Prevalence and Misperception: Exploring the Gap Between Objective and Subjective Assessment of Sleep Apnea in a Population at Increased Risk for Dementia
Abstract
1. Introduction
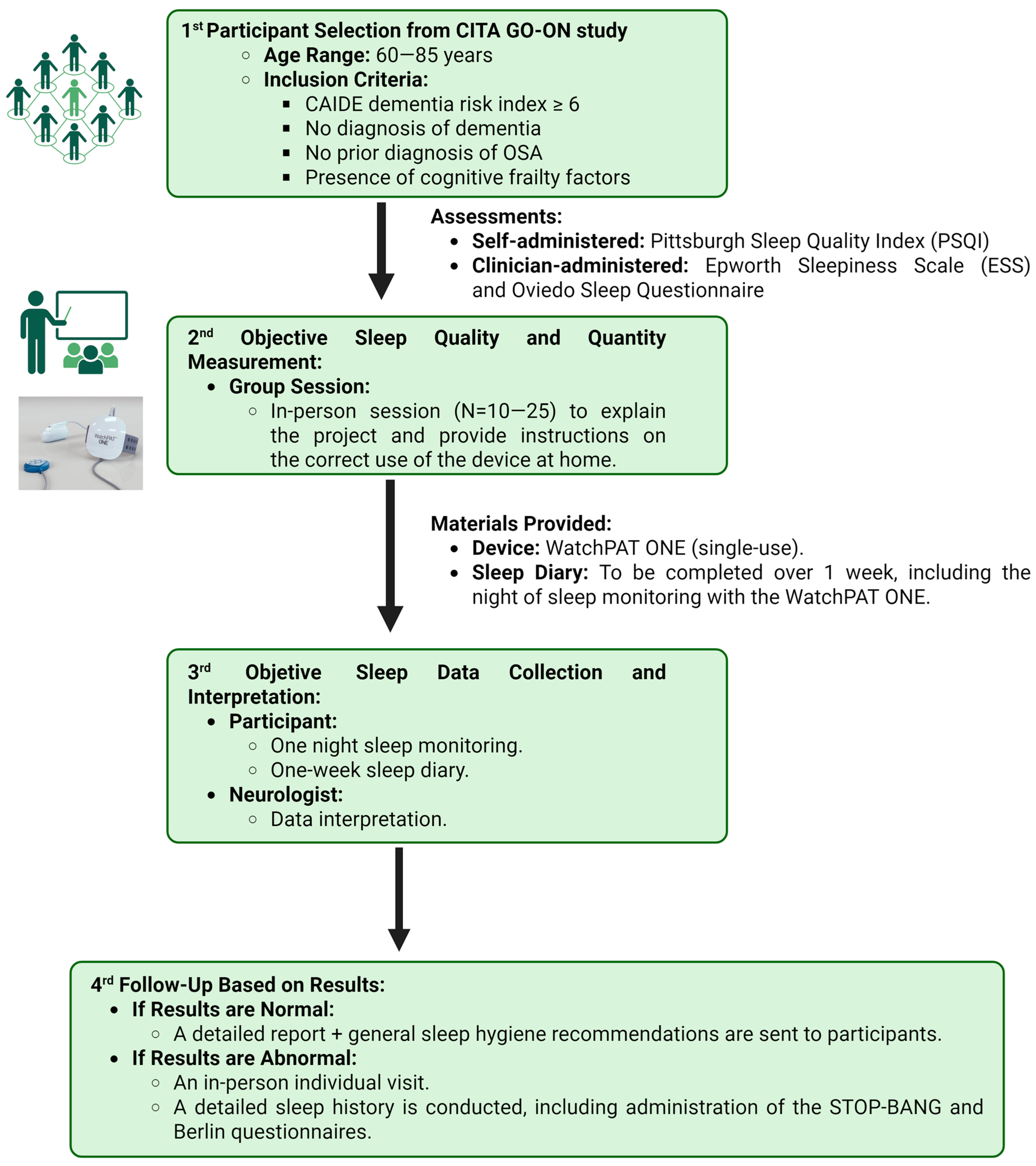
2. Methodology
2.1. Inclusion and Exclusion Criteria
2.2. Sleep Assessment Procedure
2.2.1. Subjective Sleep Quality Assessment
2.2.2. Home Sleep Study Using Wearable PAT Technology and Sleep Diary
2.2.3. Manual Correction of Sleep Record and Results Communication
2.3. Data Acquisition and Analysis
3. Results
3.1. Sample Characteristics
3.2. Subjective Sleep Assessment
3.2.1. Pittsburg Sleep Quality Index (PSQI)
3.2.2. Epworth Sleepiness Scale (ESS) and Oviedo Sleep Questionnaire
3.2.3. 7-Day Sleep Diary
3.3. Objective Sleep Assessment
4. Discussion
4.1. Limitations
4.2. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alzheimer Europe. Dementia in Europe Yearbook 2021; Alzheimer Europe: Luxembourg, 2021; p. 178. [Google Scholar]
- Nichols, E.; Steinmetz, J.D.; Vollset, S.E.; Fukutaki, K.; Chalek, J.; Abd-Allah, F.; Abdoli, A.; Abualhasan, A.; Abu-Gharbieh, E.; Akram, T.T.; et al. Estimation of the Global Prevalence of Dementia in 2019 and Forecasted Prevalence in 2050: An Analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, A.; Norton, N.; Fast, T.; Frölich, L.; Georges, J.; Holzapfel, D.; Kirabali, T.; Krolak-Salmon, P.; Rossini, P.M.; Ferretti, M.T.; et al. Global Estimates on the Number of Persons across the Alzheimer’s Disease Continuum. Alzheimers Dement. 2023, 19, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Liu, K.Y.; Costafreda, S.G.; Selbæk, G.; Alladi, S.; Ames, D.; Banerjee, S.; Burns, A.; Brayne, C.; et al. Dementia Prevention, Intervention, and Care: 2024 Report of the Lancet Standing Commission. Lancet 2024, 404, 572–628. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.; Lovier, M.A. Sleep Disturbances and Dementia Risk in Older Adults: Findings From 10 Years of National U.S. Prospective Data. Am. J. Prev. Med. 2023, 64, 781–787. [Google Scholar] [CrossRef]
- Jaqua, E.E.; Hanna, M.; Labib, W.; Moore, C.; Matossian, V. Common Sleep Disorders Affecting Older Adults. Perm. J. 2023, 27, 122–132. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, W.; Wang, M. Characteristics of Macroscopic Sleep Structure in Patients with Mild Cognitive Impairment: A Systematic Review. Front. Psychiatry 2023, 14, 1212514. [Google Scholar] [CrossRef]
- Blackman, J.; Swirski, M.; Clynes, J.; Harding, S.; Leng, Y.; Coulthard, E. Pharmacological and Non-Pharmacological Interventions to Enhance Sleep in Mild Cognitive Impairment and Mild Alzheimer’s Disease: A Systematic Review. J. Sleep Res. 2021, 30, e13229. [Google Scholar] [CrossRef]
- Pase, M.P.; Harrison, S.; Misialek, J.R.; Kline, C.E.; Cavuoto, M.; Baril, A.-A.; Yiallourou, S.; Bisson, A.; Himali, D.; Leng, Y.; et al. Sleep Architecture, Obstructive Sleep Apnea, and Cognitive Function in Adults. JAMA Netw. Open 2023, 6, e2325152. [Google Scholar] [CrossRef]
