Predictors of Prolonged Intensive Care Unit Stay and In-Hospital Mortality Following Cardiac Surgery: An Integrated Analysis from the PROCARD-ATI Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. Statistical Analysis
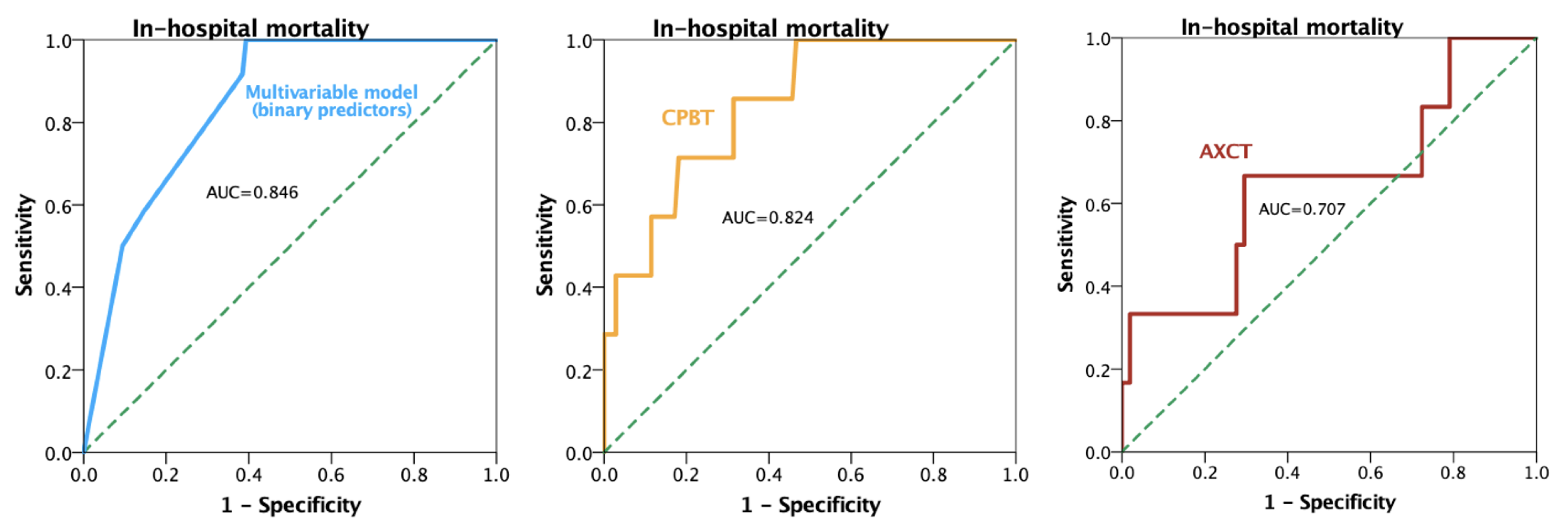
3. Results
4. Discussion
4.1. Key Findings
4.2. Predictors of Prolonged ICU Stay
4.3. Predictors of Mortality
4.4. Clinical Perspective
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| AXCT | Aortic cross-clamp time |
| CPBT | Cardiopulmonary bypass time |
| E-CABG | European Multicenter Study on Coronary Artery Bypass Grafting |
| EuroSCORE | European System for Cardiac Operative Risk Evaluation |
| ICU | Intensive care unit |
| IQR | Interquartile range |
| ROC | Receiver operator characteristics |
| STS | Society of Thoracic Surgeons |
References
- Diab, M.; Bilkhu, R.; Soppa, G.; McGale, N.; Hirani, S.P.; Newman, S.P.; Jahangiri, M. Quality of Life in Relation to Length of Intensive Care Unit Stay After Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2017, 31, 1080–1090. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Ahuja, A.; Kumar, A.; Anstey, C.; Thang, C.; Guo, L.; Shekar, K.; Ramanan, M. Outcomes of Prolonged ICU Stay for Patients Undergoing Cardiac Surgery in Australia and New Zealand. J. Cardiothorac. Vasc. Anesth. 2022, 36, 4313–4319. [Google Scholar] [CrossRef]
- Almashrafi, A.; Alsabti, H.; Mukaddirov, M.; Balan, B.; Aylin, P. Factors Associated with Prolonged Length of Stay Following Cardiac Surgery in a Major Referral Hospital in Oman: A Retrospective Observational Study. BMJ Open 2016, 6, e010764. [Google Scholar] [CrossRef] [PubMed]
- Litwinowicz, R.; Bartus, K.; Drwila, R.; Kapelak, B.; Konstanty-Kalandyk, J.; Sobczynski, R.; Wierzbicki, K.; Bartuś, M.; Chrapusta, A.; Timek, T.; et al. In-Hospital Mortality in Cardiac Surgery Patients after Readmission to the Intensive Care Unit: A Single-Center Experience with 10,992 Patients. J. Cardiothorac. Vasc. Anesth. 2015, 29, 570–575. [Google Scholar] [CrossRef]
- Almashrafi, A.; Elmontsri, M.; Aylin, P. Systematic Review of Factors Influencing Length of Stay in ICU after Adult Cardiac Surgery. BMC Health Serv. Res. 2016, 16, 318. [Google Scholar] [CrossRef] [PubMed]
- Dominici, C.; Salsano, A.; Nenna, A.; Spadaccio, C.; Barbato, R.; Mariscalco, G.; Santini, F.; Biancari, F.; Chello, M. A Nomogram for Predicting Long Length of Stay in The Intensive Care Unit in Patients Undergoing CABG: Results From the Multicenter E-CABG Registry. J. Cardiothorac. Vasc. Anesth. 2020, 34, 2951–2961. [Google Scholar] [CrossRef]
