Multi-Organ Denervation: The Past, Present and Future
Abstract
:1. Introduction
2. Evidence for SNS Over-Activation
3. Neuromodulation: An Introduction
4. Autonomic Nervous System: An Overview
Renal Innervation
5. Renal Denervation
6. Hepatic Innervation
7. Anatomical Considerations: Hepatic Denervation
8. Hepatic Denervation Studies
9. Splenic Innervation
Anatomic Considerations: Splenic Denervation
10. Splenic Denervation Studies
11. Other Denervation Sites
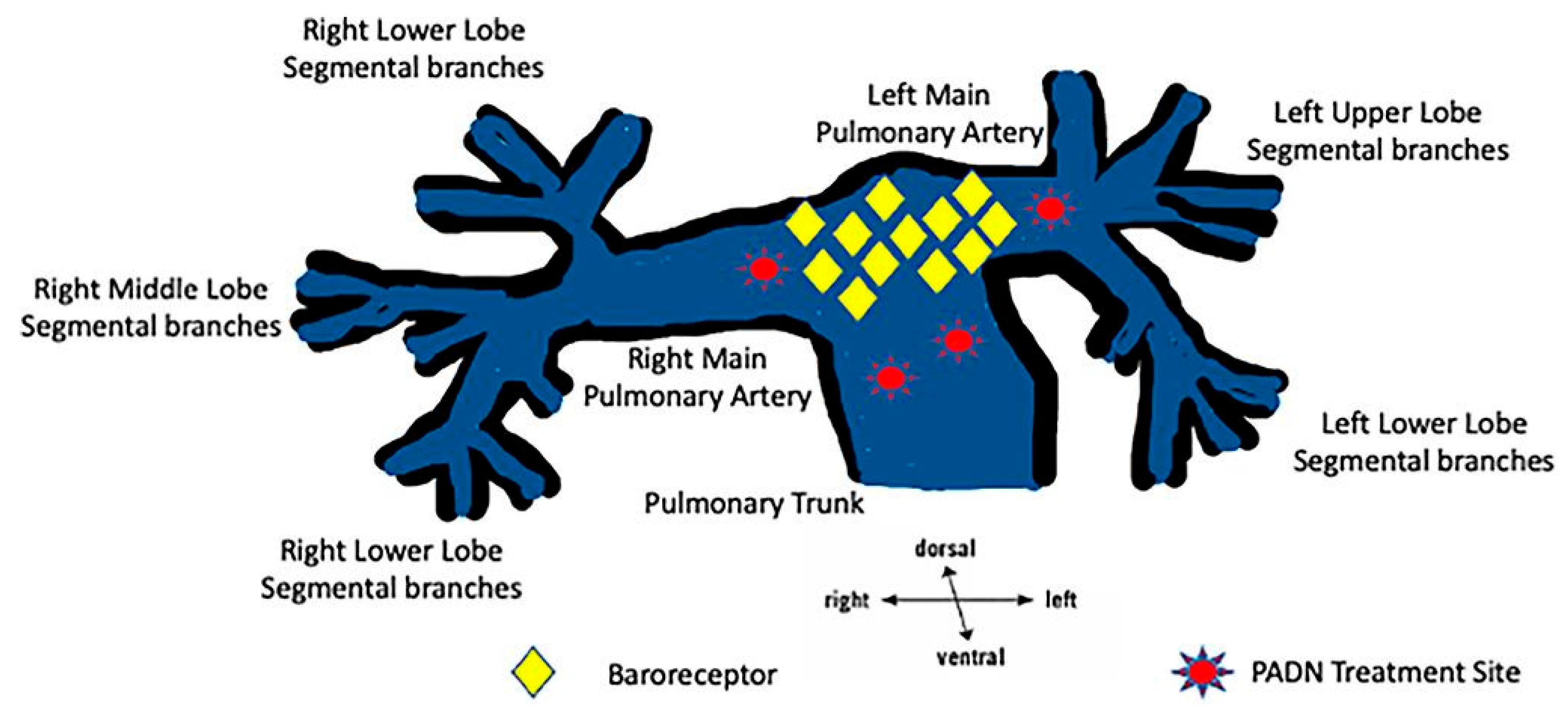
11.1. Pulmonary Artery Denervation
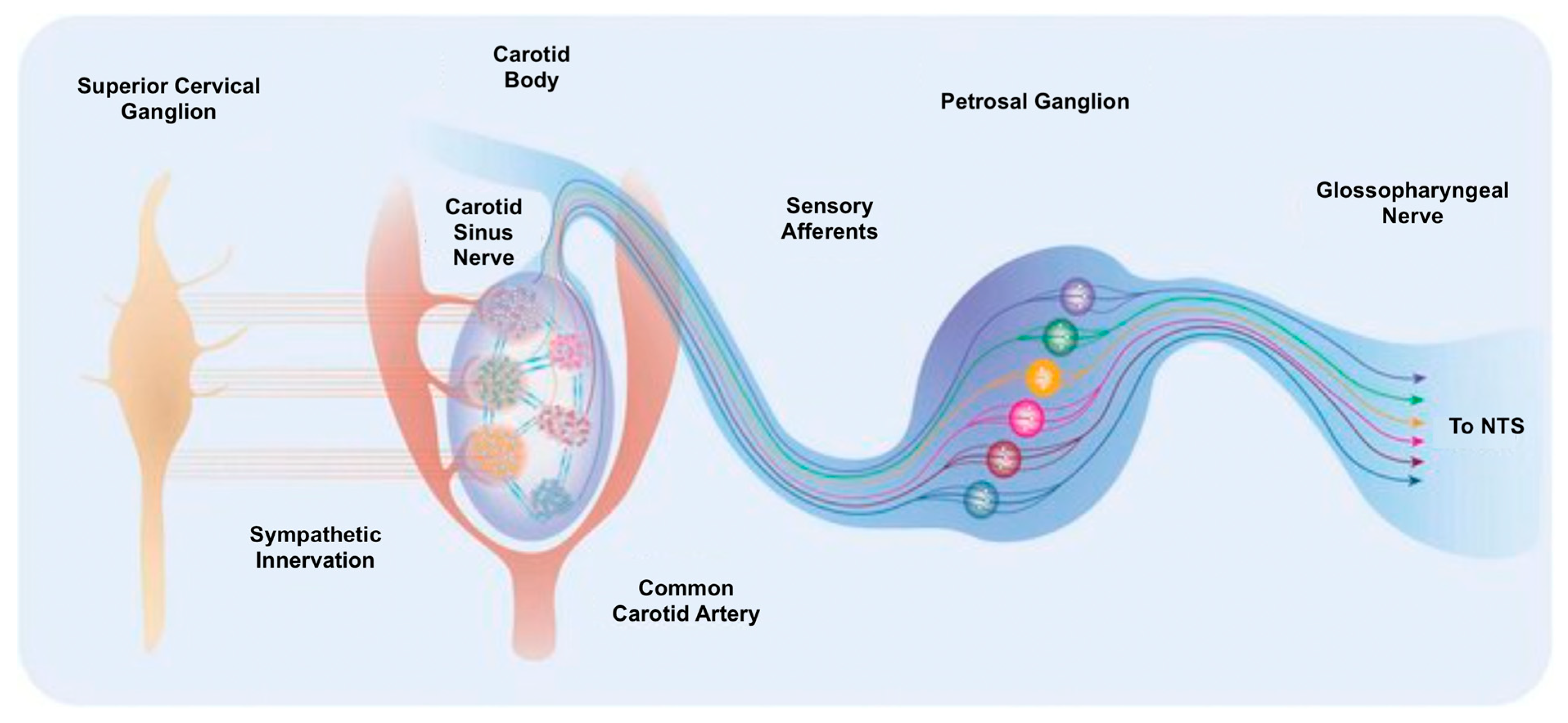
11.2. Carotid Body Denervation
12. Multiorgan Neuromodulation in Practice
13. Potential Side Effects
14. Important Considerations
15. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McCorry, L.K. Physiology of the Autonomic Nervous System. Am. J. Pharm. Educ. 2007, 71, 78. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.; Marwaha, K.; Sanvictores, T.; Awosika, A.O.; Ayers, D. Physiology, Stress Reaction. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK541120/ (accessed on 29 June 2024).
