Management of Hemodynamic and Respiratory Instability and Anesthetic Approaches in Patients Undergoing Pulmonary Thrombectomy for Pulmonary Embolism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement and Regulation
2.2. Study Design and Participants
2.3. Study Outcomes
2.4. Data Collection and Data Measurement
2.5. Data Analysis and Statistical Plan
3. Results
3.1. Underlying Conditions and PE Etiology According to the Occurrence of CA
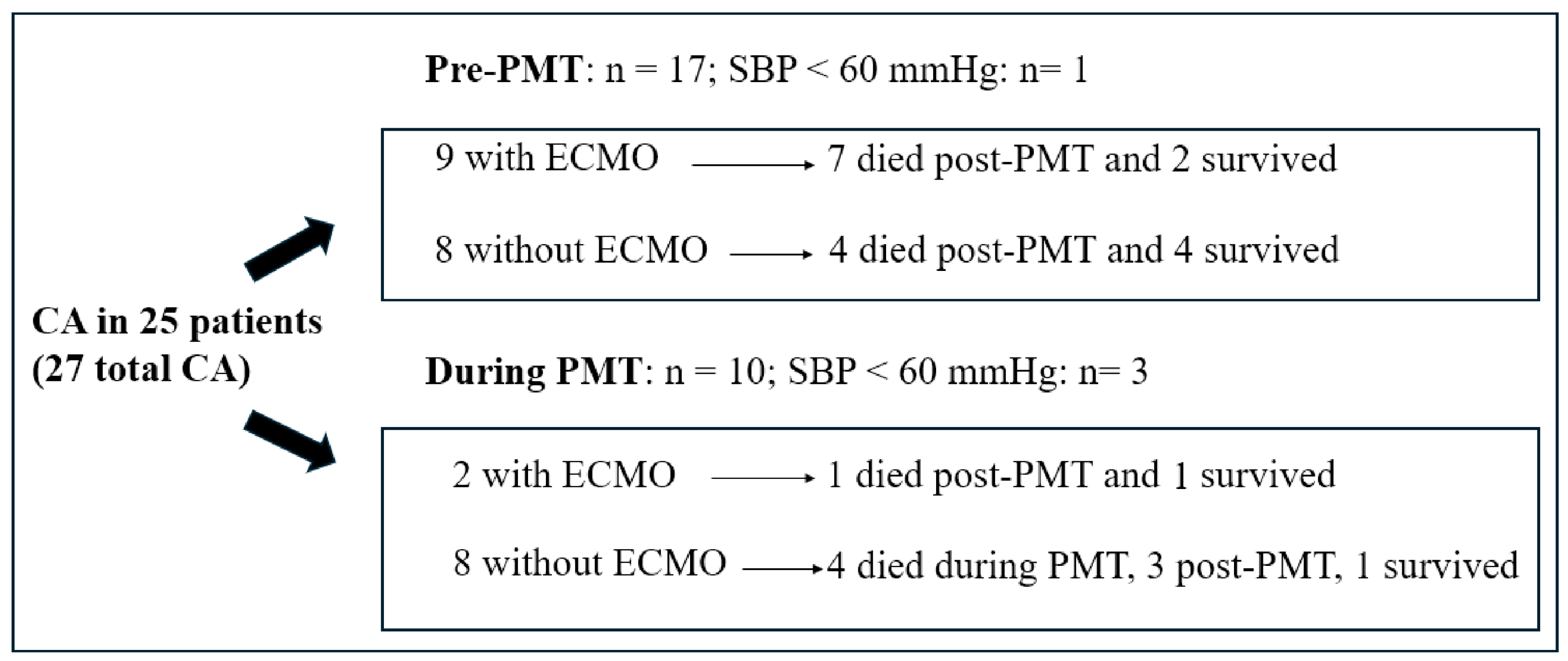
3.2. Hemodynamic and/or Respiratory Support
3.3. Anesthesia During PMT
3.4. Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PMT | Pulmonary mechanical thrombectomy |
| CA | Cardiac arrest |
| NTproBNP | N-terminal pro-B-type natriuretic peptide |
| PE | Pulmonary embolism |
| SBP | Systolic blood pressure |
| PESI | Pulmonary Embolism Severity Index |
| sPESI | simplified Pulmonary Embolism Severity Index |
| RV | Right ventricle |
| OTT | Orotracheal tube |
References
- Rivera-Lebron, B.; McDaniel, M.; Ahrar, K.; Alrifai, A.; Dudzinski, D.M.; Fanola, C.; Blais, D.; Janicke, D.; Melamed, R.; Mohrien, K.; et al. Diagnosis, treatment and follow up of acute pulmonary embolism: Consensus practice from the pert consortium. Clin. Appl. Thromb. Hemost. 2019, 25, 1076029619853037. [Google Scholar] [CrossRef]
- Giri, J.; Sista, A.K.; Weinberg, I.; Kearon, C.; Kumbhani, D.J.; Desai, N.D.; Piazza, G.; Gladwin, M.T.; Chatterjee, S.; Kobayashi, T.; et al. Interventional Therapies for acute pulmonary embolism: Current status and principles for the development of novel evidence: A scientific statement from the American Heart Association. Circulation 2019, 140, E774–E801. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.-P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef] [PubMed]
- Tu, T.; Toma, C.; Tapson, V.F.; Adams, C.; Jaber, W.A.; Silver, M.; Khandhar, S.; Amin, R.; Weinberg, M.; Engelhardt, T.; et al. A prospective, single-arm, multicenter trial of catheter-directed mechanical thrombectomy for intermediate-riskacute pulmonary embolism: The FLARE study. J. Am. Coll. Cardiovasc. Interv. 2019, 12, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Laher, A.E.; Richards, G. Cardiac arrest due to pulmonary embolism. Indian Heart J. 2018, 70, 731–735. [Google Scholar] [CrossRef]
- Bergum, D.; Nordseth, T.; Mjølstad, O.C.; Skogvoll, E.; Haugen, B.O. Causes of in-hospital cardiac arrest—Incidences and rate of recognition. Resuscitation 2015, 87, 63–68. [Google Scholar] [CrossRef]