- Nashef, S.A.M.; Roques, F.; Sharples, L.D.; Nilsson, J.; Smith, C.; Goldstone, A.R.; Lockowandt, U. Euroscore II. Eur. J. Cardio-Thorac. Surg. 2012, 41, 734–745. [Google Scholar] [CrossRef]
- Shahian, D.M.; Jacobs, J.P.; Badhwar, V.; Kurlansky, P.A.; Furnary, A.P.; Cleveland, J.C.; Lobdell, K.W.; Vassileva, C.; Wyler von Ballmoos, M.C.; Thourani, V.H.; et al. The Society of Thoracic Surgeons 2018 Adult Cardiac Surgery Risk Models: Part 1—Background, Design Considerations, and Model Development. Ann. Thorac. Surg. 2018, 105, 1411–1418. [Google Scholar] [CrossRef]
- O’Brien, S.M.; Feng, L.; He, X.; Xian, Y.; Jacobs, J.P.; Badhwar, V.; Kurlansky, P.A.; Furnary, A.P.; Cleveland, J.C.; Lobdell, K.W.; et al. The Society of Thoracic Surgeons 2018 Adult Cardiac Surgery Risk Models: Part 2—Statistical Methods and Results. Ann. Thorac. Surg. 2018, 105, 1419–1428. [Google Scholar] [CrossRef]
- Ettema, R.G.A.; Peelen, L.M.; Schuurmans, M.J.; Nierich, A.P.; Kalkman, C.J.; Moons, K.G.M. Prediction Models for Prolonged Intensive Care Unit Stay after Cardiac Surgery: Systematic Review and Validation Study. Circulation 2010, 122, 682–689. [Google Scholar] [CrossRef]
- Ong, C.S.; Reinertsen, E.; Sun, H.; Moonsamy, P.; Mohan, N.; Funamoto, M.; Kaneko, T.; Shekar, P.S.; Schena, S.; Lawton, J.S.; et al. Prediction of Operative Mortality for Patients Undergoing Cardiac Surgical Procedures without Established Risk Scores. J. Thorac. Cardiovasc. Surg. 2023, 165, 1449–1459.e15. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lin, Y.; Li, P.; Hu, Y.; Zhang, L.; Kong, G. Predicting Prolonged Length of ICU Stay through Machine Learning. Diagnostics 2021, 11, 2242. [Google Scholar] [CrossRef]
- Rotar, E.P.; Beller, J.P.; Smolkin, M.E.; Chancellor, W.Z.; Ailawadi, G.; Yarboro, L.T.; Hulse, M.; Ratcliffe, S.J.; Teman, N.R. Prediction of Prolonged Intensive Care Unit Length of Stay Following Cardiac Surgery. Semin. Thorac. Cardiovasc. Surg. 2022, 34, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Fottinger, A.; Eddeen, A.B.; Lee, D.S.; Woodward, G.; Sun, L.Y. Derivation and Validation of Pragmatic Clinical Models to Predict Hospital Length of Stay after Cardiac Surgery in Ontario, Canada: A Population-Based Cohort Study. CMAJ Open 2023, 11, E180–E190. [Google Scholar] [CrossRef] [PubMed]
- Widyastuti, Y.; Stenseth, R.; Wahba, A.; Pleym, H.; Videm, V. Length of Intensive Care Unit Stay Following Cardiac Surgery: Is It Impossible to Find a Universal Prediction Model? Interact. Cardiovasc. Thorac. Surg. 2012, 15, 825–833. [Google Scholar] [CrossRef]