- Carnagarin, R.; Lambert, G.W.; Kiuchi, M.G.; Nolde, J.M.; Matthews, V.B.; Eikelis, N.; Lambert, E.A.; Schlaich, M.P. Effects of sympathetic modulation in metabolic disease. Ann. N. Y. Acad. Sci. 2019, 1454, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Hillebrand, S.; De Mutsert, R.; Christen, T.; Maan, A.C.; Jukema, J.W.; Lamb, H.J.; De Roos, A.; Rosendaal, F.R.; Den Heijer, M.; Swenne, C.A.; et al. Body fat, especially visceral fat, is associated with electrocardiographic measures of sympathetic activation: Visceral Fat and Sympathetic Activation. Obesity 2014, 22, 1553–1559. [Google Scholar] [CrossRef]
- Rahman, A.; Hasan, A.; Nishiyama, A.; Kobori, H. Altered Circadian Timing System-Mediated Non-Dipping Pattern of Blood Pressure and Associated Cardiovascular Disorders in Metabolic and Kidney Diseases. Int. J. Mol. Sci. 2018, 19, 400. [Google Scholar] [CrossRef] [PubMed]
- Narkiewicz, K.; Van De Borne, P.J.H.; Montano, N.; Dyken, M.E.; Phillips, B.G.; Somers, V.K. Contribution of Tonic Chemoreflex Activation to Sympathetic Activity and Blood Pressure in Patients with Obstructive Sleep Apnea. Circulation 1998, 97, 943–945. [Google Scholar] [CrossRef] [PubMed]
- Somers, V.K.; Dyken, M.E.; Clary, M.P.; Abboud, F.M. Sympathetic neural mechanisms in obstructive sleep apnea. J. Clin. Investig. 1995, 96, 1897–1904. [Google Scholar] [CrossRef] [PubMed]
- Mansukhani, M.P.; Kara, T.; Caples, S.M.; Somers, V.K. Chemoreflexes, Sleep Apnea, and Sympathetic Dysregulation. Curr. Hypertens. Rep. 2014, 16, 476. [Google Scholar] [CrossRef]
- Borovac, J.A.; D’Amario, D.; Bozic, J.; Glavas, D. Sympathetic nervous system activation and heart failure: Current state of evidence and the pathophysiology in the light of novel biomarkers. World J. Cardiol. 2020, 12, 373–408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Anderson, A.S. The Sympathetic Nervous System and Heart Failure. Cardiol. Clin. 2014, 32, 33–45. [Google Scholar] [CrossRef]
- Velez-Roa, S.; Ciarka, A.; Najem, B.; Vachiery, J.-L.; Naeije, R.; Van De Borne, P. Increased Sympathetic Nerve Activity in Pulmonary Artery Hypertension. Circulation 2004, 110, 1308–1312. [Google Scholar] [CrossRef]
- Chaudhary, K.; Buddineni, J.P.; Nistala, R.; Whaley-Connell, A. Resistant Hypertension in the High-Risk Metabolic Patient. Curr. Diabetes Rep. 2011, 11, 41–46. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, T.; Abbasi, F.; Cheal, K.; Chu, J.; Lamendola, C.; Reaven, G. Use of Metabolic Markers To Identify Overweight Individuals Who Are Insulin Resistant. Ann. Intern. Med. 2003, 139, 802. [Google Scholar] [CrossRef]
- Kiuchi, M.G.; Carnagarin, R.; Matthews, V.B.; Schlaich, M.P. Multi-organ denervation: A novel approach to combat cardiometabolic disease. Hypertens. Res. 2023, 46, 1747–1758. [Google Scholar] [CrossRef]
- Bellocchi, C.; Carandina, A.; Montinaro, B.; Targetti, E.; Furlan, L.; Rodrigues, G.D.; Tobaldini, E.; Montano, N. The Interplay between Autonomic Nervous System and Inflammation across Systemic Autoimmune Diseases. Int. J. Mol. Sci. 2022, 23, 2449. [Google Scholar] [CrossRef]
- Chakravarthy, K.; Chaudhry, H.; Williams, K.; Christo, P.J. Review of the Uses of Vagal Nerve Stimulation in Chronic Pain Management. Curr. Pain Headache Rep. 2015, 19, 54. [Google Scholar] [CrossRef]
- Napadow, V.; Edwards, R.R.; Cahalan, C.M.; Mensing, G.; Greenbaum, S.; Valovska, A.; Li, A.; Kim, J.; Maeda, Y.; Park, K.; et al. Evoked Pain Analgesia in Chronic Pelvic Pain Patients Using Respiratory-Gated Auricular Vagal Afferent Nerve Stimulation. Pain Med. 2012, 13, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Janner, H.; Klausenitz, C.; Gürtler, N.; Hahnenkamp, K.; Usichenko, T.I. Effects of Electrical Transcutaneous Vagus Nerve Stimulation on the Perceived Intensity of Repetitive Painful Heat Stimuli: A Blinded Placebo- and Sham-Controlled Randomized Crossover Investigation. Anesth. Analg. 2018, 126, 2085–2092. [Google Scholar] [CrossRef] [PubMed]
- Vrijens, B.; Antoniou, S.; Burnier, M.; De La Sierra, A.; Volpe, M. Current Situation of Medication Adherence in Hypertension. Front. Pharmacol. 2017, 8, 100. [Google Scholar] [CrossRef] [PubMed]
- Burnier, M.; Egan, B.M. Adherence in Hypertension: A Review of Prevalence, Risk Factors, Impact, and Management. Circ. Res. 2019, 124, 1124–1140. [Google Scholar] [CrossRef]
- Blaschke, T.F.; Osterberg, L.; Vrijens, B.; Urquhart, J. Adherence to Medications: Insights Arising from Studies on the Unreliable Link Between Prescribed and Actual Drug Dosing Histories. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 275–301. [Google Scholar] [CrossRef] [PubMed]
- Noubiap, J.J.; Nansseu, J.R.; Lontchi-Yimagou, E.; Nkeck, J.R.; Nyaga, U.F.; Ngouo, A.T.; Tounouga, D.N.; Tianyi, F.-L.; Foka, A.J.; Ndoadoumgue, A.L.; et al. Geographic distribution of metabolic syndrome and its components in the general adult population: A meta-analysis of global data from 28 million individuals. Diabetes Res. Clin. Pract. 2022, 188, 109924. [Google Scholar] [CrossRef] [PubMed]
- Kandzari, D.E. Catheter-Based Renal Denervation Therapy: Evolution of Evidence and Future Directions. Circ. Cardiovasc. Interv. 2021, 14, e011130. [Google Scholar] [CrossRef] [PubMed]
