Myokine Secretion Dynamics and Their Role in Critically Ill Patients: A Scoping Review
Abstract
:1. Introduction
2. Methods
2.1. Data Sources and Searches
2.2. Eligibility Criteria
2.3. Study Selection
2.4. Data Extraction
2.5. Data Synthesis
3. Results
3.1. Study Selection
3.2. Study Characterisation
3.3. Population
3.4. Physical Intervention
3.5. Myokine Sampling
3.6. Overall Myokine Change
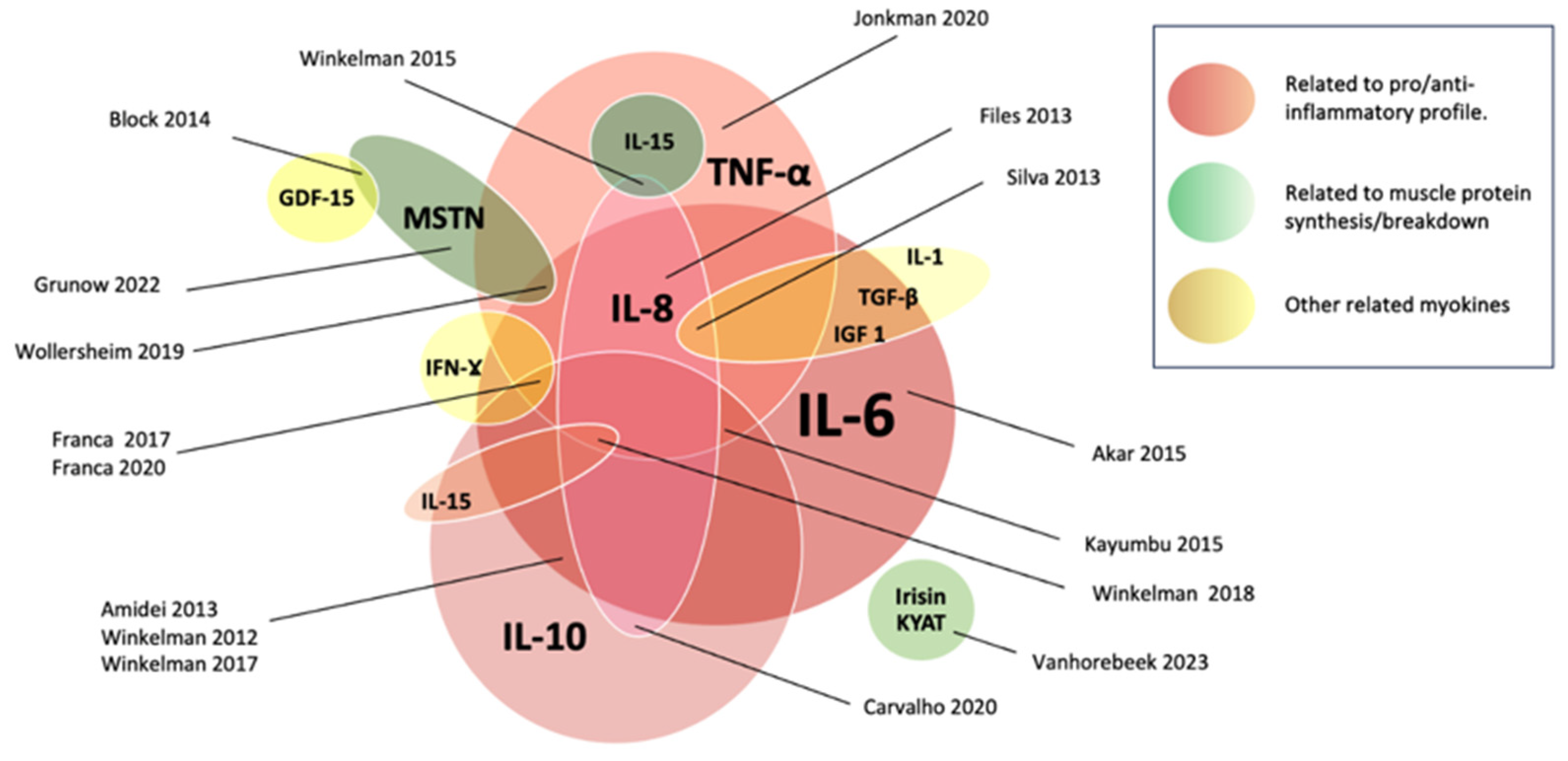
3.7. Myokine Changes and Potential Clinical Effects According to Their Role
3.7.1. Effects Related to Pro/Anti-Inflammatory Profile
3.7.2. Effects Related to Muscle Protein Synthesis/Breakdown
3.7.3. Others
3.8. Myokine Changes According to the Intervention Type
3.8.1. Passive Mobilization (PM)
3.8.2. Early Mobilisation (EM)
3.8.3. NMES/FES
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ICU | Intensive Care Unit |
| ICU-AW | Intensive Care Unit-Acquired Weakness |
| MV | Mechanical Ventilation |
| NMES | Neuromuscular Electrical Stimulation |
| JBI | Joanna Briggs Institute |
| INPLASY | International Platform of Registered Systematic Review and Meta-analysis Protocols |
| PRISMA-ScR | Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews |
| CINAHL | Cumulated Index to Nursing and Allied Health Literature |
| RCT | Randomized Controlled Trial |
| COPD | Chronic Obstructive Pulmonary Disease |
| Hz | Hertz |
| μs | Microseconds |
| mA | Milliamperes |
| ELISA | Enzyme-Linked ImmunoSorbent Assay |
| mRNA | Messenger Ribonucleic Acid |
| IL-6 | Interleukin 6 |
| IL-10 | Interleukin 10 |
| TNF-α | Tumour Necrosis Factor α |
| IL-15 | Interleukin 15 |
| IL-1 | Interleukin 1 |
| IFN-g | Interferon Gamma |
| AA1/2 | Serum Amyloid a1/2 |
| IGF-I | Insulin-like Growth Factor |
| KYAT | Kynurenine-oxoglutarate Transaminase |
| Amy1 | Total amylase |
| WBV | Whole body vibration |
| FES | Functional Electrical Stimulation |
| MSTN | Myostatin gene expression |
| GDF-15 | Growth Differentiation Factor 15 |
| FNDC5 | Fibronenctin type III Domain-Containing protein 5 |
| PM | Passive Mobilization |
| EM | Early Mobilization |
References
- Schreiber, A.; Bertoni, M.; Goligher, E.C. Avoiding Respiratory and Peripheral Muscle Injury During Mechanical Ventilation: Diaphragm-Protective Ventilation and Early Mobilization. Crit. Care Clin. 2018, 34, 357–381. [Google Scholar] [CrossRef]
- Kress, J.P.; Hall, J.B. ICU-acquired weakness and recovery from critical illness. N. Engl. J. Med. 2014, 370, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Puthucheary, Z.A.; Rawal, J.; McPhail, M.; Connolly, B.; Ratnayake, G.; Chan, P.; Hopkinson, N.S.; Phadke, R.; Dew, T.; Sidhu, P.S.; et al. Acute skeletal muscle wasting in critical illness. JAMA 2013, 310, 1591–1600. [Google Scholar] [CrossRef]
- Iwashyna, T.; Ely, W.; Smith, D.; Langa, K.M. Long-term Cognitive Impairment and Functional Disability Among Survivors of Severe Sepsis. JAMA 2010, 304, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Herridge, M.S.; Tansey, C.M.; Matté, A.; Tomlinson, G.; Diaz-Granados, N.; Cooper, A.; Guest, C.B.; Mazer, C.D.; Mehta, S.; Stewart, T.E.; et al. Functional Diasability 5 years after Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2011, 364, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Dres, M.; Goligher, E.C.; Heunks, L.M.A.; Brochard, L.J. Critical illness-associated diaphragm weakness. Intensive Care Med. 2017, 43, 1441–1452. [Google Scholar] [CrossRef]
- Van Der Schaaf, M.; Beelen, A.; Dongelmans, D.A.; Vroom, M.B.; Nollet, F. Poor functional recovery after a critical illness: A longitudinal study. J. Rehabil. Med. 2009, 41, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Gruther, W.; Benesch, T.; Zorn, C.; Paternostro-Sluga, T.; Quittan, M.; Fialka-Moser, V.; Spiss, C.; Kainberger, F.; Crevenna, R. Muscle wasting in intensive care patients: Ultrasound observation of the M. quadriceps femoris muscle layer. J. Rehabil. Med. 2008, 40, 185–189. [Google Scholar] [CrossRef]
- Levine, S.; Nguyen, T.; Taylor, N.; Friscia, M.E.; Budak, M.T.; Rothenberg, P.; Zhu, J.; Sachdeva, R.; Sonnad, S.; Kaiser, L.R.; et al. Rapid Disuse Atrophy of Diaphragm Fibers in Mechanically Ventilated Humans. N. Engl. J. Med. 2008, 358, 485–493. [Google Scholar] [CrossRef]
- Goligher, E.C.; Dres, M.; Fan, E.; Rubenfeld, G.D.; Scales, D.C.; Herridge, M.S.; Vorona, S.; Sklar, M.C.; Rittayamai, N.; Lanys, A.; et al. Mechanical ventilation-induced diaphragm atrophy strongly impacts clinical outcomes. Am. J. Respir. Crit. Care Med. 2018, 197, 204–213. [Google Scholar] [CrossRef]
- Goligher, E.C.; Fan, E.; Herridge, M.S.; Murray, A.; Vorona, S.; Brace, D.; Rittayamai, N.; Lanys, A.; Tomlinson, G.; Singh, J.M.; et al. Evolution of diaphragm thickness during mechanical ventilation: Impact of inspiratory effort. Am. J. Respir. Crit. Care Med. 2015, 192, 1080–1088. [Google Scholar] [CrossRef]
- Zayed, Y.; Kheiri, B.; Barbarawi, M.; Chahine, A.; Rashdan, L.; Chintalapati, S.; Bachuwa, G.; Al-Sanouri, I. Effects of neuromuscular electrical stimulation in critically ill patients: A systematic review and meta-analysis of randomised controlled trials. Aust. Crit. Care. 2020, 33, 203–210. [Google Scholar] [CrossRef]