- Cavuoto, M.G.; Robinson, S.R.; O’Donoghue, F.J.; Barnes, M.; Howard, M.E.; Tolson, J.; Stevens, B.; Schembri, R.; Rosenzweig, I.; Rowe, C.C.; et al. Associations Between Amyloid Burden, Hypoxemia, Sleep Architecture, and Cognition in Obstructive Sleep Apnea. J. Alzheimers Dis. 2023, 96, 149–159. [Google Scholar] [CrossRef]
- Bubu, O.M.; Andrade, A.G.; Umasabor-Bubu, O.Q.; Hogan, M.M.; Turner, A.D.; de Leon, M.J.; Ogedegbe, G.; Ayappa, I.; Jean-Louis, G.G.; Jackson, M.L.; et al. Obstructive Sleep Apnea, Cognition and Alzheimer’s Disease: A Systematic Review Integrating Three Decades of Multidisciplinary Research. Sleep Med. Rev. 2020, 50, 101250. [Google Scholar] [CrossRef]
- Tian, Q.; Sun, J.; Li, X.; Liu, J.; Zhou, H.; Deng, J.; Li, J. Association between Sleep Apnoea and Risk of Cognitive Impairment and Alzheimer’s Disease: A Meta-Analysis of Cohort-Based Studies. Sleep Breath. 2024, 28, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Osorio, R.S.; Martínez-García, M.Á.; Rapoport, D.M. Sleep Apnoea in the Elderly: A Great Challenge for the Future. Eur. Respir. J. 2022, 59, 2101649. [Google Scholar] [CrossRef] [PubMed]
- Gomes, T.; Benedetti, A.; Postuma, R.; Rizzo, D.; Baltzan, M.; Kimoff, R.J.; Kaminska, M. Comparison of Sleep Apnea Questionnaires and Reported Diagnosis in Neurological Disorders of Aging. Can. J. Neurol. Sci. 2025, 52, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Braley, T.J.; Dunietz, G.L.; Chervin, R.D.; Lisabeth, L.D.; Skolarus, L.E.; Burke, J.F. Recognition and Diagnosis of Obstructive Sleep Apnea in Older Americans. J. Am. Geriatr. Soc. 2018, 66, 1296–1302. [Google Scholar] [CrossRef]
- Oktay Arslan, B.; Uçar Hoşgör, Z.Z.; Orman, M.N. Which Screening Questionnaire Is Best for Predicting Obstructive Sleep Apnea in the Sleep Clinic Population Considering Age, Gender, and Comorbidities? Turk. Thorac. J. 2020, 21, 383–389. [Google Scholar] [CrossRef]
- Schnall, R.P.; Sheffy, J.; Penzel, T. Peripheral Arterial Tonometry–PAT Technology. Sleep Med. Rev. 2022, 61, 101566. [Google Scholar] [CrossRef]
- Campbell, C.D.; Sulaiman, I. The Role of the WatchPAT Device in the Diagnosis and Management of Obstructive Sleep Apnea. Front. Sleep 2023, 2, 1148316. [Google Scholar] [CrossRef]
- Fekedulegn, D.; Andrew, M.E.; Shi, M.; Violanti, J.M.; Knox, S.; Innes, K.E. Actigraphy-Based Assessment of Sleep Parameters. Ann. Work Expo. Health 2020, 64, 350–367. [Google Scholar] [CrossRef]
- Ecay-Torres, M.; Estanga, A.; Tainta, M.; Izagirre, A.; Garcia-Sebastian, M.; Villanua, J.; Clerigue, M.; Iriondo, A.; Urreta, I.; Arrospide, A.; et al. Increased CAIDE Dementia Risk, Cognition, CSF Biomarkers, and Vascular Burden in Healthy Adults. Neurology 2018, 91, e217–e226. [Google Scholar] [CrossRef]
- Effectiveness of a Lifestyle Multidomain Intervention to Prevent Cognitive Decline in the Basque Country. Goiz Zaindu Gipuzkoa—Cita Go-On Study. 2021. Available online: https://clinicaltrials.gov/study/NCT04840030?term=cita%20go-on&rank=1 (accessed on 2 January 2025).