- Moh’d, A.F.; Al-Odwan, H.T.; Altarabsheh, S.; Makahleh, Z.M.; Khasawneh, M.A. Predictors of Aortic Clamp Time Duration and Intensive Care Unit Length of Stay in Elective Adult Cardiac Surgery. Egypt. Heart J. 2021, 73, 92. [Google Scholar] [CrossRef]
- Cislaghi, F.; Condemi, A.M.; Corona, A. Predictors of Prolonged Mechanical Ventilation in a Cohort of 3,269 CABG Patients. Minerva Anestesiol. 2007, 73, 615–621. [Google Scholar]
- Kapadohos, T.; Angelopoulos, E.; Vasileiadis, I.; Nanas, S.; Kotanidou, A.; Karabinis, A.; Marathias, K.; Routsi, C. Determinants of Prolonged Intensive Care Unit Stay in Patients after Cardiac Surgery: A Prospective Observational Study. J. Thorac. Dis. 2017, 9, 70–79. [Google Scholar] [CrossRef]
- Hassan, A.; Anderson, C.; Kypson, A.; Kindell, L.; Ferguson, T.B.; Chitwood, W.R.; Rodriguez, E. Clinical Outcomes in Patients with Prolonged Intensive Care Unit Length of Stay after Cardiac Surgical Procedures. Ann. Thorac. Surg. 2012, 93, 565–569. [Google Scholar] [CrossRef]
- Erkut, B.; Ates, A. Investigation of the Effect of Cross-Clamp Time and Cross-Clamp Time on Troponin I Levels in Patients Undergoing Elective Coronary Artery Bypass Surgery. World J. Surg. Surg. Res. 2019, 2, 1110. [Google Scholar]
- Iino, K.; Miyata, H.; Motomura, N.; Watanabe, G.; Tomita, S.; Takemura, H.; Takamoto, S. Prolonged Cross-Clamping During Aortic Valve Replacement Is an Independent Predictor of Postoperative Morbidity and Mortality: Analysis of the Japan Cardiovascular Surgery Database. Ann. Thorac. Surg. 2017, 103, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Al-Sarraf, N.; Thalib, L.; Hughes, A.; Houlihan, M.; Tolan, M.; Young, V.; McGovern, E. Cross-Clamp Time Is an Independent Predictor of Mortality and Morbidity in Low- and High-Risk Cardiac Patients. Int. J. Surg. 2011, 9, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Shultz, B.; Timek, T.; Davis, A.T.; Heiser, J.; Murphy, E.; Willekes, C.; Hooker, R. Outcomes in Patients Undergoing Complex Cardiac Repairs with Cross Clamp Times over 300 Minutes. J. Cardiothorac. Surg. 2016, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Nissinen, J.; Biancari, F.; Wistbacka, J.-O.; Peltola, T.; Loponen, P.; Tarkiainen, P.; Virkkilä, M.; Tarkka, M. Safe Time Limits of Aortic Cross-Clamping and Cardiopulmonary Bypass in Adult Cardiac Surgery. Perfusion 2009, 24, 297–305. [Google Scholar] [CrossRef]


| Parameter | Entire Cohort (n = 130) | ICU Stay < 7 Days (n = 80) | ICU Stay ≥ 7 Day (n = 50) | p Value |
|---|---|---|---|---|
| ICU length of stay (days) | 6 [4–8] | 4 [3–6] | 9 [8–17] | <0.001 |
| Hospitalization duration (days) | 19 [14–27] | 15 [12–21] | 23 [20–35] | <0.001 |
| In-hospital death | 13 (10%) | 4 (5%) | 9 (18%) | 0.032 |