- Zanotti, E.; Felicetti, G.; Maini, M.; Fracchia, C. Peripheral muscle strength training in bed-bound patients with COPD receiving mechanical ventilation: Effect of electrical stimulation. Chest 2003, 124, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Langer, D.; Troosters, T.; Hermans, G.; Decramer, M. Early exercise in critically ill patients enhances short-term functional recovery*. Crit. Care Med. 2009, 37, 2499–2505. [Google Scholar]
- Machado A dos, S.; Pires-Neto, R.C.; Carvalho, M.T.X.; Soares, J.C.; Cardoso, D.M.; de Albuquerque, I.M. Efeito do exercício passivo em cicloergômetro na força muscular, tempo de ventilação mecânica e internação hospitalar em pacientes críticos: Ensaio clínico randomizado. J. Bras. Pneumol. 2017, 43, 134–139. [Google Scholar]
- Hickmann, C.E.; Laterre, P.-F.; Roeseler, J.; Castanares-Zapatero, D. Rôle de l’exercice précoce dans la régulation de l’inflammation chez le patient critique. Médecine Intensive Réanimation 2020, 29, 299–308. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Steensberg, A.; Fischer, C.; Keller, C.; Keller, P.; Plomgaard, P.; Febbraio, M.; Saltin, B. Searching for the exercise factor: Is IL-6 a candidate? J. Muscle Res. Cell Motil. 2003, 24, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, R. Exercise-induced myokines with therapeutic potential for muscle wasting. Front. Physiol. 2019, 10, 287. [Google Scholar] [CrossRef] [PubMed]
- Lightfoot, A.P.; Cooper, R.G. The role of myokines in muscle health and disease. Curr. Opin. Rheumatol. 2016, 28, 661–666. [Google Scholar] [CrossRef]
- Lee, J.H.; Jun, H.S. Role of myokines in regulating skeletal muscle mass and function. Front. Physiol. 2019, 10, 42. [Google Scholar] [CrossRef]
- Görgens, S.W.; Eckardt, K.; Jensen, J.; Drevon, C.A.; Eckel, J. Exercise and Regulation of Adipokine and Myokine Production. Prog. Mol. Biol. Transl. Sci. 2015, 135, 313–336. [Google Scholar]
- Wageck, B.; Nunes, G.S.; Silva, F.L.; Damasceno, M.C.P.; de Noronha, M. Application and effects of neuromuscular electrical stimulation in critically ill patients: Systematic review. Med. Intensiv. 2014, 38, 444–454. [Google Scholar] [CrossRef]
- Trethewey, S.P.; Brown, N.; Gao, F.; Turner, A.M. Interventions for the management and prevention of sarcopenia in the critically ill: A systematic review. J. Crit. Care 2019, 50, 287–295. [Google Scholar] [CrossRef]
- Dobšák, P.; Tomandl, J.; Spinarova, L.; Vitovec, J.; Dusek, L.; Novakova, M.; Jarkovsky, J.; Krejci, J.; Hude, P.; Honek, T.; et al. Effects of Neuromuscular Electrical Stimulation and Aerobic Exercise Training on Arterial Stiffness and Autonomic Functions in Patients with Chronic Heart Failure. Artif. Organs. 2012, 36, 920–930. [Google Scholar] [CrossRef]
- Peters, M.D.J.; Marnie, C.; Tricco, A.C.; Pollock, D.; Munn, Z.; Alexander, L.; McInerney, P.; Godfrey, C.M.; Khalil, H. Updated methodological guidance for the conduct of scoping reviews. JBI Evid. Synth. 2020, 18, 2119–2126. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Grainger, M.J.; Gray, C.T. Citationchaser: A tool for transparent and efficient forward and backward citation chasing in systematic searching. Res. Synth. Methods 2022, 13, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Vanhorebeek, I.; Gunst, J.; Casaer, M.P.; Derese, I.; Derde, S.; Pauwels, L.; Segers, J.; Hermans, G.; Gosselink, R.; Berghe, G.V.D. Skeletal Muscle Myokine Expression in Critical Illness, Association with Outcome and Impact of Therapeutic Interventions. J. Endocr. Soc. 2023, 7, bvad001. [Google Scholar] [CrossRef] [PubMed]
- Grunow, J.J.; Reiher, K.; Carbon, N.M.; Engelhardt, L.J.; Mai, K.; Koch, S.; Schefold, J.C.; Z’Graggen, W.; Schaller, S.J.; Fielitz, J.; et al. Muscular myostatin gene expression and plasma concentrations are decreased in critically ill patients. Crit. Care. 2022, 26, 237. [Google Scholar] [CrossRef]
- Wollersheim, T.; Grunow, J.J.; Carbon, N.M.; Haas, K.; Malleike, J.; Ramme, S.F.; Schneider, J.; Spies, C.D.; Märdian, S.; Mai, K.; et al. Muscle wasting and function after muscle activation and early protocol-based physiotherapy: An explorative trial. J. Cachexia Sarcopenia Muscle 2019, 10, 734–747. [Google Scholar] [CrossRef] [PubMed]
- Winkelman, C.; Sattar, A.; Momotaz, H.; Johnson, K.D.; Morris, P.; Rowbottom, J.R.; Thornton, J.D.; Feeney, S.; Levine, A. Dose of Early Therapeutic Mobility: Does Frequency or Intensity Matter? Biol. Res. Nurs. 2018, 20, 522–530. [Google Scholar] [CrossRef]
- Winkelman, C.; Higgins, P.A.; Chen, Y.J.K.; Levine, A.D. Cytokines in chronically critically ill patients after activity and rest. Biol. Res. Nurs. 2007, 8, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Winkelman, C.; Johnson, K.D.; Gordon, N. Associations Between Muscle-Related Cytokines and Selected Patient Outcomes in the ICU. Biol. Res. Nurs. 2015, 17, 125–134. [Google Scholar] [CrossRef]
- Winkelman, C.; Johnson, K.D.; Hejal, R.; Gordon, N.H.; Rowbottom, J.; Daly, J.; Peereboom, K.; Levine, A.D. Examining the positive effects of exercise in intubated adults in ICU: A prospective repeated measures clinical study. Intensive Crit. Care Nurs. 2012, 28, 307–318. [Google Scholar] [CrossRef]