- Carnero-Pardo, C.; Sáez-Zea, C.; Montiel-Navarro, L.; Feria-Vilar, I.; Gurpegui, M. Estudio Normativo y de Fiabilidad Del Fototest. Neurología 2011, 26, 20–25. [Google Scholar] [CrossRef]
- Rami, L.; Bosch, B.; Sanchez-Valle, R.; Molinuevo, J.L. The Memory Alteration Test (M@T) Discriminates between Subjective Memory Complaints, Mild Cognitive Impairment and Alzheimer’s Disease. Arch. Gerontol. Geriatr. 2010, 50, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Tainta, M.; Iriondo, A.; Ecay-Torres, M.; Estanga, A.; de Arriba, M.; Barandiaran, M.; Clerigue, M.; Garcia-Sebastian, M.; Villanua, J.; Izagirre, A.; et al. Brief Cognitive Tests as a Decision-Making Tool in Primary Care. A Population and Validation Study. Neurologia 2022, 39, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Rattanabannakit, C.; Risacher, S.L.; Gao, S.; Lane, K.A.; Brown, S.A.; McDonald, B.C.; Unverzagt, F.W.; Apostolova, L.G.; Saykin, A.J.; Farlow, M.R. The Cognitive Change Index as a Measure of Self and Informant Perception of Cognitive Decline: Relation to Neuropsychological Tests. J. Alzheimers Dis. 2016, 51, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Ngandu, T.; Lehtisalo, J.; Solomon, A.; Levälahti, E.; Ahtiluoto, S.; Antikainen, R.; Bäckman, L.; Hänninen, T.; Jula, A.; Laatikainen, T.; et al. A 2 Year Multidomain Intervention of Diet, Exercise, Cognitive Training, and Vascular Risk Monitoring versus Control to Prevent Cognitive Decline in at-Risk Elderly People (FINGER): A Randomised Controlled Trial. Lancet 2015, 385, 2255–2263. [Google Scholar] [CrossRef]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The barthel index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A New Instrument for Psychiatric Practice and Research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Bobes, J.; González, M.P.; Vallejo, J.; Sáiz, J.; Gibert, J.; Ayuso, J.L.; Rico, F. Oviedo Sleep Questionnaire (OSQ): A New Semistructured Interview for Sleep Disorders. Eur. Neuropsychopharmacol. 1998, 8, S162. [Google Scholar] [CrossRef]
- Guimarães, C.; Martins, M.V.; Vaz Rodrigues, L.; Teixeira, F.; Moutinho dos Santos, J. Epworth Sleepiness Scale in Obstructive Sleep Apnea Syndrome—An Underestimated Subjective Scale. Pulmonology 2012, 18, 267–271. [Google Scholar] [CrossRef]
- Itamar. Available online: https://www.itamar-medical.com/watchpat-one/ (accessed on 1 December 2024).
- Yalamanchali, S.; Farajian, V.; Hamilton, C.; Pott, T.R.; Samuelson, C.G.; Friedman, M. Diagnosis of Obstructive Sleep Apnea by Peripheral Arterial Tonometry: Meta-Analysis. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 1343–1350. [Google Scholar] [CrossRef]