- Kürkciyan, I.; Meron, G.; Sterz, F.; Janata, K.; Domanovits, H.; Holzer, M.; Berzlanovich, A.; Bankl, H.C.; Laggner, A.N. Pulmonary embolism as a cause of cardiac arrest: Presentation and outcome. Arch. Intern. Med. 2000, 160, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Peracaula, M.; Sebastian, L.; Francisco, I.; Vilaplana, M.B.; Rodríguez-Chiaradía, D.A.; Tura-Ceide, O. Decoding pulmonary embolism: Pathophysiology, diagnosis, and treatment. Biomedicines 2024, 12, 1936. [Google Scholar] [CrossRef]
- Benfor, B.; Haddad, P.; Bohle, K.; Atkins, M.D.; Lumsden, A.B.; Peden, E.K. Cardiovascular collapse during mechanical thrombectomy for acute pulmonary embolism and the role of extracorporeal membrane oxygenation in patient rescue. J. Vasc. Surgery Venous Lymphat. Disord. 2023, 11, 978–985.e3. [Google Scholar] [CrossRef]
- Horowitz, J.M.; Jaber, W.A.; Stegman, B.; Rosenberg, M.; Fanola, C.; Bhat, A.P.; Gondi, S.; Castle, J.; Ahmed, M.; Brown, M.A.; et al. Mechanical Thrombectomy for High-Risk Pulmonary Embolism: Insights from the US Cohort of the FLASH Registry. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 3, 101124. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, L.; Han, J.; Song, L.; Geng, H.; Liu, Y. Joint analysis of D-dimer, N-terminal pro b-type natriuretic peptide, and cardiac troponin I on predicting acute pulmonary embolism relapse and mortality. Sci. Rep. 2021, 11, 14909. [Google Scholar] [CrossRef] [PubMed]
- Boris, D.; Tamara, S.; Ivica, D.; Bojana, S.; Jovan, M.; Jelena, D.; Marija, B.; Sonja, S.; Ljiljana, K.; Tamara, K.-P.; et al. The significance of B-type natriuretic peptide in predicting early mortality among pulmonary embolism patients, alongside troponin: Insights from a multicentric registry. Curr. Probl. Cardiol. 2024, 49, 102437. [Google Scholar] [CrossRef] [PubMed]
- Price, L.C.; Martinez, G.; Brame, A.; Pickworth, T.; Samaranayake, C.; Alexander, D.; Garfield, B.; Aw, T.-C.; McCabe, C.; Mukherjee, B.; et al. Perioperative management of patients with pulmonary hypertension undergoing non-cardiothoracic, non-obstetric surgery: A systematic review and expert consensus statement. Br. J. Anaesth. 2021, 126, 774–790. [Google Scholar] [CrossRef] [PubMed]
- Kotani, Y.; Piersanti, G.; Maiucci, G.; Fresilli, S.; Turi, S.; Montanaro, G.; Zangrillo, A.; Lee, T.C.; Landoni, G. Etomidate as an induction agent for endotracheal intubation in critically ill patients: A meta-analysis of randomized trials. J. Crit. Care 2023, 77, 154317. [Google Scholar] [CrossRef]