- Silva, P.E.; Marqueti, R.d.C.; Livino-De-Carvalho, K.; de Araujo, A.E.T.; Castro, J.; da Silva, V.M.; Vieira, L.; Souza, V.C.; Dantas, L.O.; Cipriano, G., Jr.; et al. Neuromuscular electrical stimulation in critically ill traumatic brain injury patients attenuates muscle atrophy, neurophysiological disorders, and weakness: A randomized controlled trial. J. Intensive Care 2019, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Kayambu, G.; Boots, R.; Paratz, J. Early physical rehabilitation in intensive care patients with sepsis syndromes: A pilot randomised controlled trial. Intensive Care Med. 2015, 41, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Jonkman, A.H.; Frenzel, T.; McCaughey, E.J.; McLachlan, A.J.; Boswell-Ruys, C.L.; Collins, D.W.; Gandevia, S.C.; Girbes, A.R.J.; Hoiting, O.; Kox, M.; et al. Breath-synchronized electrical stimulation of the expiratory muscles in mechanically ventilated patients: A randomized controlled feasibility study and pooled analysis. Crit. Care. 2020, 24, 628. [Google Scholar] [CrossRef] [PubMed]
- França, E.; Gomes, J.; De Lira, J.; Amaral, T.; Vilaça, A.; Júnior, M.P.; Júnior, U.E.; Júnior, L.F.; Costa, M.; Andrade, M.; et al. Acute effect of passive cycle-ergometry and functional electrical stimulation on nitrosative stress and inflammatory cytokines in mechanically ventilated critically ill patients: A randomized controlled trial. Braz. J. Med. Biol. Res. 2020, 53, e8770. [Google Scholar] [CrossRef] [PubMed]
- de França, E.E.T.; Ribeiro, L.C.; Lamenha, G.G.; Magalhães, I.K.F.; Figueiredo, T.d.G.; Costa, M.J.C.; Júnior, U.F.E.; Feitosa, B.L.; Andrade, M.D.A.; Júnior, M.A.V.C.; et al. Oxidative stress and immune system analysis after cycle ergometer use in critical patients. Clinics 2017, 72, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Files, D.; Morris, P.; Shrestha, S.; Dhar, S.; Young, M.; Hauser, J.; Chmelo, E.; Thompson, C.; Dixon, L.; Murphy, K.; et al. Randomized, controlled pilot study of early rehabilitation strategies in acute respiratory failure. Crit. Care. 2013, 17, 198–199. [Google Scholar] [CrossRef]
- Carvalho, M.T.X.; Real, A.A.; Cabeleira, M.E.; Schiling, E.; Lopes, I.; Bianchin, J.; da Silva, A.M.V.; Annoni, R.; de Albuquerque, I.M. Acute effect of passive cycling exercise on serum levels of interleukin-8 and interleukin-10 in mechanically ventilated critically ill patients. Int. J. Ther. Rehabil. 2020, 27, 1–7. [Google Scholar] [CrossRef]
- Bloch, S.; Syburrah, T.; Rosendahl, U.; Kemp, P.; Griffiths, M.; Polkey, M. A paradoxical rise in rectus femoris myostatin (GDF-8) and GDF-15 in response to neuromuscular electrical stimulation in critical care. Thorax 2014, 69 (Suppl. S2), 2014–2016. [Google Scholar] [CrossRef]
- Amidei, C.; Sole, M.L. Physiological Responses to Passive Exercise in Adults Receiving Mechanical Ventilation. Am. J. Crit. Care 2013, 22, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Akar, O.; Günay, E.; Ulasli, S.S.; Ulasli, A.M.; Kacar, E.; Sariaydin, M.; Solak, Ö.; Celik, S.; Ünlü, M. Efficacy of neuromuscular electrical stimulation in patients with COPD followed in intensive care unit. Clin. Respir. J. 2017, 11, 743–750. [Google Scholar] [CrossRef]
- Pekkala, S.; Wiklund, P.K.; Hulmi, J.J.; Ahtiainen, J.P.; Horttanainen, M.; Pöllänen, E.; Mäkelä, K.A.; Kainulainen, H.; Häkkinen, K.; Nyman, K.; et al. Are skeletal muscle FNDC5 gene expression and irisin release regulated by exercise and related to health? J. Physiol. 2013, 591, 5393–5400. [Google Scholar] [CrossRef]
- Reza, M.M.; Subramaniyam, N.; Sim, C.M.; Ge, X.; Sathiakumar, D.; McFarlane, C.; Sharma, M.; Kambadur, R. Irisin is a pro-myogenic factor that induces skeletal muscle hypertrophy and rescues denervation-induced atrophy. Nat. Commun. 2017, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Schlittler, M.; Goiny, M.; Agudelo, L.Z.; Venckunas, T.; Brazaitis, M.; Skurvydas, A.; Kamandulis, S.; Ruas, J.L.; Erhardt, S.; Westerblad, H.; et al. Endurance exercise increases skeletal muscle kynurenine aminotransferases and plasma kynurenic acid in humans. Am. J. Physiol. Cell Physiol. 2016, 310, C836–C840. [Google Scholar] [CrossRef]
- Kleinert, M.; Clemmensen, C.; Sjøberg, K.A.; Carl, C.S.; Jeppesen, J.F.; Wojtaszewski, J.F.; Kiens, B.; Richter, E.A. Exercise increases circulating GDF15 in humans. Mol. Metab. 2018, 9, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Adela, R.; Banerjee, S.K. GDF-15 as a target and biomarker for diabetes and cardiovascular diseases: A translational prospective. J. Diabetes Res. 2015, 2015, 490842. [Google Scholar] [CrossRef] [PubMed]
- Kanzleiter, T.; Rath, M.; Görgens, S.W.; Jensen, J.; Tangen, D.S.; Kolnes, A.J.; Kolnes, K.J.; Lee, S.; Eckel, J.; Schürmann, A.; et al. The myokine decorin is regulated by contraction and involved in muscle hypertrophy. Biochem. Biophys. Res. Commun. 2014, 450, 1089–1094. [Google Scholar] [CrossRef]

| N° | Search Term |
|---|---|
| 1 | exp Cytokines/ |
| 2 | exp Chemokines/ |
| 3 | exp Interleukins/ |
| 4 | exp Biomarkers/ |
| 5 | (cytokin$ or chemokin$ or interleukin$ or miokin$ or exerkin$ or biomarker$ or biologic$ marker$).mp,tw. |