| Emergency surgery | 20 (15.4%) | 8 (10%) | 12 (24%) | 0.053 |
| Type of Surgery | 0.408 | |||
| Coronary artery bypass grafting | 33 (25.4%) | 20 (25%) | 13 (26%) | |
| Valve disease | 53 (40.8%) | 32 (40%) | 21 (42%) | |
| Other | 31 (23.8%) | 19 (24%) | 12 (24%) | |
| Combined interventions | 13 (10%) | 9 (11%) | 4 (8%) | |
| Demographic | ||||
| Age (years) | 61 [56–69] | 60 [56–69] | 65 [56–70] | 0.120 |
| Body mass index (kg/m2) | 27.5 ± 4.6 | 27.8 ± 4.5 | 27.1 ± 4.8 | 0.445 |
| Sex (men) | 86 (66.2%) | 57 (71%) | 29 (58%) | 0.132 |
| Risk factors | ||||
| Arterial hypertension | 87 (66.9%) | 58 (72.5%) | 29 (58%) | 0.887 |
| NYHA class III/IV | 26 (20%) | 12 (13.5%) | 14 (28%) | 0.088 |
| Type 2 Diabetes Mellitus | 33 (25.4%) | 25 (31%) | 8 (16%) | 0.181 |
| Smoking | 16 (12.3%) | 15 (17%) | 1 (2%) | 0.015 |
| Chronic obstructive pulmonary disease | 8 (6.2%) | 2 (2.5%) | 6 (12%) | 0.008 |
| Stage 3–5 chronic kidney disease | 9 (6.9%) | 5 (6%) | 4 (8%) | 0.432 |
| Preoperative | ||||
| Atrial fibrillation | 19 (14.6%) | 14 (16%) | 5 (10%) | 0.492 |
| Left ventricular ejection fraction (%) | 50 [45–55] | 50 [50–55] | 55 [50–55] | 0.381 |
| Tricuspid annulus plane systolic excursion (mm) | 20 [19–22] | 20 [20–21] | 20 [19–22] | 0.504 |
| Systolic pulmonary artery pressure (mmHg) | 20 [17–24] | 29 [25–42] | 26 [24–28] | 0.053 |
| Albumin (g/dL) | 3.3 ± 0.6 | 3.3 ± 0.5 | 3.1 ± 0.8 | 0.230 |
| Direct bilirubin (mg/dL) | 0.3 [0.2–0.46] | 0.30 [0.20–0.49] | 0.31 [0.17–0.40] | 0.480 |
| Total bilirubin (mg/dL) | 0.81 [0.57–1.33] | 0.80 [0.55–1.30] | 0.88 [0.60–1.42] | 0.665 |
| Alanine aminotransferase (U/L) | 24 [18–42] | 23 [18–43] | 28 [19–39] | 0.491 |
| Aspartate aminotransferase (U/L) | 28 [20–44] | 30 [21–44] | 27 [20–40] | 0.602 |
| Hemoglobin (g/dL) | 13.8 [12.6–14.7] | 13.9 [13.2–14.9] | 13.7 [12.3–14.3] | 0.212 |
| Thrombocytes (n/uL) | 215 ± 72 × 103 | 220 ± 73 × 103 | 208 ± 70 × 103 | 0.455 |
| Leucocytes (n/uL) | 7800 [6180–9826] × 103 | 7700 [6090–9400] × 103 | 8125 [6252–10,695] × 103 | 0.452 |
| Lymphocytes (n/uL) | 2000 [1590–2500] × 103 | 2000 [1680–2600] × 103 | 1880 [1325–2467] × 103 | 0.185 |
| Neutrophils (n/uL) | 5060 [3570–6480] × 103 | 5070 [3520–6540] × 103 | 5050 [3640–6300] × 103 | 0.957 |
| Serum potassium (mmol/L) | 4.4 [4–4.7] | 4.4 [4–4.7] | 4.5 [4.2–4.6] | 0.519 |
| Serum sodium (mmol/L) | 140 [138–141] | 140 [137–141] | 140 [138–141] | 0.778 |
| Errythrocyte sedimentation rate (mm/h) | 8 [5–23] | 8 [3–19] | 10 [5–39] | 0.310 |
| Fibrionogen (mg/dL) | 338 [304–390] | 342 [302–391] | 338 [297–380] | 0.630 |