- Rossler, J.; Cywinski, J.B.; Argalious, M.; Ruetzler, K.; Khanna, S. Anesthetic management in patients having catheter-based thrombectomy for acute pulmonary embolism: A narrative review. J. Clin. Anesth. 2023, 92, 111281. [Google Scholar] [CrossRef]
- Messika, J.; Goutorbe, P.; Hajage, D.; Ricard, J.-D. Severe pulmonary embolism managed with high-flow nasal cannula oxygen therapy. Eur. J. Emerg. Med. 2017, 24, 230–232. [Google Scholar] [CrossRef]
- Lacroix, G.; Pons, F.; D’Aranda, E.; Legodec, J.; Romanat, P.-E.; Goutorbe, P. High-flow oxygen, a therapeutic bridge while awaiting thrombolysis in pulmonary embolism? Am. J. Emerg. Med. 2013, 31, 463.e1–463.e2. [Google Scholar] [CrossRef]
- Corp, A.; Thomas, C.; Adlam, M. The cardiovascular effects of positive pressure ventilation. BJA Educ. 2021, 21, 202–209. [Google Scholar] [CrossRef]
- Dalla, K.; Bech-Hanssen, O.; Ricksten, S.-E. General anesthesia and positive pressure ventilation suppress left and right ventricular myocardial shortening in patients without myocardial disease—A strain echocardiography study. Cardiovasc. Ultrasound 2019, 17, 16. [Google Scholar] [CrossRef]
- Pruszczyk, P.; Klok, F.A.; Kucher, N.; Roik, M.; Meneveau, N.; Sharp, A.S.; Nielsen-Kudsk, J.N.-K.; Obradović, S.; Barco, S.; Giannini, F.; et al. Percutaneous treatment options for acute pulmonary embolism: A clinical consensus statement by the ESC working group on pulmonary circulation and right ventricular function and the European Association of Percutaneous Cardiovascular Interventions. EuroIntervention 2022, 18, e623–e638. [Google Scholar] [CrossRef]
- Lee, E.S.; Baltsen, C.D.; Stubblefield, W.B.; Granfeldt, A.; Andersen, A.; Stannek, K.; Dudzinski, D.M.; Kabrhel, C.; Lyhne, M.D. Intubation and mechanical ventilation in patients with acute pulmonary embolism: A scoping review. J. Intensive Care Med. 2024, 25, 8850666241285862. [Google Scholar] [CrossRef] [PubMed]
- Zochios, V.A.; Keeshan, A. Pulmonary embolism in the mechanically-ventilated critically ill patient: Is it different? J. Intensive Care Soc. 2013, 14, 36–44. [Google Scholar] [CrossRef]
- Carroll, B.J.; Beyer, S.E.; Mehegan, T.; Dicks, A.; Pribish, A.; Locke, A.; Godishala, A.; Soriano, K.; Kanduri, J.; Sack, K.; et al. Changes in care for acute pulmonary embolism through a multidisciplinary pulmonary embolism response team. Am. J. Med. 2020, 133, 1313–1321.e6. [Google Scholar] [CrossRef]
