170.13 ± 8.85 | 164.50 ± 9.45 | 0.015 | ||
| BMI (kg/m2) | 28.40 (26.11–30.83) | 31.43 (30.13–34.41) | 31.24 (29.05–34.52) | <0.001 | 0.010 |
| Waist (cm) | 99.00 (88.00–104.50) | 105.00 (101.00–109.00) | 103.00 (99.00–110.00) | <0.001 | 0.003 |
| Hip (cm) | 102.00 (98.00–106.00) | 108.00 (103.00–116.00) | 107.00 (101.00–115.00) | <0.001 | <0.001 |
| WHR (cm2) | 0.98 (0.89–1.01) | 0.97 (0.92–1.03) | 0.98 (0.92–1.020) | 0.431 | 0.641 |
| SABP (mmHg) | 120.00 (115.00–120.00) | 137.50 (130.00–145.00) | 130.00 (125.00–137.50) | <0.001 | <0.001 |
| DABP (mmHg) | 80.00 (70.00–85.00 | 80.00 (80.00–85.00) | 80.00 (70.00–82.50) | 0.347 | 0.021 |
| Glucose (mg/dL) | 90.00 (86.00–96.00) | 117.50 (110.00–124.50) | 115.00 (106.00–123.00) | <0.001 | 0.356 |
| HbA1c (%) | 5.15 ± 0.36 | 6.42 ± 0.73 | 6.37 ± 0.81 | <0.001 | 0.534 |
| Insulin (µIU/mL) | 7.30 (4.00–17.70) | 7.65 (3.10–12.85) | 7.65 (4.00–10.25) | 0.770 | 0.191 |
| HOMA-IR2 | 1.33 (0.52–2.11) | 1.10 (0.67–1.85) | 1.20 (0.73–1.70) | 0.739 | 0.670 |
| HOMA-S (%) | 113.10 (47.80–255.00) | 90.45 (54.45–183.50) | 85.10 (68.60–162.60) | 0.389 | 0.771 |
| HOMA-B (%) | 85.80 (57.50–160.45) | 60.40 (35.80–087.90) | 59.70 (36.45–78.95) | 0.009 | 0.774 |
| Index QUICKI (%) | 0.35 (0.32–0.41) | 0.34 (0.32–0.39) | 0.34 (0.32–0.37) | 0.396 | 0.760 |
| eGDR (mg/kg/min) | 9.74 (8.94–10.28) | 8.12 (6.64–9.23) | 8.70 (7.15–9.74) | <0.001 | 0.004 |
| Cholesterol (mg/dL) | 193.00 (173.00–223.00) | 188.00 (163.00–222.00) | 184.00 (170.00–206.00) | 0.714 | 0.180 |
| HDL-C (mg/dL) | 46.00 (41.00–52.00) | 48.70 (40.40–55.70) | 50.00 (41.20–59.40) | 0.527 | 0.077 |
| LDL-C (mg/dL) | 116.00 (103.00–148.00) | 117.90 (85.60–141.40) | 101.60 (77.40–124.00) | 0.999 | 0.008 |
| no-HDL-C (mg/dL) | 145.00 (122.00–174.60) | 142.00 (121.60–174.90) | 140.50 (115.80–157.00) | 0.860 | 0.259 |
| Triglicerydes (mg/dL) | 136.00 (107.00–176.00) | 120.00 (99.00–184.00) | 119.00 (94.00–174.90) | 0.680 | 0.434 |
| TG/HDL-C | 3.38 (1.95–4.03) | 2.45 (1.94–4.04) | 2.48 (1.76–3.74) | 0.615 | 0.205 |
| Parameter | Control (n = 26) | Patients with T2DM (n = 53) | p-Values | ||
|---|---|---|---|---|---|
| Baseline (T0) | 6 Months After Metformin Therapy (T1) | C vs. T0 | T0 vs. T1 | ||
| sCr (mg/dL) | 0.86 (0.77–0.91) | 0.82 (0.71–0.89) | 0.83 (0.73–0.92) | 0.070 | 0.006 |
| eGFR (mL/min.1.73m3) | 91.00 (82.00–100.00) | 95.00 (85.40–108.20) | 90.00 (76.00–105.00) | 0.188 | 0.004 |
| Cystatin C (µg /mL) | 1.11 (0.98–1.28) | 1.33 (1.13–1.47) | 1.15 (1.00–1.31) | 0.002 | <0.001 |
| uAlbumin (µg/mL) | 2.50 (2.50–6.40) | 3.90 (2.50–9.70) | 2.80 (2.50–6.90) | 0.257 | 0.656 |
| uCr (mg/dL) | 102.50 (64.00–129.00) | 101.00 (73.00–169.00) | 92.00 (70.00–151.00) | 0.549 | 0.598 |
| ACR (µg/mg Cr) | 5.00 (2.89–6.43) | 5.43 (3.05–8.04) | 4.31 (2.92–7.30) | 0.375 | 0.556 |
| Parameter | Patients with T2DM (n = 53) | |||
|---|---|---|---|---|
| Baseline (T0) | 6 Months After Metformin Therapy (T1) | |||
| NGAL (ng/mg Cr) | ||||
| r | p | r | p | |
| FN (ng/mg Cr) | 0.709 | <0.001 | 0.594 | <0.001 |
| LN (ng/mg Cr) | 0.646 | <0.001 | 0.479 | <0.001 |
| Parameter | Patients with T2DM (n = 53) | |||
|---|---|---|---|---|
| Baseline (T0) | 6 Months After Metformin Therapy (T1) | |||
| NGAL (ng/mg Cr) | ||||