- Hedner, J.; White, D.P.; Malhotra, A.; Herscovici, S.; Pittman, S.D.; Zou, D.; Grote, L.; Pillar, G. Sleep Staging Based on Autonomic Signals: A Multi-Center Validation Study. J. Clin. Sleep Med. 2011, 07, 301–306. [Google Scholar] [CrossRef]
- Zhang, Z.; Sowho, M.; Otvos, T.; Sperandio, L.S.; East, J.; Sgambati, F.; Schwartz, A.; Schneider, H. A Comparison of Automated and Manual Sleep Staging and Respiratory Event Recognition in a Portable Sleep Diagnostic Device with In-Lab Sleep Study. J. Clin. Sleep Med. 2020, 16, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Cruces-Artero, C.; Hervés-Beloso, C.; Martín-Miguel, V.; Hernáiz-Valero, S.; Lago-Deibe, F.I.; Montero-Gumucio, M.; Orge-Amoedo, M.; Roca-Pardiñas, J.; Clavería, A. Diagnostic accuracy of STOP-Bang questionnaire on moderate sleep apnoea in primary care. Gac. Sanit. 2019, 33, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.; Yin, J.D.C.; Tan, L.W.L.; van Dam, R.M.; Cheung, Y.Y.; Lee, C.-H. Using the Berlin Questionnaire to Predict Obstructive Sleep Apnea in the General Population. J. Clin. Sleep Med. 2017, 13, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Benz, F.; Riemann, D.; Domschke, K.; Spiegelhalder, K.; Johann, A.F.; Marshall, N.S.; Feige, B. How Many Hours Do You Sleep? A Comparison of Subjective and Objective Sleep Duration Measures in a Sample of Insomnia Patients and Good Sleepers. J. Sleep Res. 2023, 32, e13802. [Google Scholar] [CrossRef]
- Gosselin, N.; Baril, A.-A.; Osorio, R.S.; Kaminska, M.; Carrier, J. Obstructive Sleep Apnea and the Risk of Cognitive Decline in Older Adults. Am. J. Respir. Crit. Care Med. 2019, 199, 142–148. [Google Scholar] [CrossRef]
- Li, N.; Liu, Y.; Zhao, Y.; Wu, X.; Tong, J.; Hua, Y. Cerebrovascular Reactivity in Young and Old Patients with Obstructive Sleep Apnea. Sleep Med. 2020, 73, 125–129. [Google Scholar] [CrossRef]
- Chen, R.; Zhu, J.; Yang, Y.; Liao, W.; Ye, W.; Du, L.; Chen, M.; Zhang, Y.; Yao, W.; Zheng, Z. Evaluation of Five Questionnaires for Obstructive Sleep Apnea Screening in the Elderly. Sci. Rep. 2025, 15, 1689. [Google Scholar] [CrossRef]
- Fietze, I.; Laharnar, N.; Obst, A.; Ewert, R.; Felix, S.B.; Garcia, C.; Gläser, S.; Glos, M.; Schmidt, C.O.; Stubbe, B.; et al. Prevalence and Association Analysis of Obstructive Sleep Apnea with Gender and Age Differences—Results of SHIP-Trend. J. Sleep Res. 2019, 28, e12770. [Google Scholar] [CrossRef]
- Bauters, F.A.; Loof, S.; Hertegonne, K.B.; Chirinos, J.A.; De Buyzere, M.L.; Rietzschel, E.R. Sex-Specific Sleep Apnea Screening Questionnaires: Closing the Performance Gap in Women. Sleep Med. 2020, 67, 91–98. [Google Scholar] [CrossRef]