| 6 | (Angiopoietin-like 4 or ANGPTL4 or Apelin or aminoisobutyric acid or BAIBA or Brain-derived neurotrophic factor or BDNF or Chemokine ligand or C-X-C motif or Decorin or Fibroblast growth factor 21 or FGF21 or IL-6 or IL-8 or IL-10 or IL-13 or IL-15 or IL-18 or Irisin or FNDC5 or Musclin or Myonectin or C1QTNF5 or Myostatin or Leukemia inhibitory factor or LIF or Secreted protein acidic rich in cysteine or SPARC or Tumor necrosis factor$ or TNF$).mp,tw. |
| 7 | or/1–6 |
| 8 | exp intensive Care/ |
| 9 | exp critical Illness/ |
| 10 | exp Critical Care/ |
| 11 | exp Intensive Care Units/ |
| 12 | ((intensive or critic$) adj2 (care or ill$)).mp,tw. |
| 13 | exp Respiration, Artificial/ |
| 14 | exp Ventilator Weaning/ |
| 15 | ((mechanic$ or artificial) adj2 (ventilat$ or respirat$)).mp,tw. |
| 16 | or/8–15 |
| 17 | exp Electric Stimulation Therapy/ |
| 18 | exp Transcutaneous Electric Nerve Stimulation/ |
| 19 | exp Electric Stimulation/ |
| 20 | ((neuromusc$ or functional or transcutan$) adj2 (electric$ or electrotherap$)).mp,tw. |
| 21 | (electrotherap$ or electromyostimulation or electrostimulation or (electric$ adj2 stimulation)).mp,tw. |
| 22 | (NMES or FES or TENS).mp,tw. |
| 23 | or/17–22 |
| 24 | exp Exercise/ |
| 25 | exp Exercise Therapy/ |
| 26 | exp Physical Therapy Modalities/ |
| 27 | exp Occupational Therapy/ |
| 28 | exp Muscle Contraction/ |
| 29 | exp Resistance Training/ |
| 30 | (physiotherap$ or physical therap$ or kinesiotherap$ or physical rehabilitation or exercis$ or train$).mp,tw. |
| 31 | (musc$ adj2 contrac$).mp,tw. |
| 32 | or/24–31 |
| 33 | or/23,32 |
| 34 | and/7,16,33 |
| Author/ | Population | Intervention | Program | Biomarkers | Method of Evaluation | Results | Clinical Impact |
|---|---|---|---|---|---|---|---|
| Year/ | |||||||
| Country/Study Design | |||||||
| Akar et al., 2017 [45] (Turkey) RCT | COPD-conscious patients in early intubation period. | Group 1: AT exercise for upper and lower extremities and NMES (amplitude of 20–25 mA, symmetrical biphasic square waves with 6 s of contraction, 1.5 s of increase and 0.75 s of decrease, and wave frequency of 50 Hz). Group 2: only NMES. Group 3: only AT. | 5 days per week for a total of 20 sessions. | IL-6, IL-8, IL-10, and TNF-α | Blood samples were taken twice: once before the intervention, then at the end of the intervention. Serum biomarker levels were measured using commercially prepared ELISA kits. | For group 1, serum IL-6 significantly decreased after the program (p = 0.015), and significant decreases were also noted in serum IL-8 for groups 1 and 2 (p = 0.017). | After the program, lower-extremity muscle strength improved significantly in groups 1 and 2, while upper-extremity muscle strength improved significantly in all groups. The weaning time of the groups was comparable. Time to sit up assisted/unassisted in bed and at the bedside and the time to move from bed to chair were similar for all groups. |
| Amidei et al., 2013 [44] (United States). Quasi-experimental | Critically ill adults within 48 h of intubation and MV use. | PM: 20 min of 20 flexion–extension (from 70° flexion to 5° flexion) movements per minute in each leg simultaneously. Leg movements were alternated, simulating slow walking. | Single session. | IL-6 and IL-10 | Blood samples were taken at three-time points: baseline, upon intervention completion, and one hour after. Plasmatic biomarker levels were measured via commercially prepared ELISA kits. | A significant decrease in IL-6 values was found from baseline to the completion of the exercise (p = 0.03), but there was no difference 60 min later. No significant difference was noted in IL-10 values at any time. The IL-6:IL-10 ratios showed a significant decrease at the end of the exercise and again after 60 min of rest (p = 0.05). | At all study points, heart rates and mean systolic blood pressures were in normal ranges for physiological status. The Body Pain Score showed a significant decrease at all points. |
| Bloch et al., 2014 [43] (United Kingdom) Contralateral controlled trial | Patients admitted to a cardiothoracic ICU recruited before elective high-risk cardiac surgery or during ICU admission. | NMES performed twice a day for 1 h in one leg. | Every day for one week. | Myostatin and GDF-15 | Rectus femoris biopsies were taken before and after intervention (no additional information about sample processing was provided). | Myostatin and GDF-15 mRNA expressions were significantly elevated in NMES legs compared to baseline (p = 0.03 and p = 0.04, respectively) but remained unchanged in control legs. | There was no significant change in the bilateral rectus femoris cross-sectional area measured by ultrasound. |