- Casazza, F.; Becattini, C.; Bongarzoni, A.; Cuccia, C.; Roncon, L.; Favretto, G.; Zonzin, P.; Pignataro, L.; Agnelli, G. Clinical features and short term outcomes of patients with acute pulmonary embolism. The Italian pulmonary embolism registry (IPER). Thromb. Res. 2012, 130, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Becattini, C.; Agnelli, G.; Lankeit, M.; Masotti, L.; Pruszczyk, P.; Casazza, F.; Vanni, S.; Nitti, C.; Kamphuisen, P.; Vedovati, M.C.; et al. Acute pulmonary embolism: Mortality prediction by the 2014 European society of cardiology risk stratification model. Eur. Respir. J. 2016, 48, 780786. [Google Scholar] [CrossRef]
- Secemsky, E.; Chang, Y.; Jain, C.C.; Beckman, J.A.; Giri, J.; Jaff, M.R.; Rosenfield, K.; Rosovsky, R.; Kabrhel, C.; Weinberg, I. Contemporary management and outcomes of patients with massive and submassive pulmonary embolism. Am. J. Med. 2018, 131, 1506–1514.e0. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.D.; Matta, F.; Hughes, P.G.; Hughes, M.J. Nineteen-year trends in mortality of patients hospitalized in the United States with high-risk pulmonary embolism. Am. J. Med. 2021, 134, 1260–1264. [Google Scholar] [CrossRef]
- Silver, M.J.; Gibson, M.; Giri, J.; Khandhar, S.; Jaber, W.; Toma, C.; Mina, B.; Bowers, T.; Greenspon, L.; Kado, H.; et al. Outcomes in High-Risk Pulmonary Embolism Patients Undergoing FlowTriever Mechanical Thrombectomy: Results from The FLAME Study. Circ. Cardiovasc. Interv. 2023, 16, 10. [Google Scholar] [CrossRef]
- Toma, C.; Jaber, W.A.; Weinberg, M.D.; Bunte, M.C.; Khandhar, S.; Stegman, B.; Gondi, S.; Chambers, J.; Amin, R.; Leung, D.A.; et al. Acute Outcomes for the Full US Cohort of the FLASH Mechanical Thrombectomy Registry in Pulmonary Embolism. EuroIntervention 2023, 18, 1201–1212. [Google Scholar] [CrossRef]
- Kuo, W.T.; Banerjee, A.; Kim, P.S.; DeMarco, F.J., Jr.; Levy, J.R.; Facchini, F.R.; Unver, K.; Bertini, M.J.; Sista, A.; Hall, M.J.; et al. Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis (PERFECT)Initial Results from a Prospective Multicenter Registry. Chest 2015, 148, 667–673G. [Google Scholar] [CrossRef]
- Guglin, M.; Zucker, M.J.; Bazan, V.M.; Bozkurt, B.; El Banayosy, A.; Estep, J.D.; Gurley, J.; Nelson, K.; Malyala, R.; Panjrath, G.S.; et al. Venoarterial ECMO for adults: JACC scientific expert panel. J. Am. Coll. Cardiol. 2019, 73, 698–716. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.; Khalpey, Z.; Smith, R.; Burkhoff, D.; Kociol, R.D. Venoarterial extracorporeal membrane oxygenation for cardiogenic shock and cardiac arrest. Circ. Heart Fail. 2018, 11, e004905. [Google Scholar] [CrossRef]