- Pataka, A.; Kotoulas, S.; Kalamaras, G.; Schiza, S.; Sapalidis, K.; Giannakidis, D.; Michalopoulos, N.; Koulouris, C.; Aidoni, Z.; Amaniti, A.; et al. Gender Differences in Obstructive Sleep Apnea: The Value of Sleep Questionnaires with a Separate Analysis of Cardiovascular Patients. J. Clin. Med. 2020, 9, 130. [Google Scholar] [CrossRef]
- Canham, S.L.; Gallo, J.; Simoni-Wastila, L. Perceptions of Benzodiazepine Dependence among Women Age 65 and Older. J. Gerontol. Soc. Work 2014, 57, 872–888. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Nakamura, T.; Hayano, J.; Yamamoto, Y. Age and Gender Differences in Objective Sleep Properties Using Large-Scale Body Acceleration Data in a Japanese Population. Sci. Rep. 2021, 11, 9970. [Google Scholar] [CrossRef] [PubMed]
- Pantoja-Ruiz, C.; Uriza, F.; Millan, S.; Hidalgo, P.; Reyes, P.; Morrillo, C.; Hartsfield, J.K.; Otero, L. Potential Biomarkers of Mild Cognitive Impairment in Obstructive Sleep Apnea. ERJ Open Res. 2023, 9, 53. [Google Scholar] [CrossRef]
- Schwerthöffer, D.; Haselwarter, T.; Grimmer, T. Obstructive Sleep Apnea Among Patients with Mild Cognitive Impairment. J. Alzheimers Dis. 2024, 100, 809–823. [Google Scholar] [CrossRef]
- Robbins, R.; Weaver, M.D.; Sullivan, J.P.; Quan, S.F.; Gilmore, K.; Shaw, S.; Benz, A.; Qadri, S.; Barger, L.K.; Czeisler, C.A.; et al. Accuracy of Three Commercial Wearable Devices for Sleep Tracking in Healthy Adults. Sensors 2024, 24, 6532. [Google Scholar] [CrossRef]
- Ioachimescu, O.C.; Allam, J.S.; Samarghandi, A.; Anand, N.; Fields, B.G.; Dholakia, S.A.; Venkateshiah, S.B.; Eisenstein, R.; Ciavatta, M.-M.; Collop, N.A. Performance of Peripheral Arterial Tonometry-Based Testing for the Diagnosis of Obstructive Sleep Apnea in a Large Sleep Clinic Cohort. J. Clin. Sleep Med. 2020, 16, 1663–1674. [Google Scholar] [CrossRef]
- Onder, N.S.; Akpinar, M.E.; Yigit, O.; Gor, A.P. Watch Peripheral Arterial Tonometry in the Diagnosis of Obstructive Sleep Apnea: Influence of Aging. Laryngoscope 2012, 122, 1409–1414. [Google Scholar] [CrossRef]
- Bradicich, M.; Sievi, N.A.; Grewe, F.A.; Gasperetti, A.; Kohler, M.; Schwarz, E.I. Nocturnal Heart Rate Variability in Obstructive Sleep Apnoea: A Cross-Sectional Analysis of the Sleep Heart Health Study. J. Thorac. Dis. 2020, 12, S129–S138. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Wu, S.-M.; Kuan, Y.-C.; Lin, Y.-T.; Hsu, C.-R.; Hsu, W.-H.; Liu, Y.-S.; Majumdar, A.; Stettler, M.; Yang, C.-M.; et al. Associations between Risk of Alzheimer’s Disease and Obstructive Sleep Apnea, Intermittent Hypoxia, and Arousal Responses: A Pilot Study. Front. Neurol. 2022, 13, 1038735. [Google Scholar] [CrossRef]