| Carvalho et al., 2020 [42] (Brazil) Prospective study | Mechanically ventilated patients within 48 h of intubation—haemodynamically stable with deep sedation. | 20 consecutive minutes of PM using EC at a fixed pedalling rate of 20 cycles/minute (20 min). | Single session. | IL-8 and IL-10 | Blood samples were taken at three time points: before the intervention, immediately after the intervention, and one hour after it. Serum biomarker levels were assessed using commercially prepared ELISA kits. | IL-8 decreased significantly 1 h after the session compared to baseline (p = 0.001), but there were no significant changes after the 20 min-session. Similarly, IL-10 showed a significant increase immediately after the end of the session and 60 min after (p < 0.001). | NR |
| Files et al., 2013 [41] (United States) RCT | Acute respiratory failure ICU patients. | ER: two physical therapy sessions per day, with the second session comprising resistance training. UC: one session. | Every day for one week. | TNF-α, IL-6, and IL-8 | Blood was drawn for cytokines through day 7 (no additional information about sample processing was provided). | Biomarkers through day 7 were did not differ between groups despite similar baseline acuity and inflammatory profiles. | There was no significant change in grip strength, dynamometry, or Short Physical Performance (SPPB). However, the latter showed a minimal clinically significant difference. |
| Franca et al., 2017 [40] (Brazil) RCT | Mechanically ventilated patients. | Group 1: PM through EC on lower limbs with speed adjusted to 30 cycles per minute for 20 min. Group 2: Control, without intervention. | Single session. | L-6, IL-10, IFN-γ, and TNF-α | Blood samples were taken twice: once before intervention, then one hour after intervention. Samples were stored in collection tubes containing dipotassium ethylenediamine tetra-acetic acid. Plasmatic levels were assessed using commercially prepared ELISA kits. | No significant changes in serum levels before and after for the two groups. | NR |
| Franca et al., 2020 [39] (Brazil) RCT | Patients admitted to ICU. | Group 1: Control. Group 2: PM through EC with 30 cycles per minute for 20 min. Group 3: FES (500 ms pulse width, 50 Hz, 2 s of elevation, 5 s of support, and 2 s of descent with a 1:1 ON/OFF time; 20 min for each stimulated quadricep muscle). Group 4: EC and FES. | Single session. | IL-6, IL-10, IFN-γ, and TNF-α | Blood samples were taken twice: once before intervention, then one hour after intervention. Samples were stored in collection tubes containing dipotassium ethylenediamine tetra-acetic acid. Then, they were diluted in 10 mL falcon tubes for nitrosative stress analysis, determining nitric oxide production in cells and assessing serum biomarkers using commercially prepared ELISA kits. | IFN-γ, IL-6, and IL-10 levels did not significantly decrease before and after the intervention. TNF-α levels were reduced after the PM intervention (p = 0.049). | NR |
| Jonkman et al., 2020 [38] (Netherlands) Prospective study (feasibility study) | Mixed ICU population. | FES: For external and internal oblique and transversus abdominis muscles [30 Hz, pulse width of 352 μs, and maximum intensity of 100 mA (according to visual muscle contraction)]. Control: Sham NMES without muscle contraction (frequency of 10 Hz, pulse width of 352 μs, and intensity of 10 mA). | FES was applied for 30 min, twice daily, five days per week until patients were weaned from mechanical ventilation—but no longer than six weeks. | TNF-α, IL-1 receptor antagonist, IL-1β, IL-6, IL-8, and IL-10 | Blood samples were collected at baseline and on day 3 for plasmatic biomarker analysis using a Multiplex Luminex assay | There was a between-group difference in the change in pro-inflammatory marker TNF-α, with a trend toward a decrease for the active group versus no change in the sham group. | The total abdominal expiratory muscle thickness changed on day 3, favouring the FES group. No difference was found on day 5 or over days. Using a survival analysis approach, median ventilation duration and ICU length of stay were shorter for the FES group (p-values of 0.07 and 0.03, respectively). No difference was observed between median mechanical ventilation use and median ICU length of stay. |
| Kayambu et al., 2015 [27] (Australia) Prospective double-blinded RCT | Septic patients admitted to ICU. | ER: Individualised early physical rehabilitation strategies including NMES (vastus tedialis, vastus lateralis, tibialis anterior, and brachioradialis; 40–45 Hz at 20–25 mA, pulse width of 400 μs (12 s on and 6 s off)), PM, AT, sitting out of bed, transfers, ambulation, and other mobilisation techniques. Control: standard care. | ER program for 30 min, once or twice daily, until discharge from the ICU and within 48 h of the diagnosis of sepsis. | IL-6, IL-10, and TNF-α | Blood samples were collected on days 1, 3, 5, and 7 after the trial’s initiation and after ICU discharge. Biomarkers were measured from plasma samples using Milliplex cytokine panels from Millipore and a Luminex 100 assay. | A reduction in mean IL-6 scores was observed in the ER group pre and post exercise across days 1, 3, 5, and 7, but no statistically significant differences were found between groups. Overall, the mean change in IL-10 from baseline to ICU discharge was significantly higher for the ER group (p = 0.04). For TNF-α, there was a significant decrease between groups from baseline to ICU discharge (p < 0.01). | No differences were found in the physical function and mobility scores. In the ER group, self-reported quality of life improved in the domains of physical function and physical role compared to those receiving standard care. No differences were found in ICU exercise capacity (PFIT score) or MRC score. |