- Kapur, N.K.; Bader, Y.H. Percutaneous circulatory assist devices for right ventricular failure. Interv. Cardiol. Clin. 2013, 2, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Kapur, N.K.; Esposito, M.L.; Bader, Y.; Morine, K.J.; Kiernan, M.S.; Pham, D.T.; Burkhoff, D. Mechanical circulatory support devices for acute right ventricular failure. Circulation 2017, 136, 314–326. [Google Scholar] [CrossRef]
- Kempny, A.; McCabe, C.; Dimopoulos, K.; Price, L.C.; Wilde, M.; Limbrey, R.; Gatzoulis, M.A.; Wort, S.J. Incidence, mortality and bleeding rates associated with pulmonary embolism in England between 1997 and 2015. Int. J. Cardiol. 2019, 277, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Swol, J.; Buchwald, D.; Strauch, J.; Schildhauer, T.A. Extracorporeal life support (ECLS) for cardiopulmonary resuscitation (CPR) with pulmonary embolism in surgical patients—A case series. Perfusion 2016, 31, 54–59. [Google Scholar] [CrossRef]
- Maj, G.; Melisurgo, G.; De Bonis, M.; Pappalardo, F. ECLS management in pulmonary embolism with cardiac arrest: Which strategy is better? Resuscitation 2014, 85, e175–e176. [Google Scholar] [CrossRef]
- Wu, M.Y.; Liu, Y.C.; Tseng, Y.H.; Chang, Y.S.; Lin, P.J.; Wu, T.I. Pulmonary embolectomy in high-risk acute pulmonary embolism: The effectiveness of a comprehensive therapeutic algorithm including extracorporeal life support. Resuscitation 2013, 84, 1365–1370. [Google Scholar] [CrossRef]
- Yusuff, H.; Zochios, V.; Vuylsteke, A. Extracorporeal membrane oxygenation in acute massive pulmonary embolism: A systematic review. Perfusion 2015, 30, 611–616. [Google Scholar] [CrossRef]
- Giraud, R.; Banfi, C.; Siegenthaler, N.; Bendjelid, K. Massive pulmonary embolism leading to cardiac arrest: One pathology, two different ECMO modes to assist patients. J. Clin. Monit. Comput. 2016, 30, 933–937. [Google Scholar] [CrossRef]
- Pascual García, S.; Castell Herrera, A.; Cuesta Pérez, J.J.; Rodriguez Perojo, A.; Abad Fernández, A.; Río Ramirez, M.T. Terapia dirigida por catéter en embolia pulmonar de alto riesgo: Análisis de 9 casos Catheter directed therapy in high-risk pulmonary embolism: Analysis of 9 cases. Med. Clin. 2024, 163, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Tsangaris, A.; Alexy, T.; Kalra, R.; Kosmopoulos, M.; Elliott, A.; Bartos, J.A.; Yannopoulos, D. Overview of veno-arterial extracorporeal membrane oxygenation (VA-ECMO) support for the management of cardiogenic shock. Front. Cardiovasc. Med. 2021, 8, 686558. [Google Scholar] [CrossRef] [PubMed]