| C-reactive protein (mg/L) | 3.37 [1.8–6] | 3.3 [1.7–6] | 3.9 [2–6] | 0.839 |
| Creatinin (mg/dL) | 0.93 [0.81–1.08] | 0.92 [0.8–1.07] | 0.95 [0.81–1.16] | 0.746 |
| Urea (mg/dL) | 39 [29–49] | 38 [27–49] | 40 [31–51] | 0.467 |
| NT-proBNP (pg/mL) | 873 [198–1775] | 765 [142–2829] | 873 [475–1505] | 0.450 |
| Creatine kinase (U/L) | 583 [342–810] | 633 [461–820] | 385 [66–648] | 0.022 |
| Intraoperative | ||||
| Cardiopulmonary bypass time (minutes) | 185 [150–249] | 170 [144–219] | 245 [177–354] | <0.001 |
| Aortic cross-clamp time (minutes) | 111 [86–164] | 104 [81–124] | 149 [106–223] | <0.001 |
| Postoperative | ||||
| Vasopressors at ICU admission | 47 (36.2%) | 24 (30%) | 23 (46%) | 0.065 |
| Inotropes at ICU admission | 51 (39.3%) | 28 (35%) | 23 (46%) | 0.211 |
| Left ventricular ejection fraction (%) | 50 [45–55] | 50 [45–55] | 50 [45–50] | 0.379 |
| Systolic pulmonary artery pressure (mmHg) | 24 [20–31] | 20 [20–25] | 20 [19–24] | 0.649 |
| Albumin (g/dL) | 3.2 ± 0.4 | 3.3 ± 0.4 | 3.1 ± 0.4 | 0.065 |
| Direct bilirubin (mg/dL) | 0.5 [0.3–1.16] | 0.43 [0.29–1.13] | 0.6 [0.39–1.2] | 0.227 |
| Total bilirubin (mg/dL) | 1 [0.7–2.1] | 0.9 [0.65–2] | 14 [0.83–2.59] | 0.092 |
| Alanine aminotransferase (U/L) | 31 [20–52] | 29 [20–47] | 36 [22–77] | 0.348 |
| Aspartate aminotransferase (U/L) | 61 [38–95] | 59 [35–80] | 67 [49–129] | 0.089 |
| Hemoglobin (g/dL) | 9.4 [8.4–10.5] | 9.4 [8.4–10.5] | 9.1 [8.6–10.4] | 0.708 |
| Thrombocytes (n/uL) | 152 [91–179] × 103 | 160 [110–188] × 103 | 108 [77–159] × 103 | 0.010 |
| Leucocytes (n/uL) | 11,000 [8475–13,450] × 103 | 10,080 [8400–12,750] × 103 | 11,600 [8450–15,100] × 103 | 0.176 |
| Lymphocytes (n/uL) | 900 [600–1600] × 103 | 900 [600–1600] × 103 | 800 [600–1600] × 103 | 0.431 |
| Neutrophils (n/uL) | 8750 [6900–10,800] × 103 | 8350 [6467–10,600] × 103 | 9650 [7922–13,225] × 103 | 0.032 |
| Serum potassium (mmol/L) | 4 [3.7–4.4] | 4 [3.7–4.3] | 4.2 [3.7–4.5] | 0.288 |
| Serum sodium (mmol/L) | 141 [138–143] | 141 [138–143] | 142 [140–144] | 0.293 |
| Errythrocyte sedimentation rate (mm/h) | 19.5 [7.3–33.3] | 24 [9–40] | 16.5 [7–25.5] | 0.096 |
| Fibrionogen (mg/dL) | 507 [412–562] | 524 [433–589] | 452 [366–530] | 0.053 |
| C-reactive protein (mg/L) | 121 [56–196] | 123 [56–199] | 119 [55–198] | 0.918 |
| Creatinin (mg/dL) | 0.95 [0.73–1.23] | 0.9 [0.71–1.19] | 1.04 [0.79–1.58] | 0.089 |
| Urea (mg/dL) | 40 [31–51] | 39 [29–49] | 42 [32–59] | 0.152 |
| NT-proBNP (pg/mL) | 2915 [1948–5110] | 2628 [2146–3379] | 4466 [1540–11,152] | 0.174 |
| Creatine kinase (U/L) | 620 [398–1262] | 523 [318–944] | 719 [504–1621] | 0.067 |
| Parameter | OR [95% CI] | p Value |