| r | p | r | p | |
| sCr (mg/dL) | 0.056 | 0.699 | −0.063 | 0.659 |
| Cystatin C (µg /mL) | 0.120 | 0.498 | 0.022 | 0.904 |
| eGFR (mL/min.1.73m3) | −0.290 | 0.039 | −0.182 | 0.200 |
| uAlbumin (µg/mL) | −0.049 | 0.740 | −0189 | 0.188 |
| ACR (µg/mg Cr) | 0.323 | 0.023 | 0.287 | 0.046 |
| FN (ng/mg Cr) | ||||
| sCr (mg/dL) | −0.091 | 0.519 | −0.086 | 0.540 |
| Cystatin C (µg /mL) | 0.118 | 0.493 | 0.112 | 0.517 |
| eGFR (mL/min.1.73m3) | −0.053 | 0.706 | −0.069 | 0.624 |
| uAlbumin (µg/mL) | 0.032 | 0.826 | −0.074 | 0.600 |
| ACR (µg/mg Cr) | 0.384 | 0.005 | 0.470 | <0.001 |
| LN (ng/mg Cr) | ||||
| sCr (mg/dL) | 0.010 | 0.945 | 0.225 | 0.105 |
| Cystatin C (µg /mL) | 0.081 | 0.640 | 0.372 | 0.026 |
| eGFR (mL/min.1.73m3) | −0.003 | 0.984 | −0.188 | 0.178 |
| uAlbumin (µg/mL) | 0.102 | 0.475 | −0.050 | 0.723 |
| ACR (µg/mg Cr) | 0.422 | 0.002 | 0.208 | 0.143 |
| Parameter | Patients with T2DM (n = 53) | |
|---|---|---|
| % Change NGAL | ||
| r | p | |
| % change FN | 0.619 | <0.001 |
| % change LN | 0.566 | <0.001 |
| % change sCr | 0.040 | 0.783 |
| % change Cystatin C | −0.082 | 0.646 |
| % change eGFR | 0.017 | 0.905 |
| % change uAlbumin | −0.040 | 0.608 |
| % change ACR | 0.273 | 0.061 |
| Parameter | Patients with T2DM (n = 53) at Baseline (T0) | ||
|---|---|---|---|
| NGAL (ng/mg Cr) | |||
| β | p | Total Predict Model | |
| BMI (kg/m2) | 0.249 | 0.005 | R2: 0.162; estimated R2: 2.968 |
| SABP (mmHg) | 0.025 | 0.481 | p = 0.016 |
| FN (ng/mg Cr) | |||
| BMI (kg/m2) | 1.068 | 0.010 | R2: 0.153; estimated R2: 13.824 |
| SABP (mmHg) | −0.212 | 0.200 | p = 0.017 |
| LN (ng/mg Cr) | |||
| BMI (kg/m2) | 12.802 | 0.203 | R2: 0.070; estimated R2: 346.219 |
| SABP (mmHg) | −5.732 | 0.166 | p = 0.178 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szeremeta, A.; Jura-Półtorak, A.; Grim, A.; Kuźnik-Trocha, K.; Olczyk, P.; Ivanova, D.; Kiselova-Kaneva, Y.; Olczyk, K.; Komosińska-Vassev, K. Changes in Urinary NGAL, FN, and LN Excretion in Type 2 Diabetic Patients Following Anti-Diabetic Therapy with Metformin. J. Clin. Med. 2025, 14, 1088. https://doi.org/10.3390/jcm14041088
Szeremeta A, Jura-Półtorak A, Grim A, Kuźnik-Trocha K, Olczyk P, Ivanova D, Kiselova-Kaneva Y, Olczyk K, Komosińska-Vassev K. Changes in Urinary NGAL, FN, and LN Excretion in Type 2 Diabetic Patients Following Anti-Diabetic Therapy with Metformin. Journal of Clinical Medicine. 2025; 14(4):1088. https://doi.org/10.3390/jcm14041088
Chicago/Turabian StyleSzeremeta, Anna, Agnieszka Jura-Półtorak, Alicja Grim, Kornelia Kuźnik-Trocha, Paweł Olczyk, Diana Ivanova, Yoana Kiselova-Kaneva, Krystyna Olczyk, and Katarzyna Komosińska-Vassev. 2025. "Changes in Urinary NGAL, FN, and LN Excretion in Type 2 Diabetic Patients Following Anti-Diabetic Therapy with Metformin" Journal of Clinical Medicine 14, no. 4: 1088. https://doi.org/10.3390/jcm14041088
APA StyleSzeremeta, A., Jura-Półtorak, A., Grim, A., Kuźnik-Trocha, K., Olczyk, P., Ivanova, D., Kiselova-Kaneva, Y., Olczyk, K., & Komosińska-Vassev, K. (2025). Changes in Urinary NGAL, FN, and LN Excretion in Type 2 Diabetic Patients Following Anti-Diabetic Therapy with Metformin. Journal of Clinical Medicine, 14(4), 1088. https://doi.org/10.3390/jcm14041088