| Silva et al., 2019 [36] (Brazil) RCT | Critically ill traumatic brain injury (TBI) patients. | NMES group: ER plus NMES once a day (400 μs and 100 Hz with 5 s on and 25 s off, eliciting 50 contractions per day. The current intensity was titrated to evoke maximum contractions in the quadriceps, hamstring, tibialis anterior, and gastrocnemius). Control: ER including PM, AT, sitting out of bed, transfers, and ambulation. | NMES was applied daily (one time) for 25 min for 14 days. ER was applied for 10–30 min. | IGF-1, TGF-β, IL-1β, IL-6, IL-8, IL-10, and TNF-α | Blood samples were collected 24 h after admission and on days 3, 7, and 14. Serum levels of IGF-1 and TGF-β were measured using commercially prepared ELISA kits, while circulating concentrations of IL-1β, IL-6, IL-8, IL-10, and TNF-α were assessed through a multiplex flow cytometry technique. | Plasma IGF-1, TGF-β, and IL-10 presented a statistically significant time–effect interaction (p = 0.01, 0.03, and 0.05, respectively). Meanwhile, TNF-α, IL-1 β, IL-6, and IL-8 did not present differences regarding group/intervention interaction. | The control group showed a significant reduction in muscle thickness of the tibialis anterior and rectus femoris, while it was preserved in the NMES group. The control group also had a higher incidence of neuromuscular electrophysiological disorders. NMES determined an increase in the evoked peak force versus the control group. |
| Winkelman et al., 2007 [33] (United States) Prospective correlational and exploratory | Patients receiving MV for more than 3 days and with an ICU stay of 5 to 15 days. | AT: Baseline activity or all movement initiated by participants. ER: Therapeutic activity, both active (i.e., patient-initiated) and passive (i.e., initiated and supported by a healthcare worker). All therapeutic motions were recorded. | Minimum duration of 10 min for PM. Data and the type and duration of activity and PM were collected within one 24-h period (two separate 4-h blocks). | IL-6 and IL-10 | Blood samples were taken twice under a 24 h margin: once after a period of 10 min of activity, then immediately after a period of 60 min of uninterrupted rest. The serum was analysed using commercially available antibodies, with duplicate readings for each cytokine. Then, after adding an enzyme-conjugated polyclonal detection antibody, the colourimetric substrate was assessed by a single experienced technician. | The average ratio of IL-6 to IL-10 improved after 14.7 min of passive physical activity. Significant differences existed between the average resting and activity values of IL-6 (p < 0.001) but not for IL-10. | IL-6 and IL-10 and their ratio were not associated with patient outcomes of weaning success or length of stay. High levels of IL-6 were associated with mortality. |
| Winkelman et al., 2012 [35] (United States) Prospective, exploratory, repeated measures interventional study | Subjects admitted to a medical or surgical ICU. | Control: No exercise was planned, nor did participants routinely receive exercise from a physical therapist. ER: In-bed exercise or initiated out-of-bed exercise based on the protocol’s criteria (ability to follow three out of five directions, lift both arms off the bed, and/or lift each leg off the bed). | ER was provided for 20 min once daily for 2–7 days. | IL-6 and IL-10 | Blood samples were taken twice daily on 3 contiguous study days after enrolment and again on day 7 after enrolment: once after a period of 30 min of rest, then immediately after cessation of the intervention to assess serum biomarker levels using a meso-scale discovery multiplex antibody electrochemiluminescence approach. | After controlling for resting IL-6, age, group, gender, race, BMI, and severity, there were no associations between IL-6 and either mode or duration of exercise. However, a significant association between IL-10 and exercise duration was found: the more substantial the duration, the lower the IL-10 during both control and intervention (p = 0.03). The mode of exercise was not associated with changes in IL-10. Change in IL-6 was associated with self-reported fatigue but not pain (p = 0.02). | Participants enrolled in the intervention period experienced 5 fewer days of ICU hospitalisation than the control group, despite higher acuity on ICU admission. The duration of mechanical ventilation was not different between groups. |