|---|---|---|
| Demographic and Clinical | ||
| Age | 1.019 [0.983–1.058] | 0.306 |
| Body mass index | 0.970 [0.896–1.049] | 0.442 |
| Sex | 1.795 [0.855–3.767] | 0.122 |
| Arterial hypertension | 0.929 [0.334–2.578] | 0.887 |
| Type 2 Diabetes Mellitus | 0.533 [0.211–1.349] | 0.533 |
| Smoking | 8.942 [1.126–71.045] | 0.038 |
| Chronic obstructive pulmonary disease | 0.137 [0.026–0.722] | 0.019 |
| Atrial fibrillation | 1.476 [0.484–4.506] | 0.494 |
| Emergency surgery | 0.384 [0.142–1.035] | 0.058 |
| Preoperative | ||
| Left ventricular ejection fraction | 1.016 [0.952–1.086] | 0.627 |
| Tricuspid annulus plane systolic excursion | 1.052 [0.970–1.142] | 0.221 |
| Systolic pulmonary artery pressure | 0.942 [0.879–1.010] | 0.092 |
| Albumin | 0.582 [0.241–1.406] | 0.229 |
| Direct bilirubin | 0.342 [0.045–2.599] | 0.300 |
| Total bilirubin | 1.365 [0.747–2.496] | 0.312 |
| Alanine transaminase | 1.006 [0.995–1.016] | 0.292 |
| Aspartate transaminase | 1.002 [0.997–1.006] | 0.447 |
| Hemoglobin | 1.000 [1.000–1.000] | 0.451 |
| Thrombocytes | 0.998 [0.992–1.004] | 0.451 |
| Leucocytes | 1.111 [0.969–1.275 | 0.131 |
| Lymphocytes | 0.751 [0.392–1.439] | 0.388 |
| Neutrophils | 0.984 [0.915–1.058] | 0.661 |
| Serum potassium | 0.992 [0.562–1.751] | 0.978 |
| Serum sodium | 0.605 [0.935–1.040] | 0.605 |
| Errythrocyte sedimentation rate | 1.048 [0.987–1.112] | 0.126 |
| Fibrionogen | 0.999 [0.994–1.004] | 0.634 |
| C-reactive protein | 0.974 [0.923–1.029] | 0.349 |
| Creatinin | 0.832 [0.488–1.416] | 0.497 |
| Urea | 1.005 [0.981–1.031] | 0.675 |
| NT-proBNP | 1.000 [0.999–1.000] | 0.951 |
| Creatine kinase | 1.000 [0.998–1.001] | 0.415 |
| Intraoperative | ||
| Cardiopulmonary bypass time | 1.011 [1.005–1.016] | <0.001 |
| Aortic cross-clamp time | 1.014 [1.007–1.022] | <0.001 |
| Postoperative | ||
| Vasopressors at ICU admission | 0.503 [0.242–1.048] | 0.066 |
| Inotropes at ICU admission | 1.582 [0.769–3.255] | 0.213 |
| Left ventricular ejection fraction | 0.966 [0.867–1.076] | 0.527 |
| Systolic pulmonary artery pressure | 0.892 [0.670–1.186] | 0.431 |
| Albumin | 0.339 [0.106–1.091] | 0.070 |
| Direct bilirubin | 0.967 [0.836–1.118] | 0.648 |
| Total bilirubin | 1.273 [0.887–1.827] | 0.190 |
| Alanine transaminase | 1.001 [0.999–1.004] | 0.320 |
| Aspartate transaminase | 1.000 [0.999–1.000] | 0.688 |
| Hemoglobin | 1.000 [1.000–1.000] | 0.669 |
| Thrombocytes | 0.990 [0.981–0.998] | 0.019 |
| Leucocytes | 1.110 [0.989–1.246] | 0.076 |
| Lymphocytes | 0.955 [0.810–1.126] | 0.585 |
| Neutrophils | 1.157 [1.010–1.324] | 0.035 |
| Serum potassium | 1.372 [0.622–3.026] | 0.433 |