- Kazim, S.F.; Sharma, A.; Saroja, S.R.; Seo, J.H.; Larson, C.S.; Ramakrishnan, A.; Wang, M.; Blitzer, R.D.; Shen, L.; Peña, C.J.; et al. Chronic Intermittent Hypoxia Enhances Pathological Tau Seeding, Propagation, and Accumulation and Exacerbates Alzheimer-like Memory and Synaptic Plasticity Deficits and Molecular Signatures. Biol. Psychiatry 2022, 91, 346–358. [Google Scholar] [CrossRef]
- Martinez-Garcia, M.A.; Oscullo, G.; Ponce, S.; Pastor, E.; Orosa, B.; Catalán, P.; Martinez, A.; Hernandez, L.; Muriel, A.; Chiner, E.; et al. Effect of Continuous Positive Airway Pressure in Very Elderly with Moderate-to-Severe Obstructive Sleep Apnea Pooled Results from Two Multicenter Randomized Controlled Trials. Sleep Med. 2022, 89, 71–77. [Google Scholar] [CrossRef]
| Female (n = 157) | Male (n = 165) | |||
|---|---|---|---|---|
| Age (mean ± SD) | 72.1 ± 6.4 years | 70.73 ± 5.86 years | p < 0.04 * | |
| Syndromic diagnosis | CU | 131 (83.4%) | 146 (88.5%) | p = 0.297 |
| SMC | 9 (5.7%) | 9 (5.5%) | ||
| MCI | 17 (10.8%) | 10 (6.1%) | ||
| HTA | 70 (44.6%) | 105 (63.6%) | p = 0.001 * | |
| DLP | 69 (43.9%) | 78 (47.3%) | p = 0.550 | |
| DM | 16 (10.2%) | 21 (12.7%) | p = 0.476 | |
| Tobacco | Non-smoker | 88 (56.1%) | 66 (40%) | p = 0.015 * |
| Former smoker | 60 (38.2%) | 87 (52.7%) | ||
| Current smoker | 9 (5.7%) | 12 (7.3%) | ||
| Pacs/years | 22.1 ± 22.9 | 22.9 ± 19.2 | p = 0.824 | |
| WAU | 5.7 ± 7.4 | 13.3 ± 14 | p < 0.001 * | |
| BMI | 26.9 ± 4.8 | 27.2 ± 4.3 | p = 0.453 | |
| CAIDE index | 7.6 ± 1.6 | 7.8 ± 1.4 | p = 0.266 | |
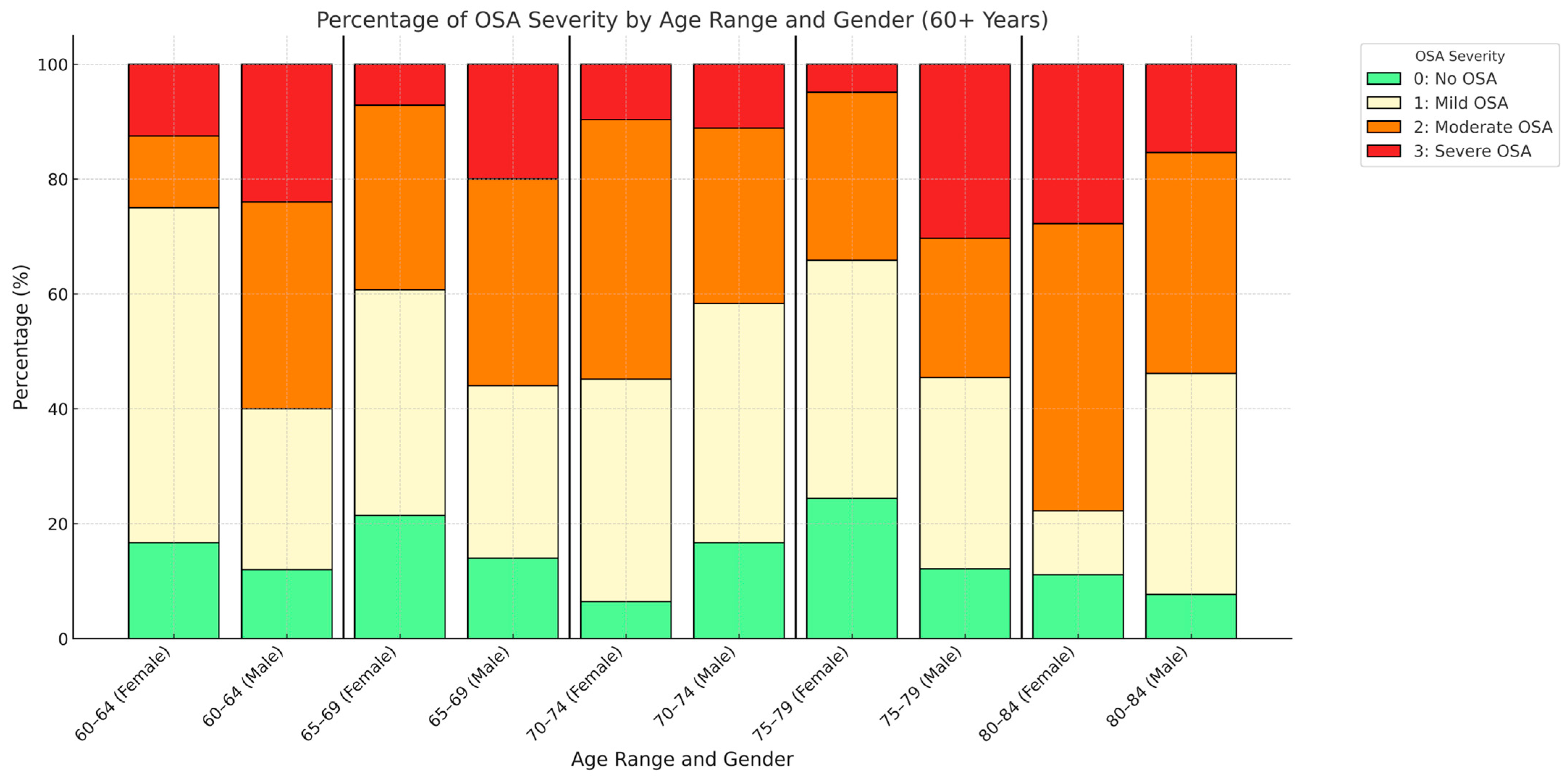
| No OSA (n = 45) | Mild OSA (n = 109) | Moderate OSA (n = 98) | Severe OSA (n = 47) | ||||
|---|---|---|---|---|---|---|---|
| Sex (female %) | 53.33% | 51.38% | 47.96% | 31.91% | p = 0.112 | ||
| Age (years) | 71.49 ± 6.32 | 70.67 ± 5.93 | 71.77 ± 6 | 71.51 ± 6.91 | p = 0.618 | ||
| Syndromic diagnosis | CU | 95.56% | 85.32% | 86.73% | 80.85% | p = 0.125 | |
| SMC | 0% | 9.17% | 3.06% | 8.51% | |||
| MCI | 4.44% | 5.5% | 10.20% | 10.63% | |||
| HTA | 46.67% | 52.29% | 57.14% | 63.83% | p = 0.353 | ||
| DLP | 40% | 52.29% | 42.86% | 42.55% | p = 0.391 | ||
| DM | 6.67% | 11.93% | 11.22% | 17.02% | p = 0.490 | ||
| BMI | 24.41 ± 5.41 | 26.36 ± 3.76 | 27.47 ± 3.93 | 30.46 ± 4.91 | p < 0.001 * | ||
| Tobacco | Non-smoker | 48.89% | 45.87% | 47.96% | 46.80% | p = 0.238 | |
| Former smoker | 35.56% | 49.54% | 45.92% | 48.93% | |||
| Current smoker | 15.56% | 4.59% | 6.12% | 4.26% | |||
| WAU | 8.98 ± 11.02 | 10.04 ± 12.88 | 8.05 ± 9.39 | 12.19 ± 13.89 | p = 0.205 | ||