| Winkelman et al., 2015 [34] (United States) Prospective, exploratory, repeated-measures interventional study. | Patients receiving MV for > 48 h with no plan to extubate in the subsequent 48 h. | ER: All participants received a protocolised activity. Level 1: unconscious patients receiving PM; level 2: conscious patients receiving combined active/PM; level 3: weight-bearing transfer to a chair, advancing to dangling; level 4: advancing to standing and ambulation. | ER was provided for 20 min once daily for three days. | IL-8, IL-15, and TNF-α | Blood samples were taken twice daily on 3 contiguous study days: once after a period of at least 30 min of rest, then immediately after cessation of the intervention to assess serum biomarker levels using a meso-scale discovery multiplex anti-body electrochemiluminescence approach. | No significance for IL-8, IL-15, and TNF-α was found in terms of within-subject variations. No association with ICU severity of illness (APACHE 3) on admission was observed. TNF-α values were significantly associated with the type of activity (in bed vs. out of bed). Low TNF-α values were related to out-of-bed activity, and high TNF-α values were associated with in-bed activity (p = 0.02). | Among the 23 participants in whom muscle strength was measured, 8 (35%) scored < 24 on the MMT and values consistent with ICUAW. Among the 32 participants in whom we measured ADLs, 24 (75%) were moderately to severely dependent on ADLs (i.e., KATZ score < 4) the day after ICU discharge. |
| Winkelman et al., 2018 [32] (United States) RCT study with repeated measures and blinded assessment of outcomes | Patients receiving MV for 36 h and study expected to require 24 h more. | Low ER: In-bed range of motion (no resistance) or passive transfer to a chair, providing full support. Moderate ER: Sitting at the edge of the bed, standing, pivoting transfer to bedside chair, marching in place at the bedside, or walking. | ETM was sustained daily until ICU discharge. The comparison group comprised patients receiving one or two daily ER sessions. | IL-6, IL-8, IL-10, IL-15, and TNF-α | Blood samples were taken twice daily on 3 contiguous study days: once after a period of rest, then 20 min after cessation of the intervention to assess serum biomarker levels using a meso-scale discovery multiplex antibody electrochemiluminescence approach | There were no significant differences in baseline values for any biomarker comparing once- or twice-a-day sessions at any intensity. There was no significant association of inflammatory biomarkers with the intensity of ER. | ICU LOS was significantly reduced for patients who received ER sessions twice daily compared to once. MMT scores and dynamometer handgrip values were more outstanding on days 1 and 3 among participants who received moderate-intensity ER than those receiving low ER. Similarly, reduced delirium on days 1 and 3 was found for participants receiving moderate-intensity ER. |
| Wollersheim et al., 2019 [31] (Germany) Exploratory RCT single-centre trial | Mechanically ventilated patients with sepsis-related MODS (SOFA score ≥ 9 within the first 72 h after ICU admission). | Control: Common ER performed. ER+ muscle-activating measures: The intervention group added NMES or WBV to the ER. NMES was performed bilaterally on eight different muscles, starting on enrolment day (maximum intensity of 70 mA until visible/palpable muscle contraction). WBV used 20 cycles (alternating stimulation, 26 Hz, and amplitude of 15 mm), with 1 min pause following each 1 min stimulation cycle. Common PT: PT performed without a clear protocol. | Common PT was performed only on weekdays. ER, NMES, and whole-body vibration (WBV) were carried out daily throughout the ICU and lasted until day 28, in addition to protocol-based physiotherapy. NMES lasted 20 min. | Myostatin, MSTN, IL6, TNF-α, and SAA1/2 | Blood samples were taken on day 14 after ICU admission to assess myostatin plasma levels using commercially prepared ELISA kits. Additionally, on day 15, muscle biopsies were sampled from the patient’s vastus lateralis to quantify gene expression via real-time polymerase chain reactions and protein concentration via western blot analyses. | MSTN and myostatin plasma levels significantly decreased in both groups, and the patients remained unaffected by the intervention. IL-6 and SAA1/2 significantly increased the above values for healthy references, while TNF-α was like healthy references for the intervention and control groups, without differences between these two groups. Comparing standard PT with both groups, a significantly increased gene expression for TNF-α (p < 0.001) and a considerable decrease in SAA1/2 (p = 0.008) were found. TNF expression was increased in standard PT in contrast to healthy values. | ICU-AW occurred within the entire cohort, and muscle-activating measures did not improve muscle strength or function at first awakening or 12-month follow-up. No signs of necrosis or inflammatory infiltration were present in the histological analysis. The myocyte cross-sectional area in the intervention group was significantly larger in comparison with the control group (type I, +10%; type IIa, +13%; type IIb, +3%) and CPP (type I, +36%; type IIa, +49%; type IIb, +65%). |