| Serum sodium | 1.067 [0.956–1.191] | 0.248 |
| Errythrocyte sedimentation rate | 0.969 [0.934–1.006] | 0.096 |
| Fibrinogen | 0.996 [0.992–1.001] | 0.087 |
| C-reactive protein | 1.000 [0.995–1.006] | 0.945 |
| Creatinin | 1.310 [0.686–2.504] | 0.413 |
| Urea | 1.012 [0.989–1.035] | 0.312 |
| NT-proBNP | 1.000 [1.000–1.000] | 0.060 |
| Creatine kinase | 1.000 [1.000–1.000] | 0.892 |
| Creatinine increase compared to baseline | 5.978 [1.336–26.752] | 0.019 |
| Parameter | OR [95% CI] | p Value |
|---|---|---|
| Cardiopulmonary bypass time | 0.985 [0.964–1.006] | 0.153 |
| Aortic cross-clamp time | 1.046 [1.014–1.080] | 0.005 |
| Chronic obstructive pulmonary disease | 0.237 [0.025–2.228] | 0.208 |
| Postoperative thrombocyte count | 0.985 [0.970–1.001] | 0.061 |
| Postoperative neutrophil count | 1.172 [0.990–1.386] | 0.065 |
| Creatinine increase compared to baseline | 6.560 [0.879–48.963] | 0.441 |
| Parameter | OR [95% CI] | p Value |
|---|---|---|
| Demographic and Clinical | ||
| Age | 1.017 [0.957–1.081] | 0.581 |
| Sex | 1.429 [0.426–4.792] | 0.564 |
| Arterial hypertension | 0.687 [0.068–6.970] | 0.751 |
| Emergency surgery | 0.082 [0.022–0.298] | <0.001 |
| Preoperative | ||
| Left ventricular ejection fraction | 0.991 [0.850–1.154] | 0.903 |
| Total bilirubin | 0.088 [0.001–12.143] | 0.334 |
| Alanine transaminase | 0.972 [0.872–1.038] | 0.605 |
| Aspartate transaminase | 0.997 [0.961–1.035] | 0.875 |
| Hemoglobin | 1.000 [0.999–1.001] | 0.875 |
| Thrombocytes | 1.004 [0.984–1.024] | 0.721 |
| Leucocytes | 0.956 [0.573–1.593] | 0.863 |
| Lymphocytes | 0.566 [0.032–9.983] | 0.698 |
| Neutrophils | 0.604 [0.129–2.826] | 0.522 |
| Serum potassium | 0.546 [0.048–6.187] | 0.625 |
| Serum sodium | 0.936 [0.729–1.202] | 0.603 |
| C-reactive protein | 0.998 [0.866–1.151] | 0.981 |
| Creatinin | 0.933 [0.196–4.433] | 0.930 |
| Urea | 1.032 [0.982–1.085] | 0.211 |
| Creatine kinase | 0.996 [0.987–1.004] | 0.338 |
| Intraoperative | ||
| Cardiopulmonary bypass time | 1.012 [1.005–1.019] | 0.001 |
| Aortic cross-clamp time | 1.010 [1.001–1.018] | 0.021 |
| Postoperative | ||
| Vasopressors at ICU admission | 0.154 [0.039–0.603] | 0.007 |
| Inotropes at ICU admission | 0.603 [0.183–1.985] | 0.405 |
| Left ventricular ejection fraction | 1.346 (0.811–2.235) | 0.250 |
| ICU length of stay | 1.095 [1.035–1.158] | 0.001 |
| Albumin | 0.042 [0.000–6.391] | 0.217 |
| Direct bilirubin | 0.007 [0.000–1.338] | 0.503 |
| Total bilirubin | 0.039 [0.000–80.817] | 0.405 |
| Alanine transaminase | 0.875 [0.699–1.094] | 0.241 |
| Aspartate transaminase | 0.966 [0.876–1.064] | 0.480 |