- Sahli, S.D.; Kaserer, A.; Braun, J.; Halbe, M.; Dahlem, Y.; Spahn, M.A.; Rossler, J.; Kruger, B.; Maisano, F.; Spahn, D.R.; et al. Predictors associated with mortality of extracorporeal life support therapy for acute heart failure: Single-center experience with 679 patients. J. Thorac. Dis. 2022, 14, 1960–1971. [Google Scholar] [CrossRef] [PubMed]
- Luedemann, W.M.; Zickler, D.; Kruse, J.; Koerner, R.; Lenk, J.; Erxleben, C.; Torsello, G.F.; Fehrenbach, U.; Jonczyk, M.; Guenther, R.W.; et al. Percutaneous large-bore pulmonary thrombectomy with the FlowTrierver Device: Initial experience in intermediate-high and high-risk patients. Cardiovasc. Interv. Radiol. 2022, 46, 35–42. [Google Scholar] [CrossRef]
- Raza, H.A.; Horowitz, J.; Yuriditsky, E. Indigo® aspiration system for thrombectomy in pulmonary embolism. Futur. Cardiol. 2023, 19, 469–475. [Google Scholar] [CrossRef]

| Early Mortality Risk | Indicators of Risk | ||||
|---|---|---|---|---|---|
| Shock or Hypotension * | PESI Class III-V or sPESI ≥ 1 ** | Signs of RV Dysfunction on an Imaging Test | Cardiac Laboratory Biomarkers | ||
| High | + | + | + | + | |
| Intermediate | Intermediate high | − | + | + | + |
| Intermediate low | − | + | Either 1 (or none) positive | ||
| Low | − | − | Assessment optional: if assessed, both negative | ||
| Pre-Thrombectomy Parameters | CA | p-Value | ||
|---|---|---|---|---|
| Total | Non-CA | CA | ||
| n = 98 | n = 73 | n = 25 | ||
| Hypertension | 56 (57.1) | 39 (53.4) | 17 (68) | p = 0.20 |
| Diabetes mellitus | 15 (15.3) | 11 (15.1) | 4 (16) | p = 0.91 |
| Cardiopathy | 17 (17.3) | 12 (16.4) | 5 (20) | p = 0.68 |
| Respiratory diseases | 26 (26.5) | 17 (23.3) | 7 (28) | p = 0.21 |
| Renal failure | 14 (14.3) | 10 (13.7) | 4 (16) | p = 0.77 |
| Cancer history | 22 (22.4) | 16 (21.9) | 6 (24) | p = 0.82 |
| Pulmonary hypertesion | 41 (41.8) | 33 (45.2) | 8 (32) | p = 0.24 |
| Current cancer | 18 (18.4) | 11 (15.1) | 7 (28) | p = 0.18 |
| Undetermined cause of PE | 40 (40.8) | 31 (42.4) | 9 (36) | |
| COVID-19 | 10 (10.2) | 6 (8.2) | 4 (16) | |
| Obesity-sedentary lifestyle | 8 (8.2) | 7 (9.6) | 1 (4) | |
| Morbid obesity | 3 (3.1) | 3 (4.1) | 0 (0) | |
| Traumatism-fractures | 7 (7.1) | 7 (9.6) | 0 (0) | |
| Postoperative | 6 (6.1) | 3 (4.1) | 3 (12) | |
| Contraceptives | 2 (2) | 2 (2.7) | 0 (0) | |
| Coagulation disorders | 2 (2) | 2 (2.7) | 0 (0) | |
| Ruptured hydatid cyst | 1 (1) | 0 (0) | 1 (4) | |