| Vanhorebeek et al., 2023 [29] (Belgium) Retrospective analysis (data from two RCTs). | Participants in the Early Parenteral Nutrition Completing Enteral Nutrition in Adult Critically Ill Patients (EPaNIC RCT) study were included. The second study included patients who participated in the NMES in Critically Ill Patients (NESCI) study. | In the EPaNIC study, standard PT was provided to both groups (with and without nutritional intervention). In the NESCI study, adult patients between days 2 and 4 after ICU admission received PT and ER according to a standardised local “Start to move as soon as possible” protocol. Randomization determined whether the quadriceps of the dominant or the nondominant leg were selected for stimulation. | PT and ER were provided daily according to unit protocols. The NMES session lasted for 1 h daily for 7 consecutive days (50 min at the highest tolerable intensity with a visible muscle response; 8.5 s ON, 45 Hz, pulse of 350 μs, 1.5 s ramp up, 1 s ramp down, and 12-s OFF phase). | FNDC5, KYAT1, KYAT3, AMY1A, AMY1B, AMY1C, AMY2A, and AMY2B | Muscle biopsies were taken on day 8±1 after ICU admission. The de Bergström technique was used on the patient’s vastus lateralis to assess mRNA expression. mRNA extraction was performed using an in-house protocol. Quantification was performed via real-time polymerase chain reaction. | In critically ill patients, mRNA expression of FNDC5, KYAT1, and total amylases in muscle was 34% to 80% lower than in controls, whereas no significant effect was observed for KYAT3. NMES that resulted in good muscle contraction increased FNDC5 expression compared with unstimulated muscle but did not affect the other myokines. | In univariable analysis, ICU non-survivors showed lower FNDC5 expression than ICU survivors. In multivariable analysis, lower FNDC5 expression remained independently associated with a higher risk of death in the ICU. ICU survivors who needed more than 7 days of intensive care after collecting the muscle biopsy had lower expressions of FNDC5 and amylases than ICU survivors who needed a shorter ICU stay. Patients who acquired clinically relevant muscle weakness in the ICU had a lower expression of FNDC5 than patients who did not develop such weakness. |
| Grunow and cols, 2022 [30] (Germany) Retrospective cohort analysis (data from two prospective clinical studies) | Patients in both studies fulfilled the same inclusion criteria: age > 18, MV, and high risk for developing ICU-AW (SOFA score ≥ 9 within the first 72 h after ICU admission). | Common PT (sPT) or PT guided by daily mobilisation goals (pPT) provided by a stepwise approach. Level 1 (no mobilization) was used until level 5 (intensified therapy with activities of daily living) or pPT+ muscle activating measures (NMES and/or WBV). | For 14–15 days, muscle-activating measures were carried out daily throughout the ICU stay up to day 28, in addition to protocol-based physiotherapy. NMES was performed bilaterally on eight different muscle groups for 20 min. WBV was performed daily for 20 cycles. | Myostatin and MSTN | Blood samples were collected on days 4, 8, and 14 using commercially prepared ELISA kits to determine myostatin plasma levels. Additionally, muscle biopsies were sampled from the patient’s vastus lateralis on day 15 after ICU admission to isolate mRNA expression of MSTN via TRIzol reagent, then quantified by real-time quantitative polymerase chain reaction. | Patients were categorised into two subgroups on day 14 according to myostatin plasma levels: increased/decreased levels when compared to healthy controls. Critically ill patients showed significantly reduced MSTN gene expression in skeletal muscle compared to healthy controls (p = 0.004). Reduced myostatin plasma levels were observed during the first 2 weeks of the ICU stay (p < 0.001) and were pronounced during the early phase of ICU treatment but with a significant increase over time (p < 0.001). | Muscular MSTN gene expression on day 15 after ICU admission was significantly lower in ICU-AW patients than in controls and patients without ICU-AW. Low myostatin plasma concentrations on day 8 correlated with reduced muscle strength at first awakening, while no correlation was observed at ICU discharge. MSTN gene expression decreased in all critically ill patients. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jalil, Y.; Damiani, L.F.; García-Valdés, P.; Basoalto, R.; Gallastegui, J.; Gutierrez-Arias, R. Myokine Secretion Dynamics and Their Role in Critically Ill Patients: A Scoping Review. J. Clin. Med. 2025, 14, 2892. https://doi.org/10.3390/jcm14092892
Jalil Y, Damiani LF, García-Valdés P, Basoalto R, Gallastegui J, Gutierrez-Arias R. Myokine Secretion Dynamics and Their Role in Critically Ill Patients: A Scoping Review. Journal of Clinical Medicine. 2025; 14(9):2892. https://doi.org/10.3390/jcm14092892
Chicago/Turabian StyleJalil, Yorschua, L. Felipe Damiani, Patricio García-Valdés, Roque Basoalto, Julen Gallastegui, and Ruvistay Gutierrez-Arias. 2025. "Myokine Secretion Dynamics and Their Role in Critically Ill Patients: A Scoping Review" Journal of Clinical Medicine 14, no. 9: 2892. https://doi.org/10.3390/jcm14092892
APA StyleJalil, Y., Damiani, L. F., García-Valdés, P., Basoalto, R., Gallastegui, J., & Gutierrez-Arias, R. (2025). Myokine Secretion Dynamics and Their Role in Critically Ill Patients: A Scoping Review. Journal of Clinical Medicine, 14(9), 2892. https://doi.org/10.3390/jcm14092892