| Hemoglobin | 1.000 [0.997–1.003] | 0.951 |
| Thrombocytes | 0.920 [0.816–1.037] | 0.172 |
| Leucocytes | 1.171 [0.823–1.664] | 0.380 |
| Lymphocytes | 0.879 [0.180–4.295] | 0.873 |
| Neutrophils | 1.208 [0.851–1.716] | 0.290 |
| Serum potassium | 1.756 [0.133–23.218] | 0.669 |
| Serum sodium | 1.095 [0.639–1.879] | 0.740 |
| Errythrocyte sedimentation rate | 0.900 [0.698–1.161] | 0.419 |
| Fibrinogen | 0.996 [0.982–1.011] | 0.614 |
| C-reactive protein | 1.033 [0.987–1.082] | 0.163 |
| Creatinin | 1.548 [0.429–5.591] | 0.505 |
| Urea | 1.026 [0.976–1.078] | 0.323 |
| NT-proBNP | 1.000 [1.000–1.000] | 0.105 |
| Creatine kinase | 0.999 [0.993–1.005] | 0.679 |
| Parameter | OR [95% CI] | p Value |
|---|---|---|
| Cardiopulmonary bypass time | 1.030 [1.003–1.057] | 0.030 |
| Aortic cross-clamp time | 0.965 [0.934–0.997] | 0.034 |
| Emergency surgery | 0.043 [0.002–0.863] | 0.040 |
| Vasopressors at ICU admission | 0.001 [0.001–1.000] | 0.996 |
| ICU length of stay | 0.149 [0.009–2.462] | 0.183 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hădăreanu, C.-D.; Hădăreanu, D.-R.; Stoiculescu, F.-M.; Berceanu, M.-C.; Donoiu, I.; Istrătoaie, O.; Florescu, C.; Novac, M.-B.; Raicea, V.-C. Predictors of Prolonged Intensive Care Unit Stay and In-Hospital Mortality Following Cardiac Surgery: An Integrated Analysis from the PROCARD-ATI Study. J. Clin. Med. 2025, 14, 2747. https://doi.org/10.3390/jcm14082747
Hădăreanu C-D, Hădăreanu D-R, Stoiculescu F-M, Berceanu M-C, Donoiu I, Istrătoaie O, Florescu C, Novac M-B, Raicea V-C. Predictors of Prolonged Intensive Care Unit Stay and In-Hospital Mortality Following Cardiac Surgery: An Integrated Analysis from the PROCARD-ATI Study. Journal of Clinical Medicine. 2025; 14(8):2747. https://doi.org/10.3390/jcm14082747
Chicago/Turabian StyleHădăreanu, Călin-Dinu, Diana-Ruxandra Hădăreanu, Flavia-Mihaela Stoiculescu, Mihaela-Corina Berceanu, Ionuț Donoiu, Octavian Istrătoaie, Cristina Florescu, Marius-Bogdan Novac, and Victor-Cornel Raicea. 2025. "Predictors of Prolonged Intensive Care Unit Stay and In-Hospital Mortality Following Cardiac Surgery: An Integrated Analysis from the PROCARD-ATI Study" Journal of Clinical Medicine 14, no. 8: 2747. https://doi.org/10.3390/jcm14082747
APA StyleHădăreanu, C.-D., Hădăreanu, D.-R., Stoiculescu, F.-M., Berceanu, M.-C., Donoiu, I., Istrătoaie, O., Florescu, C., Novac, M.-B., & Raicea, V.-C. (2025). Predictors of Prolonged Intensive Care Unit Stay and In-Hospital Mortality Following Cardiac Surgery: An Integrated Analysis from the PROCARD-ATI Study. Journal of Clinical Medicine, 14(8), 2747. https://doi.org/10.3390/jcm14082747