- Smithwick, R.H. Splanchnicectomy for Essential Hypertension: Results in 1266 Cases. J. Am. Med. Assoc. 1953, 152, 1501. [Google Scholar] [CrossRef]
- Symplicity HTN-2 Investigators. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): A randomised controlled trial. Lancet 2010, 376, 1903–1909. [Google Scholar] [CrossRef]
- Davis, M.I.; Filion, K.B.; Zhang, D.; Eisenberg, M.J.; Afilalo, J.; Schiffrin, E.L.; Joyal, D. Effectiveness of renal denervation therapy for resistant hypertension: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2013, 62, 231–241. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, Q.; Lu, Y.; Li, Y.; Zhang, L.; Zhang, J.; Xing, Q.; Lv, W.; Cheng, X.; Zhang, G.; et al. Renal Denervation Reduced Ventricular Arrhythmia After Myocardial Infarction by Inhibiting Sympathetic Activity and Remodeling. J. Am. Heart Assoc. 2018, 7, e009938. [Google Scholar] [CrossRef] [PubMed]
- Verloop, W.L.; Spiering, W.; Vink, E.E.; Beeftink, M.M.A.; Blankestijn, P.J.; Doevendans, P.A.; Voskuil, M. Denervation of the Renal Arteries in Metabolic Syndrome: The DREAMS-Study. Hypertension 2015, 65, 751–757. [Google Scholar] [CrossRef]
- Warchol-Celinska, E.; Prejbisz, A.; Kadziela, J.; Florczak, E.; Januszewicz, M.; Michalowska, I.; Dobrowolski, P.; Kabat, M.; Sliwinski, P.; Klisiewicz, A.; et al. Renal Denervation in Resistant Hypertension and Obstructive Sleep Apnea: Randomized Proof-of-Concept Phase II Trial. Hypertension 2018, 72, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Schmieder, R.E. Renal denervation in patients with chronic kidney disease: Current evidence and future perspectives. Nephrol. Dial. Transplant. 2023, 38, 1089–1096. [Google Scholar] [CrossRef]
- Fukuta, H.; Goto, T.; Wakami, K.; Ohte, N. Effects of catheter-based renal denervation on heart failure with reduced ejection fraction: A systematic review and meta-analysis. Heart Fail. Rev. 2017, 22, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, D.; Zhu, X.; Liu, D.; Cao, Q.; Pan, T.; Zhang, Q.; Gu, X.; Li, L.; Teng, G. Interventional metabology: A review of bariatric arterial embolization and endovascular denervation for treating metabolic disorders. J. Diabetes 2023, 15, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lin, X.; Jiao, Y.; Yang, D.; Li, Z.; Zhu, L.; Li, Y.; Yin, S.; Li, Q.; Xu, S.; et al. A brain-to-liver signal mediates the inhibition of liver regeneration under chronic stress in mice. Nat. Commun. 2024, 15, 10361. [Google Scholar] [CrossRef] [PubMed]
- Tzafriri, A.R.; Garcia-Polite, F.; Keating, J.; Melidone, R.; Knutson, J.; Markham, P.; Edelman, E.R.; Mahfoud, F. Morphometric analysis of the human common hepatic artery reveals a rich and accessible target for sympathetic liver denervation. Sci. Rep. 2022, 12, 1413. [Google Scholar] [CrossRef] [PubMed]
- Ferris, H.A.; Kahn, C.R. Unraveling the Paradox of Selective Insulin Resistance in the Liver: The Brain-Liver Connection. Diabetes 2016, 65, 1481–1483. [Google Scholar] [CrossRef] [PubMed]
- Kandlikar, S.S.; Fink, G.D. Splanchnic sympathetic nerves in the development of mild DOCA-salt hypertension. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1965–H1973. [Google Scholar] [CrossRef]
- Zhen, Z.; Liao, S.-Y.; Zhu, Z.-Y.; Sijia, S.; Au, K.-W.; Lai, W.-H.; Tsang, A.; Hai, J.S.H.; Tse, H.-F. Catheter-Based Splanchnic Denervation for Treatment of Hypertensive Cardiomyopathy. Hypertension 2019, 74, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Rothman, A.M.K.; Vachiery, J.-L.; Howard, L.S.; Mikhail, G.W.; Lang, I.M.; Jonas, M.; Kiely, D.G.; Shav, D.; Shabtay, O.; Avriel, A.; et al. Intravascular Ultrasound Pulmonary Artery Denervation to Treat Pulmonary Arterial Hypertension (TROPHY1). JACC Cardiovasc. Interv. 2020, 13, 989–999. [Google Scholar] [CrossRef]
- Ribeiro, M.J.; Sacramento, J.F.; Gonzalez, C.; Guarino, M.P.; Monteiro, E.C.; Conde, S.V. Carotid body denervation prevents the development of insulin resistance and hypertension induced by hypercaloric diets. Diabetes 2013, 62, 2905–2916. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.D.; Limberg, J.K. Is carotid body denervation the silver bullet for heart failure? J. Physiol. 2014, 592, 1179–1180. [Google Scholar] [CrossRef]
- Waxenbaum, J.A.; Reddy, V.; Varacallo, M. Anatomy, Autonomic Nervous System. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK539845/ (accessed on 29 June 2024).
- Sanvictores, T.; Jozsa, F.; Tadi, P. Neuroanatomy, Autonomic Nervous System Visceral Afferent Fibers and Pain. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK560843/ (accessed on 29 June 2024).
- McCausland, C.; Carey, F.J.; Sajjad, H. Anatomy, Back, Splanchnic Nerve. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK549856/ (accessed on 29 June 2024).
- García-Touchard, A.; Sañudo, J.R. Renal Denervation. Importance of Knowledge of Sympathetic Nervous System Anatomy in Refining the Technique. Rev. Esp. Cardiol. Engl. Ed. 2019, 72, 531–534. [Google Scholar] [CrossRef]
- Leslie, S.W.; Sajjad, H. Anatomy, Abdomen and Pelvis, Renal Artery. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK459158/ (accessed on 29 June 2024).