| Single ventricle | 1 (1) | 1 (1.4) | 0 (0) | |
| PE severity: | p < 0.001 | |||
| High | 34 (35.1) | 15 (20.8) | 19 (76) | |
| Intermediate/high | 62 (63.2) | 57 (78) | 5 (20) | |
| Low | 2 (2.1) | 1 (1.4) | 1 (4) | |
| SBP < 90 mmHg | 35 (35.7) | 16 (21.9) | 19 (76) | p < 0.001 |
| Heart rate > 110 bpm | 33 (33.7) | 27 (37) | 6 (24) | p = 0.23 |
| Oxygen saturation < 90% | 21 (21.4) | 12 (16.4) | 9 (36) | p = 0.04 |
| Bilateral PE | 91 (94.8) | 68 (95.8) | 23 (92) | p = 0.46 |
| Unilateral PE | 4 (4.1) | 2 (2.8) | 2 (8) | p = 0.25 |
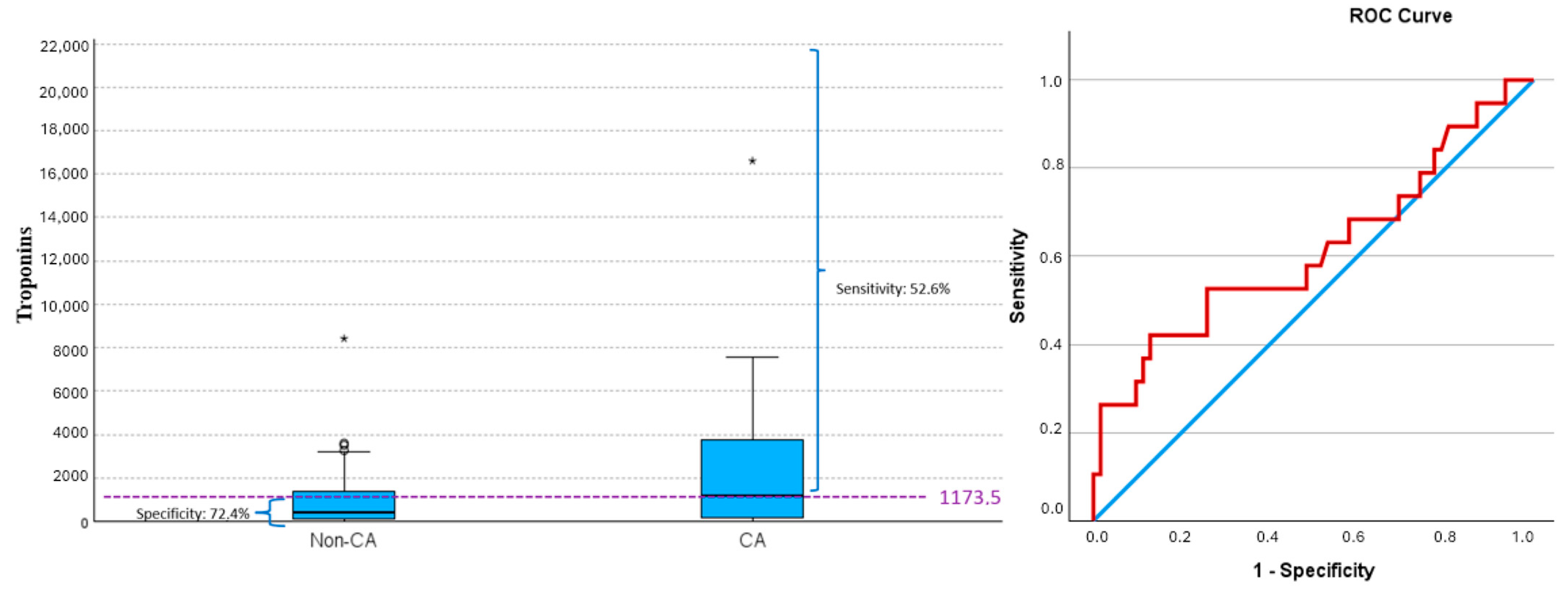
| NT-proBNP | (70) 5467.44 ± 7548.07 | (58) 4142.34 ± 5683.71 | (12) 11,872.08 ± 11,660.86 | p = 0.03 |
| Troponins | (81) 1890.17 ± 5628.99 | (62) 968.37 ± 1394.48 | (19) 4898.16 ± 11,034.37 | p = 0.13 |
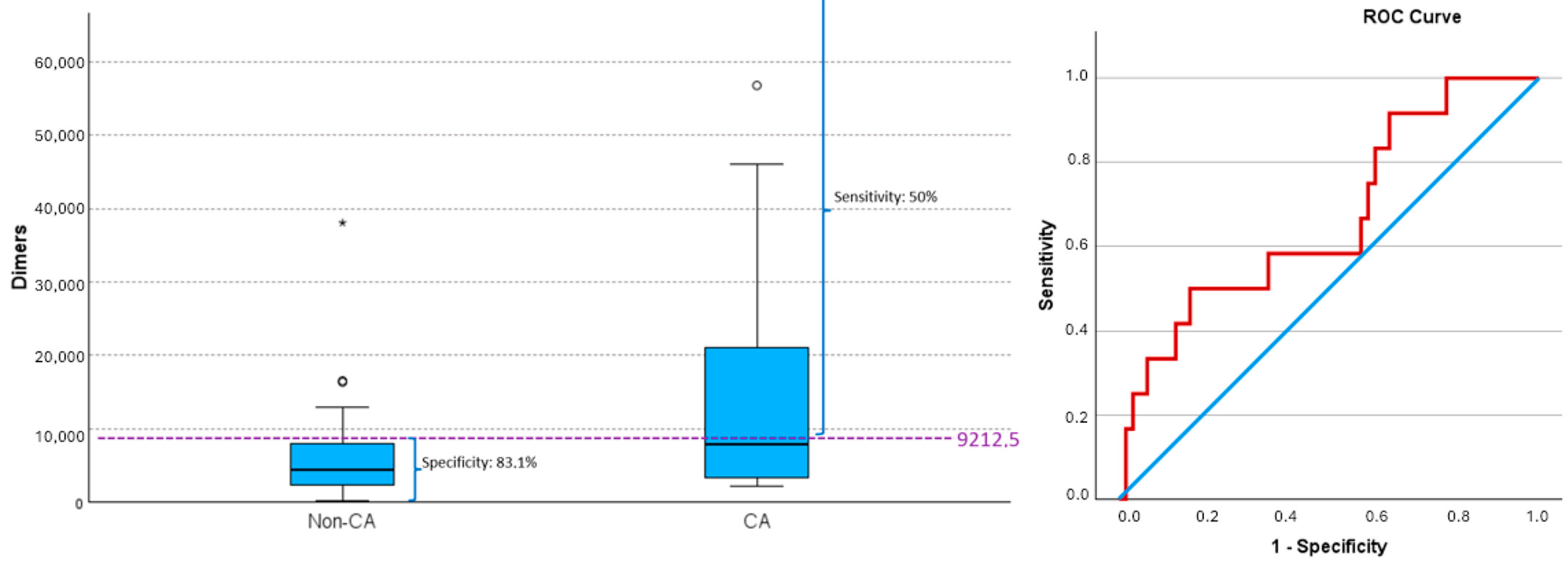
| D-dimers | (71) 8772.96 ± 14,747.68 | (59) 7397 ± 13,680.47 | (12) 15,536 ± 18,362.53 | p = 0.07 |
| SPS (mmHg) | (33) 46.70 ± 14.06 | (26) 47.69 ±14.60 | (13) 43 ± 12.11 | p = 0.47 |
| Anesthesia | n = 98 (100%) | Hemodynamic Instability (n = 13) | Airway Devices | n = 98 (100%) |
|---|---|---|---|---|
| Sedation | 53 (54.1) | None | Ventimask® Nasal glasses Oxygen mask with reservoir bag OptiflowTM nasal high flow therapy Oxygen mask with reservoir bag + ECMO | 19 (19.38) 20 (20.4) 8 (8.16) 5 (5.10) 1 (1) |
| Continuation of general anesthesia initiated prior to PMT | 23 (23.5) | 3 CA (1 ECMO) 1 SBP < 60 mmHg | Orotracheal tube (before procedure) OTT + ECMO (both before procedure) OTT before procedure + ECMO during procedure | 13 (13.26) 9 (9.2) 1 (1) |
| Sedation at the start, with conversion to general anesthesia during PMT | 10 (10.2) | 5 CA 2 SBP < 60 mmHg | Oxygen mask with reservoir bag at the start + OTT during procedure Ventimask® at the start + OTT during procedure Nasal glasses at the start + OTT during procedure OptiflowTM nasal high flow therapy at the start + OTT during | 4 (4.08) 3 (3.1) 2 (2) 1 (1) |
| None | 7 (7.1) | None | Oxygen mask with reservoir bag OptiflowTM nasal high flow therapy Ventimask® at the start + oxygen mask with reservoir bag during Ventimask® | 1 (1) 2 (2) 1 (1) 3 (3.1) |
| Elective general anesthesia at the beginning of the PMT | 4 (4.1) | 1 CA (1 ECMO) | OTT at the start OTT at the start + ECMO during | 3 (3.1) 1 (1) |
| Start without anesthesia, followed by general anaesthesia during PMT | 1 (1) | 1 CA | Ventimask® at the start + OTT during procedure | 1 (1) |
| Anesthesia | Risk of PE | |||
|---|---|---|---|---|