| CAIDE index | 7.31 ± 1.06 | 7.59 ± 1.55 | 7.81 ± 1.48 | 8.32 ± 1.49 | p = 0.006 * | ||
| M@T | 41.31 ± 4.48 | 41.26 ± 5.82 | 40.71 ± 4.58 | 39.17 ± 6.01 | p = 0.129 | ||
| Fototest | 38.67 ± 6.34 | 38.49 ± 6.27 | 37.68 ± 5.76 | 38.19 ± 4.82 | p = 0.729 | ||
| Saykin 12 items | 24.44 ± 8.22 | 24.64 ± 7.62 | 23.31 ± 7.84 | 25.11 ± 13.88 | p = 0.633 | ||
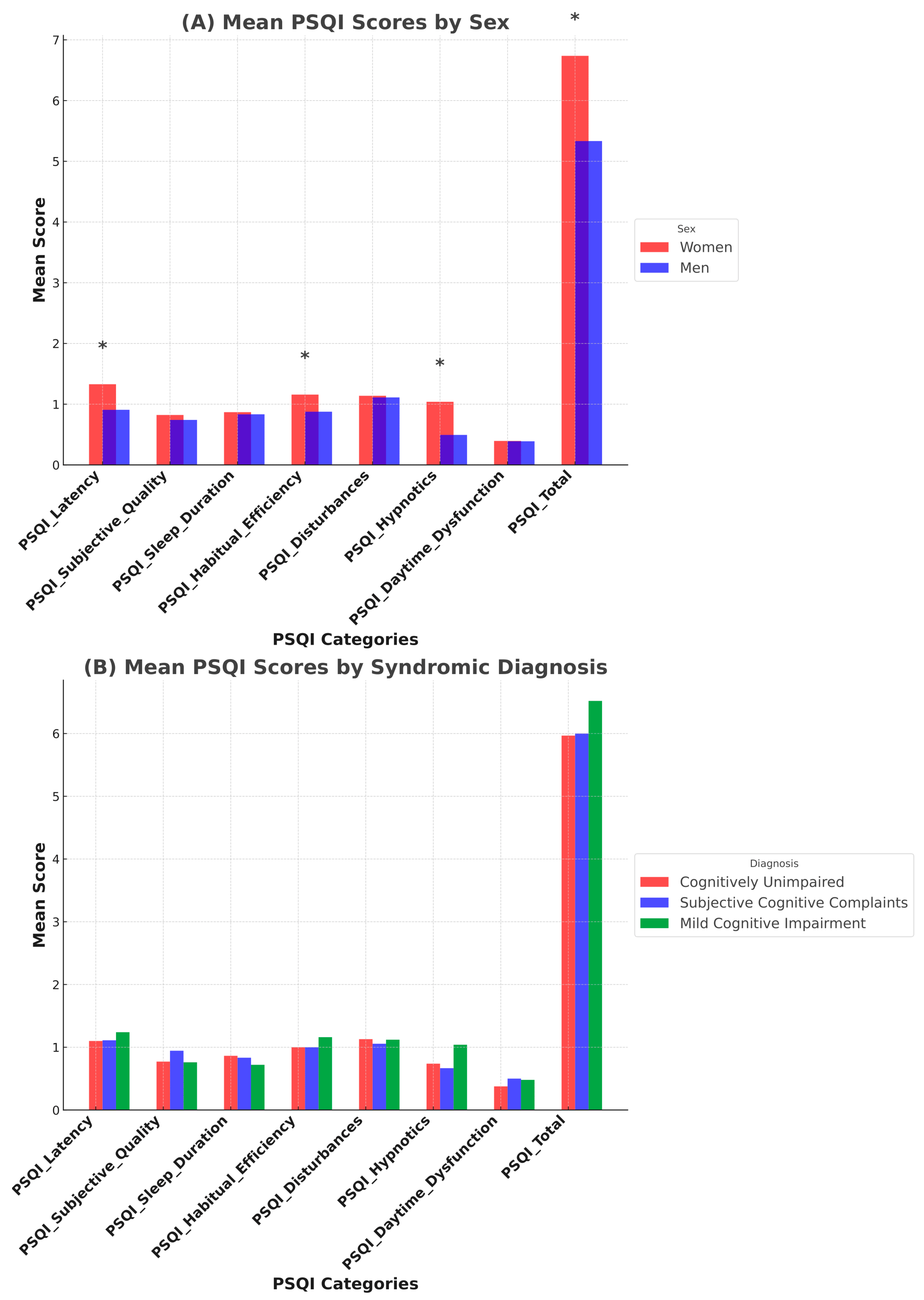
| PSQI | Sleep latency | 1.16 ± 0.98 | 1.21 ± 0.99 | 1.08 ± 1.02 | 0.98 ± 0.99 | p = 0.568 | |
| Subjective sleep quality | 0.89 ± 0.95 | 0.79 ± 0.89 | 0.76 ± 0.91 | 0.72 ± 0.79 | p = 0.832 | ||
| Sleep duration | 0.8 ± 0.63 | 0.89 ± 0.65 | 0.79 ± 0.73 | 0.93 ± 0.78 | p = 0.574 | ||
| Sleep efficiency | 0.98 ± 1.01 | 1.05 ± 1.03 | 1.04 ± 1.09 | 0.96 + 1.09 | p = 0.954 | ||
| Sleep disturbances | 1.11 ± 0.52 | 1.17 ± 0.52 | 1.07 ± 0.51 | 1.15 ± 0.47 | p = 0.601 | ||
| Hypnotic use | 0.82 ± 1.30 | 0.69 ± 1.22 | 0.87 ± 1.33 | 0.62 ± 1.15 | p = 0.626 | ||
| Daytime dysfunction | 0.38 ± 0.58 | 0.34 ± 0.53 | 0.36 ± 0.59 | 0.36 ± 0.67 | p = 0.984 | ||
| Total score | 6.11 ± 3.89 | 6.09 ± 3.85 | 5.89 ± 3.70 | 5.59 ± 3.03 | p = 0.871 | ||
| Oviedo | Subjective satisfaction | 4.96 ± 1.48 | 4.55 ± 1.63 | 4.62 ± 1.59 | 4.57 ± 1.59 | p = 0.538 | |
| Insomnia | 14.2 ± 6.56 | 16.82 ± 7.28 | 15.62 ± 6.64 | 20 ± 35.92 | p = 0.295 | ||
| Hypersomnia | 3.47 ± 1.73 | 3.95 ± 1.99 | 4.04 ± 2.46 | 3.74 ± 1.42 | p = 0.434 | ||
| Total score | 22.62 ± 6.56 | 25.36 ± 7.36 | 24.36 ± 6.83 | 23.40 ± 5.91 | p = 0.110 | ||
| ESS | 2.71 ± 3.09 | 2.60 ± 2.53 | 3.04 ± 3.25 | 3.75 ± 2.99 | p = 0.151 | ||
| Hypnotic use (%) | 26.67 | 22.94 | 32.65 | 25.54 | p = 0.466 | ||
| Benzodiazepines (%) | 24.44 | 17.43 | 27.55 | 21.28 | p = 0.364 | ||
| No OSA (n = 45) | Mild OSA (n = 109) | Moderate OSA (n = 98) | Severe OSA (n = 47) | ||
|---|---|---|---|---|---|
| Usage Time of WatchPAT ONE (hours) | 8.17 ± 1.03 | 8.20 ± 1.03 | 8.34 ± 0.96 | 7.99 ± 1.02 | p = 0.273 |
| AHI | 2.82 ± 1.19 | 10.23 ± 2.87 | 21.39 ± 4.42 | 42.15 ± 9.88 | p < 0.001 * |
| ODI3 | 3.73 ± 1.68 | 12.04 ± 3.71 | 23.07 ± 4.85 | 43.76 ± 10.01 | p < 0.001 * |
| Minimum satO2 (%) | 86.62 ± 5.11 | 83.72 ± 6.09 | 82.29 ± 6.03 | 76.64 ± 9.04 | p < 0.001 * |
| Mean satO2 (%) | 94.51 ± 1.31 | 93.66 ± 1.66 | 93.16 ± 1.98 | 92.64 ± 1.39 | p < 0.001 * |
| Maximum satO2 (%) | 98.29 ± 0.92 | 98.22 ± 1.12 | 97.94 ± 1.55 | 98.38 ± 0.85 | p = 0.136 |
| Nadir satO2 (%) | 92.87 ± 1.19 | 92.06 ± 1.87 | 91.55 ± 1.30 | 90.32 ± 1.87 | p < 0.001 * |
| Minimum heart rate | 47.33 ± 7.05 | 46.18 ± 6.88 | 43.70 ± 8.15 | 44.67 ± 6.29 | p = 0.016 * |
| Mean heart rate | 60.18 ± 7.73 | 58.83 ± 8.50 | 57.69 ± 8.51 | 59.23 ± 8.51 | p = 0.374 |