- Sata, Y.; Head, G.A.; Denton, K.; May, C.N.; Schlaich, M.P. Role of the Sympathetic Nervous System and Its Modulation in Renal Hypertension. Front. Med. 2018, 5, 82. [Google Scholar] [CrossRef] [PubMed]
- Hering, L.; Rahman, M.; Potthoff, S.A.; Rump, L.C.; Stegbauer, J. Role of α2-Adrenoceptors in Hypertension: Focus on Renal Sympathetic Neurotransmitter Release, Inflammation, and Sodium Homeostasis. Front. Physiol. 2020, 11, 566871. [Google Scholar] [CrossRef]
- Fountain, J.H.; Kaur, J.; Lappin, S.L. Physiology, Renin Angiotensin System. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK470410/ (accessed on 29 June 2024).
- Rey-García, J.; Townsend, R.R. Renal Denervation: A Review. Am. J. Kidney Dis. 2022, 80, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Katsurada, K.; Shinohara, K.; Aoki, J.; Nanto, S.; Kario, K. Renal denervation: Basic and clinical evidence. Hypertens. Res. 2022, 45, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Castagna, F.; Mondellini, G.M.; Pinsino, A.; McDonnell, B.J.; Stöhr, E.J.; Gaudig, A.; Amlani, A.; Nwokocha, J.; Te-Frey, R.; Takeda, K.; et al. Lack of Nocturnal Blood Pressure Reduction Increases the Risk of Stroke in Patients on Left Ventricular Assist Device Support. J. Heart Lung Transplant. 2020, 39, S395. [Google Scholar] [CrossRef]
- Nadeau, J.O.; Fang, J.; Kapral, M.K.; Silver, F.L.; Hill, M.D. Outcome after Stroke upon Awakening. Can. J. Neurol. Sci. 2005, 32, 232–236. [Google Scholar] [CrossRef]
- Haider, S.A.; Wagener, M.; Iqbal, T.; Shahzad, S.; Del Sole, P.A.; Leahy, N.; Murphy, D.; Sharif, R.; Ullah, I.; Sharif, F. Does renal denervation require cardiovascular outcome-driven data? Hypertens. Res. 2024, 47, 2633–2643. [Google Scholar] [CrossRef] [PubMed]
- Esler, M.D.; Krum, H.; Schlaich, M.; Schmieder, R.E.; Böhm, M.; Sobotka, P.A. Renal Sympathetic Denervation for Treatment of Drug-Resistant Hypertension: One-Year Results from the Symplicity HTN-2 Randomized, Controlled Trial. Circulation 2012, 126, 2976–2982. [Google Scholar] [CrossRef] [PubMed]
- Azizi, M.; Sapoval, M.; Gosse, P.; Monge, M.; Bobrie, G.; Delsart, P.; Midulla, M.; Mounier-Véhier, C.; Courand, P.-Y.; Lantelme, P.; et al. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): A multicentre, open-label, randomised controlled trial. Lancet 2015, 385, 1957–1965. [Google Scholar] [CrossRef] [PubMed]
- Fengler, K.; Rommel, K.-P.; Blazek, S.; Besler, C.; Hartung, P.; Von Roeder, M.; Petzold, M.; Winkler, S.; Höllriegel, R.; Desch, S.; et al. A Three-Arm Randomized Trial of Different Renal Denervation Devices and Techniques in Patients with Resistant Hypertension (RADIOSOUND-HTN). Circulation 2019, 139, 590–600. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Kandzari, D.E.; O’Neill, W.W.; D’Agostino, R.; Flack, J.M.; Katzen, B.T.; Leon, M.B.; Liu, M.; Mauri, L.; Negoita, M.; et al. A Controlled Trial of Renal Denervation for Resistant Hypertension. N. Engl. J. Med. 2014, 370, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Kandzari, D.E.; Bhatt, D.L.; Brar, S.; Devireddy, C.M.; Esler, M.; Fahy, M.; Flack, J.M.; Katzen, B.T.; Lea, J.; Lee, D.P.; et al. Predictors of blood pressure response in the SYMPLICITY HTN-3 trial. Eur. Heart J. 2015, 36, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Sakakura, K.; Ladich, E.; Cheng, Q.; Otsuka, F.; Yahagi, K.; Fowler, D.R.; Kolodgie, F.D.; Virmani, R.; Joner, M. Anatomic Assessment of Sympathetic Peri-Arterial Renal Nerves in Man. J. Am. Coll. Cardiol. 2014, 64, 635–643. [Google Scholar] [CrossRef]
- Kasprzycki, K.; Petkow-Dimitrow, P.; Krawczyk-Ożóg, A.; Bartuś, S.; Rajtar-Salwa, R. Anatomic Variations of Renal Arteries as an Important Factor in the Effectiveness of Renal Denervation in Resistant Hypertension. J. Cardiovasc. Dev. Dis. 2023, 10, 371. [Google Scholar] [CrossRef] [PubMed]
- Kandzari, D.E.; Mahfoud, F.; Weber, M.A.; Townsend, R.; Parati, G.; Fisher, N.D.L.; Lobo, M.D.; Bloch, M.; Böhm, M.; Sharp, A.S.P.; et al. Clinical Trial Design Principles and Outcomes Definitions for Device-Based Therapies for Hypertension: A Consensus Document from the Hypertension Academic Research Consortium. Circulation 2022, 145, 847–863. [Google Scholar] [CrossRef]
- Barbato, E.; Azizi, M.; Schmieder, R.E.; Lauder, L.; Böhm, M.; Brouwers, S.; Bruno, R.M.; Dudek, D.; Kahan, T.; Kandzari, D.E.; et al. Renal denervation in the management of hypertension in adults. A clinical consensus statement of the ESC Council on Hypertension and the European Association of Percutaneous Cardiovascular Interventions (EAPCI). EuroIntervention 2023, 18, 1227–1243. [Google Scholar] [CrossRef] [PubMed]
- Ogoyama, Y.; Tada, K.; Abe, M.; Nanto, S.; Shibata, H.; Mukoyama, M.; Kai, H.; Arima, H.; Kario, K. Effects of renal denervation on blood pressures in patients with hypertension: A systematic review and meta-analysis of randomized sham-controlled trials. Hypertens. Res. 2022, 45, 210–220. [Google Scholar] [CrossRef]
- Mahfoud, F.; Kandzari, D.E.; Kario, K.; Townsend, R.R.; Weber, M.A.; Schmieder, R.E.; Tsioufis, K.; Pocock, S.; Dimitriadis, K.; Choi, J.W.; et al. Long-term efficacy and safety of renal denervation in the presence of antihypertensive drugs (SPYRAL HTN-ON MED): A randomised, sham-controlled trial. Lancet 2022, 399, 1401–1410. [Google Scholar] [CrossRef]
- Sesa-Ashton, G.; Nolde, J.M.; Muente, I.; Carnagarin, R.; Lee, R.; Macefield, V.G.; Dawood, T.; Sata, Y.; Lambert, E.A.; Lambert, G.W.; et al. Catheter-Based Renal Denervation: 9-Year Follow-Up Data on Safety and Blood Pressure Reduction in Patients with Resistant Hypertension. Hypertension 2023, 80, 811–819. [Google Scholar] [CrossRef]