| Total | Low | Intermediate/High | High | |
| Total | 98 (100) | 2 (100) | 62 (100) | 34 (100) |
| Sedation | 53 (54.6) | 0 (0) | 45 (73.8) | 8 (23.5) |
| Continuation of general anesthesia initiated prior to PMT | 23 (23.7) | 2 (100) | 2 (3.22) | 19 (55.9) |
| Sedation at the start, with conversion to general anesthesia during PMT | 10 (10.2) | 0 (0) | 8 (12.9) | 2 (5.9) |
| None | 7 (7.2) | 0 (0) | 4 (6.45) | 3 (8.8) |
| Elective general anesthesia at the beginning of the PMT | 4 (4.1) | 0 (0) | 2 (3.22) * | 2 (5.9) |
| Start without anesthesia, followed by general anesthesia during PMT | 1 (1) | 0 (0) | 1 (1.61) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Suárez, S.; Camacho Oviedo, J.; Suriñach Caralt, J.M.; Grao Roca, M.; Dammala Liyanage, I.M.; Pérez Lafuente, M.; Mena Muñoz, E.; González Junyent, C.; Martínez-Martínez, M.; Barnés Navarro, D.; et al. Management of Hemodynamic and Respiratory Instability and Anesthetic Approaches in Patients Undergoing Pulmonary Thrombectomy for Pulmonary Embolism. J. Clin. Med. 2025, 14, 2704. https://doi.org/10.3390/jcm14082704
González-Suárez S, Camacho Oviedo J, Suriñach Caralt JM, Grao Roca M, Dammala Liyanage IM, Pérez Lafuente M, Mena Muñoz E, González Junyent C, Martínez-Martínez M, Barnés Navarro D, et al. Management of Hemodynamic and Respiratory Instability and Anesthetic Approaches in Patients Undergoing Pulmonary Thrombectomy for Pulmonary Embolism. Journal of Clinical Medicine. 2025; 14(8):2704. https://doi.org/10.3390/jcm14082704
Chicago/Turabian StyleGonzález-Suárez, Susana, John Camacho Oviedo, José Maria Suriñach Caralt, Maria Grao Roca, Isuru M. Dammala Liyanage, Mercedes Pérez Lafuente, Elisabeth Mena Muñoz, Carla González Junyent, María Martínez-Martínez, Daniel Barnés Navarro, and et al. 2025. "Management of Hemodynamic and Respiratory Instability and Anesthetic Approaches in Patients Undergoing Pulmonary Thrombectomy for Pulmonary Embolism" Journal of Clinical Medicine 14, no. 8: 2704. https://doi.org/10.3390/jcm14082704
APA StyleGonzález-Suárez, S., Camacho Oviedo, J., Suriñach Caralt, J. M., Grao Roca, M., Dammala Liyanage, I. M., Pérez Lafuente, M., Mena Muñoz, E., González Junyent, C., Martínez-Martínez, M., Barnés Navarro, D., & Ruíz-Rodríguez, J. C. (2025). Management of Hemodynamic and Respiratory Instability and Anesthetic Approaches in Patients Undergoing Pulmonary Thrombectomy for Pulmonary Embolism. Journal of Clinical Medicine, 14(8), 2704. https://doi.org/10.3390/jcm14082704