| Maximum heart rate | 92.64 ± 9.29 | 89.62 ± 15.22 | 90.11 ± 13.96 | 94.91 ± 14.86 | p = 0.172 |
| Nocturnal heart rate variability | 45.31 ± 10.81 | 43.44 ± 13.62 | 46.44 ± 13.62 | 50.45 ± 19.69 | p = 0.045 * |
| Sleep time with WatchPAT ONE (hours) | 7.28 ± 0.93 | 7.23 ± 0.87 | 7.35 ± 1.07 | 7.28 ± 0.97 | p = 0.831 |
| Sleep efficiency (%) | 89.05 ± 3.82 | 87.64 ± 4.25 | 86.93 ± 8.83 | 89.05 ± 5.25 | p = 0.131 |
| REM sleep (%) | 15.61 ± 3.47 | 15.87 ± 3.4 | 15.95 ± 7.69 | 14.34 ± 3.85 | p = 0.343 |
| Light NREM sleep (%) | 63.99 ± 6.86 | 64.52 ± 7.95 | 69.16 ± 9.06 | 74.91 ± 7.34 | p < 0.001 * |
| Deep NREM sleep (%) | 20.54 ± 6.07 | 19.14 ± 4.84 | 15.27 ± 7.13 | 11.03 ± 4.92 | p < 0.001 * |
| Sleep latency (min) | 17.11 ± 9.41 | 17.13 ± 12.42 | 17.54 ± 7.95 | 14.51 ± 7.15 | p = 0.352 |
| Number of awakenings | 6.44 ± 3.14 | 8.11 ± 4.29 | 9.35 ± 6.78 | 10 ± 7.60 | p = 0.009 * |
| WASO (wake after sleep onset) (min) | 36 ± 18.33 | 44.40 ± 22.89 | 44.32 ± 25.27 | 36.43 ± 26.27 | p = 0.058 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altuna, M.; García-Sebastián, M.; Ecay-Torres, M.; Saldias, J.; Cañada, M.; Estanga, A.; López, C.; Tainta, M.; Iriondo, A.; Arriba, M.; et al. Prevalence and Misperception: Exploring the Gap Between Objective and Subjective Assessment of Sleep Apnea in a Population at Increased Risk for Dementia. J. Clin. Med. 2025, 14, 2607. https://doi.org/10.3390/jcm14082607
Altuna M, García-Sebastián M, Ecay-Torres M, Saldias J, Cañada M, Estanga A, López C, Tainta M, Iriondo A, Arriba M, et al. Prevalence and Misperception: Exploring the Gap Between Objective and Subjective Assessment of Sleep Apnea in a Population at Increased Risk for Dementia. Journal of Clinical Medicine. 2025; 14(8):2607. https://doi.org/10.3390/jcm14082607
Chicago/Turabian StyleAltuna, Miren, Maite García-Sebastián, Mirian Ecay-Torres, Jon Saldias, Marta Cañada, Ainara Estanga, Carolina López, Mikel Tainta, Ane Iriondo, Maria Arriba, and et al. 2025. "Prevalence and Misperception: Exploring the Gap Between Objective and Subjective Assessment of Sleep Apnea in a Population at Increased Risk for Dementia" Journal of Clinical Medicine 14, no. 8: 2607. https://doi.org/10.3390/jcm14082607
APA StyleAltuna, M., García-Sebastián, M., Ecay-Torres, M., Saldias, J., Cañada, M., Estanga, A., López, C., Tainta, M., Iriondo, A., Arriba, M., Ros, N., & Martínez-Lage, P. (2025). Prevalence and Misperception: Exploring the Gap Between Objective and Subjective Assessment of Sleep Apnea in a Population at Increased Risk for Dementia. Journal of Clinical Medicine, 14(8), 2607. https://doi.org/10.3390/jcm14082607