- Townsend, R.R.; Mahfoud, F.; Kandzari, D.E.; Kario, K.; Pocock, S.; Weber, M.A.; Ewen, S.; Tsioufis, K.; Tousoulis, D.; Sharp, A.S.P.; et al. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): A randomised, sham-controlled, proof-of-concept trial. Lancet 2017, 390, 2160–2170. [Google Scholar] [CrossRef]
- Böhm, M.; Kario, K.; Kandzari, D.E.; Mahfoud, F.; Weber, M.A.; Schmieder, R.E.; Tsioufis, K.; Pocock, S.; Konstantinidis, D.; Choi, J.W.; et al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): A multicentre, randomised, sham-controlled trial. Lancet 2020, 395, 1444–1451. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.A.; Schmieder, R.E.; Kandzari, D.E.; Townsend, R.R.; Mahfoud, F.; Tsioufis, K.; Kario, K.; Pocock, S.; Tatakis, F.; Ewen, S.; et al. Hypertension urgencies in the SPYRAL HTN-OFF MED Pivotal trial. Clin. Res. Cardiol. 2022, 111, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Böhm, M.; Fahy, M.; Hickey, G.L.; Pocock, S.; Brar, S.; DeBruin, V.; Weber, M.A.; Mahfoud, F.; Kandzari, D.E. A re-examination of the SPYRAL HTN-OFF MED Pivotal trial with respect to the underlying model assumptions. Contemp. Clin. Trials Commun. 2021, 23, 100818. [Google Scholar] [CrossRef] [PubMed]
- Matthews, V.B.; Elliot, R.H.; Rudnicka, C.; Hricova, J.; Herat, L.; Schlaich, M.P. Role of the sympathetic nervous system in regulation of the sodium glucose cotransporter 2. J. Hypertens. 2017, 35, 2059–2068. [Google Scholar] [CrossRef]
- Cherrington, A.D. Banting Lecture 1997. Control of glucose uptake and release by the liver in vivo. Diabetes 1999, 48, 1198–1214. [Google Scholar] [CrossRef] [PubMed]
- Mahfoud, F.; Schlaich, M.; Kindermann, I.; Ukena, C.; Cremers, B.; Brandt, M.C.; Hoppe, U.C.; Vonend, O.; Rump, L.C.; Sobotka, P.A.; et al. Effect of Renal Sympathetic Denervation on Glucose Metabolism in Patients with Resistant Hypertension: A Pilot Study. Circulation 2011, 123, 1940–1946. [Google Scholar] [CrossRef] [PubMed]
- Kądziela, J.; Warchoł-Celińska, E.; Prejbisz, A.; Januszewicz, A.; Witkowski, A.; Tsioufis, K. Renal denervation—Can we press the “ON” button again? Adv. Interv. Cardiol. 2018, 14, 321–327. [Google Scholar] [CrossRef]
- Pan, T.; Guo, J.; Teng, G. Renal Denervation: A Potential Novel Treatment for Type 2 Diabetes Mellitus? Medicine 2015, 94, e1932. [Google Scholar] [CrossRef]
- Witkowski, A.; Prejbisz, A.; Florczak, E.; Kądziela, J.; Śliwiński, P.; Bieleń, P.; Michałowska, I.; Kabat, M.; Warchoł, E.; Januszewicz, M.; et al. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension 2011, 58, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Tsioufis, C.; Dimitriadis, K.; Kasiakogias, A.; Kalos, T.; Liatakis, I.; Koutra, E.; Nikolopoulou, L.; Kordalis, A.; Ella, R.O.; Lau, E.O.-Y.; et al. Effects of multielectrode renal denervation on elevated sympathetic nerve activity and insulin resistance in metabolic syndrome. J. Hypertens. 2017, 35, 1100–1108. [Google Scholar] [CrossRef]
- Sharp, A.S.P.; Kinnaird, T.; Curzen, N.; Ayyub, R.; Alfonso, J.E.; Mamas, M.A.; Vanden Bavière, H. Cost-effectiveness of intravascular ultrasound-guided percutaneous intervention in patients with acute coronary syndromes: A UK perspective. Eur. Heart J.-Qual. Care Clin. Outcomes 2024, 10, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Negrete, K. Anatomy and Function of Autonomic Innervation of the Liver. Pegasus Rev. UCF Undergrad. Res. J. 2020, 11, 3. [Google Scholar]
- Mizuno, K.; Ueno, Y. Autonomic Nervous System and the Liver. Hepatol. Res. 2017, 47, 160–165. [Google Scholar] [CrossRef]
- Yu, S.; Huang, S.; Ding, Y.; Wang, W.; Wang, A.; Lu, Y. Transient receptor potential ion-channel subfamily V member 4: A potential target for cancer treatment. Cell Death Dis. 2019, 10, 497. [Google Scholar] [CrossRef]
- Ueno, T.; Bioulac-Sage, P.; Balabaud, C.; Rosenbaum, J. Innervation of the sinusoidal wall: Regulation of the sinusoidal diameter. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2004, 280A, 868–873. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.J.; Alpini, G.; Glaser, S. Hepatic Nervous System and Neurobiology of the Liver. Compr. Physiol. 2013, 3, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Kan, Z.; Madoff, D. Liver Anatomy: Microcirculation of the Liver. Semin. Interv. Radiol. 2008, 25, 077–085. [Google Scholar] [CrossRef]
- Eipel, C.; Abshagen, K.; Vollmar, B. Regulation of hepatic blood flow: The hepatic arterial buffer response revisited. World J. Gastroenterol. 2010, 16, 6046–6057. [Google Scholar] [CrossRef] [PubMed]
- Choe, W.-S.; Song, W.H.; Jeong, C.W.; Choi, E.-K.; Oh, S. Anatomic Conformation of Renal Sympathetic Nerve Fibers in Living Human Tissues. Sci. Rep. 2019, 9, 4831. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.G.P.; Bhatt, C.R.; Patel, S.V.; Mehta, C.D. Morphometric Study of Coeliac Trunk Specific Reference to Hepatic Artery Pattern in the West-Indian Population. Indian. J. Surg. 2014, 76, 359–362. [Google Scholar] [CrossRef]
- Huang, Q.; Pang, M.; Zeng, Q.; He, X.; Zheng, R.; Ge, M.; Li, K. The frequency and risk factors of major complications after thermal ablation of liver tumours in 2,084 ablation sessions. Front. Surg. 2022, 9, 1010043. [Google Scholar] [CrossRef]
- Kiuchi, M.G.; Ganesan, K.; Keating, J.; Carnagarin, R.; Matthews, V.B.; Herat, L.Y.; Goh, G.; Adams, L.; Schlaich, M.P. Combined renal and common hepatic artery denervation as a novel approach to reduce cardiometabolic risk: Technical approach, feasibility and safety in a pre-clinical model. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2021, 110, 740–753. [Google Scholar] [CrossRef] [PubMed]
- Hazlehurst, J.M.; Woods, C.; Marjot, T.; Cobbold, J.F.; Tomlinson, J.W. Non-alcoholic fatty liver disease and diabetes. Metabolism 2016, 65, 1096–1108. [Google Scholar] [CrossRef] [PubMed]
- Carnagarin, R.; Tan, K.; Adams, L.; Matthews, V.B.; Kiuchi, M.G.; Gavidia, L.M.L.; Lambert, G.W.; Lambert, E.A.; Herat, L.Y.; Schlaich, M.P. Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD)—A Condition Associated with Heightened Sympathetic Activation. Int. J. Mol. Sci. 2021, 22, 4241. [Google Scholar] [CrossRef]
- Sigala, B.; McKee, C.; Soeda, J.; Pazienza, V.; Morgan, M.; Lin, C.I.; Selden, C.; Borght, S.V.; Mazzoccoli, G.; Roskams, T.; et al. Sympathetic Nervous System Catecholamines and Neuropeptide Y Neurotransmitters Are Upregulated in Human NAFLD and Modulate the Fibrogenic Function of Hepatic Stellate Cells. PLoS ONE 2013, 8, e72928. [Google Scholar] [CrossRef]
- Kraft, G.; Vrba, A.; Scott, M.; Allen, E.; Edgerton, D.S.; Williams, P.E.; Vafai, S.B.; Azamian, B.R.; Cherrington, A.D. Sympathetic Denervation of the Common Hepatic Artery Lessens Glucose Intolerance in the Fat- and Fructose-Fed Dog. Diabetes 2019, 68, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Bruinstroop, E.; Eliveld, J.; Foppen, E.; Busker, S.; Ackermans, M.T.; Fliers, E.; Kalsbeek, A. Hepatic denervation and dyslipidemia in obese Zucker (fa/fa) rats. Int. J. Obes. 2015, 39, 1655–1658. [Google Scholar] [CrossRef]
- Hurr, C.; Simonyan, H.; Morgan, D.A.; Rahmouni, K.; Young, C.N. Liver sympathetic denervation reverses obesity-induced hepatic steatosis. J. Physiol. 2019, 597, 4565–4580. [Google Scholar] [CrossRef]
- Amir, M.; Yu, M.; He, P.; Srinivasan, S. Hepatic Autonomic Nervous System and Neurotrophic Factors Regulate the Pathogenesis and Progression of Non-alcoholic Fatty Liver Disease. Front. Med. 2020, 7, 62. [Google Scholar] [CrossRef]
- Kato, H.; Shimazu, T. Effect of Autonomic Denervation on DNA Synthesis During Liver Regeneration After Partial Hepatectomy. Eur. J. Biochem. 1983, 134, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, H.; Zhang, Y.; Liu, Y.; Zhou, T.; Zhou, B.; Zhang, Y.; Chen, R.; Xing, J.; He, L.; et al. Current Perspectives of Neuroendocrine Regulation in Liver Fibrosis. Cells 2022, 11, 3783. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, C.F.M.G.; Salvadori, R.; Feldner, A.C.D.C.A.; Aguiar, M.O.D.; Gonzalez, A.M.; Branco, G.R.; Superbia, M.; Lai, M.; Mota, D.D.O.; Ferraz, M.L.C.G.; et al. Autonomic dysfunction is common in liver cirrhosis and is associated with cardiac dysfunction and mortality: Prospective observational study. Sao Paulo Med. J. 2022, 140, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Colle, I.; Van Vlierberghe, H.; Troisi, R.; De Hemptinne, B. Transplanted liver: Consequences of denervation for liver functions. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2004, 280A, 924–931. [Google Scholar] [CrossRef]
- Koopman, F.A.; Stoof, S.P.; Straub, R.H.; Van Maanen, M.A.; Vervoordeldonk, M.J.; Tak, P.P. Restoring the Balance of the Autonomic Nervous System as an Innovative Approach to the Treatment of Rheumatoid Arthritis. Mol. Med. 2011, 17, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Verlinden, T.J.M.; Van Dijk, P.; Hikspoors, J.; Herrler, A.; Lamers, W.H.; Köhler, S.E. Innervation of the human spleen: A complete hilum-embedding approach. Brain Behav. Immun. 2019, 77, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, T.; Liao, L.; Fan, X.; Chang, L.; Hashimoto, K. Brain-spleen axis in health and diseases: A review and future perspective. Brain Res. Bull. 2022, 182, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain–Gut Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry 2018, 9, 44. [Google Scholar] [CrossRef]
- Lori, A.; Perrotta, M.; Lembo, G.; Carnevale, D. The Spleen: A Hub Connecting Nervous and Immune Systems in Cardiovascular and Metabolic Diseases. Int. J. Mol. Sci. 2017, 18, 1216. [Google Scholar] [CrossRef]
- Moraes, D.M.V.D.; Gutierres, A.; Colleoni Neto, R.; Lindemann, I.L.; Rottenfusser, R.; Carlotto, J.R.M. Anatomy of the splenic artery: What does the surgeon need to know? Rev. Colégio Bras. Cir. 2022, 49, e20223294. [Google Scholar] [CrossRef]
- Gupta, I.; Cassará, A.M.; Tarotin, I.; Donega, M.; Miranda, J.A.; Sokal, D.M.; Ouchouche, S.; Dopson, W.; Matteucci, P.; Neufeld, E.; et al. Quantification of clinically applicable stimulation parameters for precision near-organ neuromodulation of human splenic nerves. Commun. Biol. 2020, 3, 577. [Google Scholar] [CrossRef]
- Cleypool, C.G.J.; Lotgerink Bruinenberg, D.; Roeling, T.; Irwin, E.; Bleys, R.L.A.W. Splenic artery loops: Potential splenic plexus stimulation sites for neuroimmunomodulatory-based anti-inflammatory therapy? Clin. Anat. 2021, 34, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, D.; Lorton, D. Sympathetic Nerve Hyperactivity in the Spleen: Causal for Nonpathogenic-Driven Chronic Immune-Mediated Inflammatory Diseases (IMIDs)? Int. J. Mol. Sci. 2018, 19, 1188. [Google Scholar] [CrossRef] [PubMed]
- Osborn, J.W.; Fink, G.D. Region-specific changes in sympathetic nerve activity in angiotensin II-salt hypertension in the rat. Exp. Physiol. 2010, 95, 61–68. [Google Scholar] [CrossRef]
- Sattler, J.; Tu, J.; Stoner, S.; Li, J.; Buttgereit, F.; Seibel, M.J.; Zhou, H.; Cooper, M.S. Role of 11β-HSD type 1 in abnormal HPA axis activity during immune-mediated arthritis. Endocr. Connect. 2018, 7, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Donegà, M.; Fjordbakk, C.T.; Kirk, J.; Sokal, D.M.; Gupta, I.; Hunsberger, G.E.; Crawford, A.; Cook, S.; Viscasillas, J.; Stathopoulou, T.-R.; et al. Human-relevant near-organ neuromodulation of the immune system via the splenic nerve. Proc. Natl. Acad. Sci. USA 2021, 118, e2025428118. [Google Scholar] [CrossRef] [PubMed]
- Albaghdadi, M.; Garcia-Polite, F.; Zani, B.; Keating, J.; Melidone, R.; Spognardi, A.; Markham, P.; Tzafriri, A. Splenic artery denervation: Target micro-anatomy, feasibility, and early preclinical experience. Transl. Res. 2019, 213, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Brudey, C.; Park, J.; Wiaderkiewicz, J.; Kobayashi, I.; Mellman, T.A.; Marvar, P.J. Autonomic and inflammatory consequences of posttraumatic stress disorder and the link to cardiovascular disease. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2015, 309, R315–R321. [Google Scholar] [CrossRef]
- Elkhatib, S.K.; Moshfegh, C.M.; Watson, G.F.; Schwab, A.D.; Katsurada, K.; Patel, K.P.; Case, A.J. Splenic denervation attenuates repeated social defeat stress-induced T-lymphocyte inflammation. Biol. Psychiatry Glob. Open Sci. 2021, 1, 190–200. [Google Scholar] [CrossRef]
- Kummer, W. Pulmonary Vascular Innervation and Its Role in Responses to Hypoxia: Size Matters! Proc. Am. Thorac. Soc. 2011, 8, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.G.; Miserlis, D.; Hart, J.P. Current status of pulmonary artery denervation. Front. Cardiovasc. Med. 2022, 9, 972256. [Google Scholar] [CrossRef]
- Rothman, A.M.K.; Arnold, N.D.; Chang, W.; Watson, O.; Swift, A.J.; Condliffe, R.; Elliot, C.A.; Kiely, D.G.; Suvarna, S.K.; Gunn, J.; et al. Pulmonary Artery Denervation Reduces Pulmonary Artery Pressure and Induces Histological Changes in an Acute Porcine Model of Pulmonary Hypertension. Circ. Cardiovasc. Interv. 2015, 8, e002569. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-L.; Zhang, H.; Xie, D.-J.; Zhang, J.; Zhou, L.; Rothman, A.M.K.; Stone, G.W. Hemodynamic, functional, and clinical responses to pulmonary artery denervation in patients with pulmonary arterial hypertension of different causes: Phase II results from the Pulmonary Artery Denervation-1 study. Circ. Cardiovasc. Interv. 2015, 8, e002837. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wei, Y.; Zhang, C.; Yang, Z.; Kan, J.; Gu, H.; Fan, F.; Gu, H.; Wang, Q.; Xie, D.; et al. Pulmonary Artery Denervation for Pulmonary Arterial Hypertension. JACC Cardiovasc. Interv. 2022, 15, 2412–2423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, J.; Chen, M.; Xie, D.-J.; Kan, J.; Yu, W.; Li, X.-B.; Xu, T.; Gu, Y.; Dong, J.; et al. Pulmonary Artery Denervation Significantly Increases 6-Min Walk Distance for Patients with Combined Pre- and Post-Capillary Pulmonary Hypertension Associated with Left Heart Failure: The PADN-5 Study. JACC Cardiovasc. Interv. 2019, 12, 274–284. [Google Scholar] [CrossRef]
- Rothman, A.; Vachiery, J.-L.E.; Howard, L.S.; Mikhail, G.; Lang, I.M.; Jonas, M.; Kiely, D.G.; Abriel, A.; Lewis, G.D.; Rosenzweig, E.B.; et al. Percutaneous Endovascular Ultrasound Pulmonary Artery Denervation for the Treatment of Pulmonary Arterial Hypertension: 12-Month Results of the Trophy 1 Study. Am. J. Respir. Crit. Care Med. 2020, 201, A7278. [Google Scholar]
- Romanov, A.; Cherniavskiy, A.; Novikova, N.; Edemskiy, A.; Ponomarev, D.; Shabanov, V.; Losik, D.; Elesin, D.; Stenin, I.; Mikheenko, I.; et al. Pulmonary Artery Denervation for Patients with Residual Pulmonary Hypertension After Pulmonary Endarterectomy. J. Am. Coll. Cardiol. 2020, 76, 916–926. [Google Scholar] [CrossRef]
- Gold, O.M.S.; Bardsley, E.N.; Ponnampalam, A.P.; Pauza, A.G.; Paton, J.F.R. Cellular basis of learning and memory in the carotid body. Front. Synaptic Neurosci. 2022, 14, 902319. [Google Scholar] [CrossRef]
- Badoer, E. The Carotid Body a Common Denominator for Cardiovascular and Metabolic Dysfunction? Front. Physiol. 2020, 11, 1069. [Google Scholar] [CrossRef] [PubMed]
- Platero-Luengo, A.; González-Granero, S.; Durán, R.; Díaz-Castro, B.; Piruat, J.I.; García-Verdugo, J.M.; Pardal, R.; López-Barneo, J. An O2-sensitive glomus cell-stem cell synapse induces carotid body growth in chronic hypoxia. Cell 2014, 156, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Tse, A.; Yan, L.; Lee, A.K.; Tse, F.W. Autocrine and paracrine actions of ATP in rat carotid body. Can. J. Physiol. Pharmacol. 2012, 90, 705–711. [Google Scholar] [CrossRef]
- Nurse, C.A. Synaptic and paracrine mechanisms at carotid body arterial chemoreceptors. J. Physiol. 2014, 592, 3419–3426. [Google Scholar] [CrossRef] [PubMed]
- Pardal, R.; Ortega-Sáenz, P.; Durán, R.; López-Barneo, J. Glia-like stem cells sustain physiologic neurogenesis in the adult mammalian carotid body. Cell 2007, 131, 364–377. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.-K.; Tang, W.-Y.; Amorim, M.R.; Sham, J.S.-K.; Polotsky, V.Y. Carotid body denervation improves hyperglycemia in obese mice. J. Appl. Physiol. 2024, 136, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Marcus, N.J.; Del Rio, R.; Schultz, E.P.; Xia, X.-H.; Schultz, H.D. Carotid body denervation improves autonomic and cardiac function and attenuates disordered breathing in congestive heart failure. J. Physiol. 2014, 592, 391–408. [Google Scholar] [CrossRef]
- Search for: Other Terms: Renal Denervation|List Results|ClinicalTrials.gov. Available online: https://clinicaltrials.gov/search?term=renal%20denervation&viewType=Table#classicRedirect (accessed on 7 July 2024).
- Marchesini, G.; Forlani, G.; Cerrelli, F.; Manini, R.; Natale, S.; Baraldi, L.; Ermini, G.; Savorani, G.; Zocchi, D.; Melchionda, N. WHO and ATPIII proposals for the definition of the metabolic syndrome in patients with Type 2 diabetes. Diabet. Med. 2004, 21, 383–387. [Google Scholar] [CrossRef] [PubMed]
- John, R.S.; Dixon, B.; Hendrix, J.M.; Shienbaum, R. Celiac Plexus Block. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK531469/ (accessed on 30 June 2024).
- Quarti-Trevano, F.; Seravalle, G.; Dell’Oro, R.; Mancia, G.; Grassi, G. Autonomic Cardiovascular Alterations in Chronic Kidney Disease: Effects of Dialysis, Kidney Transplantation, and Renal Denervation. Curr. Hypertens. Rep. 2021, 23, 10. [Google Scholar] [CrossRef]
- Booth, L.C.; De Silva, R.A.U.; Pontes, R.B.; Yao, S.T.; Hood, S.G.; Lankadeva, Y.R.; Kosaka, J.; Eikelis, N.; Lambert, G.W.; Schlaich, M.P.; et al. Renal, Cardiac, and Autonomic Effects of Catheter-Based Renal Denervation in Ovine Heart Failure. Hypertension 2021, 78, 706–715. [Google Scholar] [CrossRef]
- Brinkman, D.J.; Simon, T.; Ten Hove, A.S.; Zafeiropoulou, K.; Welting, O.; Van Hamersveld, P.H.P.; Willemze, R.A.; Yim, A.Y.F.L.; Verseijden, C.; Hakvoort, T.B.M.; et al. Electrical stimulation of the splenic nerve bundle ameliorates dextran sulfate sodium-induced colitis in mice. J. Neuroinflamm. 2022, 19, 155. [Google Scholar] [CrossRef]
- Pisano, G.; Fracanzani, A.L.; Caccamo, L.; Donato, M.F.; Fargion, S. Cardiovascular risk after orthotopic liver transplantation, a review of the literature and preliminary results of a prospective study. World J. Gastroenterol. 2016, 22, 8869. [Google Scholar] [CrossRef]
- Jordan, J.; Tank, J. How Sympathetic Is Sympathetic Enough? Hypertension 2020, 76, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Kantauskaite, M.; Vonend, O.; Yakoub, M.; Heilmann, P.; Maifeld, A.; Minko, P.; Schimmöller, L.; Antoch, G.; Müller, D.N.; Schmidt, C.; et al. The Effect of Renal Denervation on T Cells in Patients with Resistant Hypertension. Int. J. Mol. Sci. 2023, 24, 2493. [Google Scholar] [CrossRef] [PubMed]
- Oliveras, A.; Armario, P.; Sans, L.; Clarà, A.; Vázquez, S.; Molina, L.; Pareja, J.; De La Sierra, A.; Pascual, J. Organ damage changes in patients with resistant hypertension randomized to renal denervation or spironolactone: The DENERVHTA (Denervación en Hipertensión Arterial) study. J. Clin. Hypertens. 2018, 20, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Verloop, W.L.; Vink, E.E.; Spiering, W.; Blankestijn, P.J.; Doevendans, P.A.; Bots, M.L.; Vonken, E.; Voskuil, M.; Leiner, T. Effects of renal denervation on end organ damage in hypertensive patients. Eur. J. Prev. Cardiol. 2015, 22, 558–567. [Google Scholar] [CrossRef]
- Kiuchi, M.G.; Schlaich, M.P.; Chen, S.; Villacorta, H.; Ho, J.K.; Carnagarin, R.; Matthews, V.B.; Lugon, J.R. Relevance of Targeting the Distal Renal Artery and Branches with Radiofrequency Renal Denervation Approaches—A Secondary Analysis from a Hypertensive CKD Patient Cohort. J. Clin. Med. 2019, 8, 581. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.H.; Choi, S.-H. Current Status and Future Perspectives of Renal Denervation. Korean Circ. J. 2021, 51, 717. [Google Scholar] [CrossRef] [PubMed]
- Roubsanthisuk, W.; Kunanon, S.; Chattranukulchai, P.; Panchavinnin, P.; Wongpraparut, N.; Chaipromprasit, J.; Pienvichitr, P.; Ayudhya, R.K.N.; Sukonthasarn, A.; on behalf of Thai Hypertension Society. 2022 Renal denervation therapy for the treatment of hypertension: A statement from the Thai Hypertension Society. Hypertens. Res. 2023, 46, 898–912. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.; Hubbard, B.; Sakaoka, A.; Rousselle, S.; Tellez, A.; Jiang, X.; Kario, K.; Hohl, M.; Böhm, M.; Mahfoud, F. Procedural and anatomical predictors of renal denervation efficacy using two radiofrequency renal denervation catheters in a porcine model. J. Hypertens. 2018, 36, 2453–2459. [Google Scholar] [CrossRef]
- Ettehad, D.; Emdin, C.A.; Kiran, A.; Anderson, S.G.; Callender, T.; Emberson, J.; Chalmers, J.; Rodgers, A.; Rahimi, K. Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta-analysis. Lancet 2016, 387, 957–967. [Google Scholar] [CrossRef]



| Denervation Site(s) | Targeted Condition |
|---|---|
| Renal artery | Hypertension [26] |
| Arrhythmia [27] | |
| Dysglycaemia [28] | |
| Obstructive sleep apnoea [29] | |
| Chronic kidney disease [30] | |
| Heart failure [31] | |
| Common hepatic artery | Dysglycaemia [32] |
| Dyslipidaemia/insulin resistance [33] | |
| Hypertension [34] | |
| Liver cirrhosis [35] | |
| Splenic artery | Hypertension [36] |
| Hypertrophic cardiomyopathy [37] | |
| Rheumatoid arthritis [37] | |
| Pulmonary artery | Pulmonary arterial hypertension [38] |
| Carotid body | Dysglycaemia/insulin resistance [39] |
| Heart failure [40] | |
| Obstructive sleep apnoea [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haider, S.A.; Sharif, R.; Sharif, F. Multi-Organ Denervation: The Past, Present and Future. J. Clin. Med. 2025, 14, 2746. https://doi.org/10.3390/jcm14082746
Haider SA, Sharif R, Sharif F. Multi-Organ Denervation: The Past, Present and Future. Journal of Clinical Medicine. 2025; 14(8):2746. https://doi.org/10.3390/jcm14082746
Chicago/Turabian StyleHaider, Syedah Aleena, Ruth Sharif, and Faisal Sharif. 2025. "Multi-Organ Denervation: The Past, Present and Future" Journal of Clinical Medicine 14, no. 8: 2746. https://doi.org/10.3390/jcm14082746
APA StyleHaider, S. A., Sharif, R., & Sharif, F. (2025). Multi-Organ Denervation: The Past, Present and Future. Journal of Clinical Medicine, 14(8), 2746. https://doi.org/10.3390/jcm14082746








