LOLATAO—An Artificial-Intelligence-Based Virtual Assistant for Clinical Follow-Up of Patients with Non-Valvular Atrial Fibrillation (AF) Undergoing Oral Anticoagulant Therapy (OAT): A Feasibility Study
Abstract
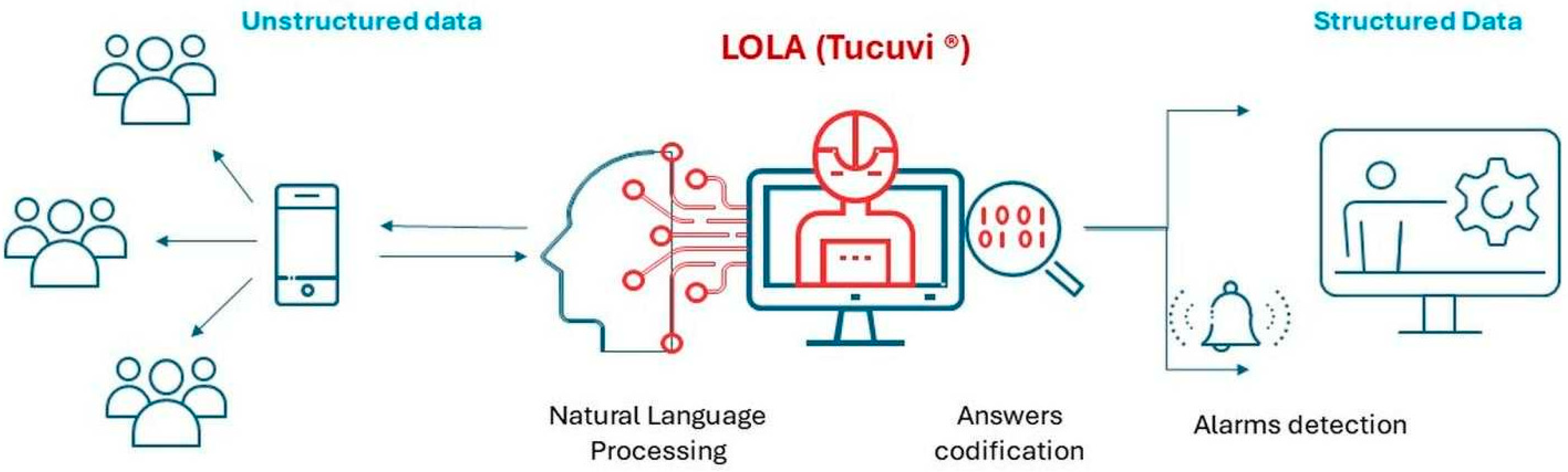
1. Introduction
2. Material and Methods
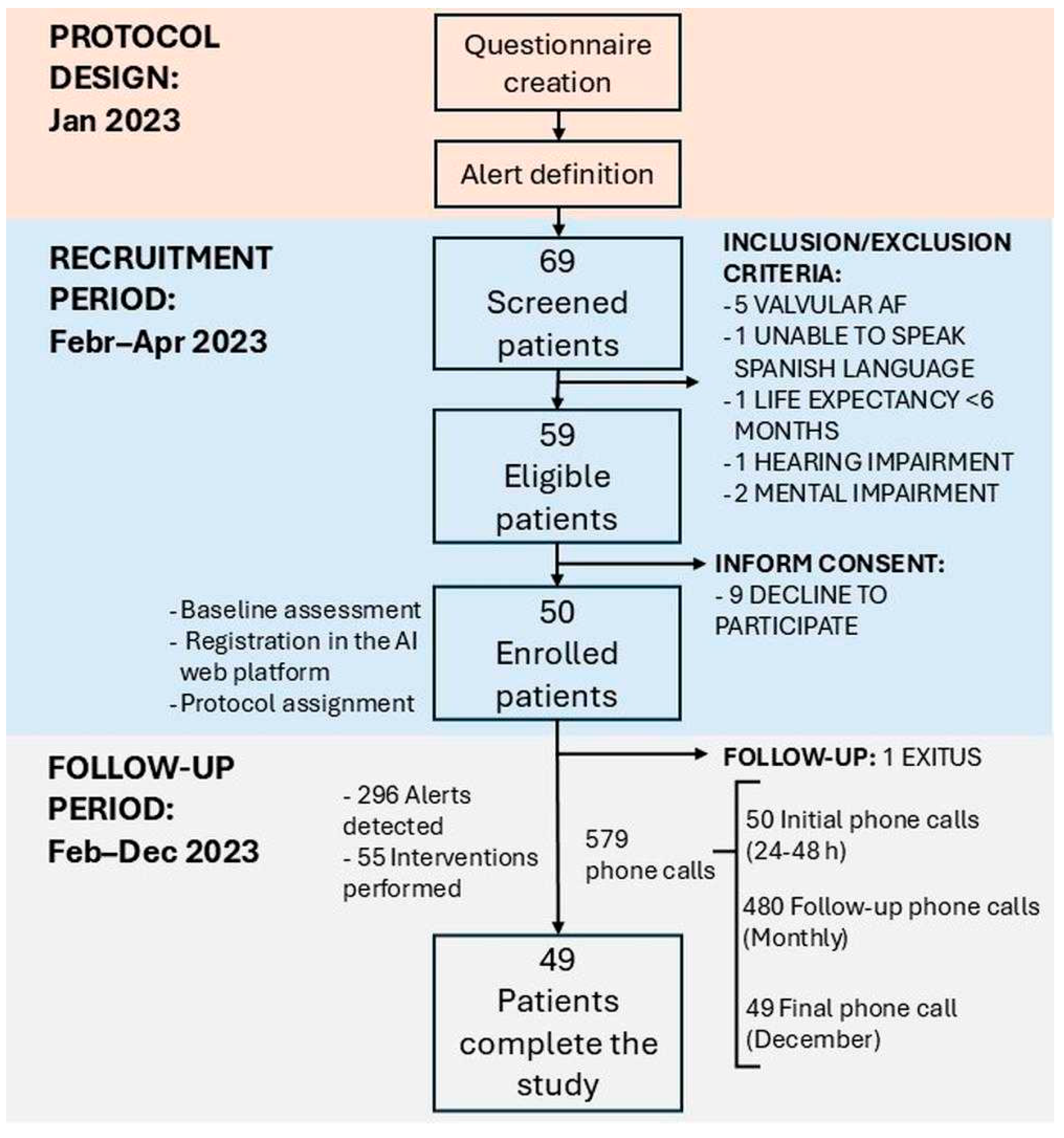
2.1. Study Design and Population
2.2. Statistical Methods
3. Results
| Demographics | ||||||
| Age (mean, range) = 75 ± 16 | AVKs (n = 16) | DOACs (n = 33) | Total (n = 49) | |||
| Male n = 12 | Female n = 4 | Male n = 29 | Female n = 11 | Male n = 33 | Female n = 16 | |
| Type of DOAC (n = 33) | ||||||
| Edoxaban (n, %) | 7 (21%) | |||||
| Rivaroxaban (n, %) | 11 (33%) | |||||
| Apixaban (n, %) | 12 (36%) | |||||
| Dabigatran (n, %) | 3 (9%) | |||||
| OAT Adherence | ||||
| Medication doses missed | AVKs (n = 16) | DOACs (n = 33) | Total (n = 49) | |
| 0 | 10 (63%) | 18 (55%) | 28 (58%) | |
| 1 | 4 (25%) | 5 (15%) | 9 (18%) | |
| 2 | 1 (6%) | 7 (21%) | 8 (16%) | |
| ≥3 | 1 (6%) | 3 (9%) | 4 (8%) | |
| Health Events | Concordance (n, %) | |||
| ≥1 Bleeding event | 14 (88%) | 5 (15%) | 19 (39%) | 19 (100%) |
| Bridging therapy | 1 (6%) | 8 (24%) | 9 (18%) | 9 (100%) |
| Renal function impairment (TFG < 60) | - | 14 (42%) | NA | 8 (57%) |
| Unknown TRT | 15 (94%) | NA | NA | 15 (100%) |
| TRT < 65% | 12 (75%) | NA | NA | 9 (75%) |
| Healthcare service use | ||||
| Emergency department | 5 (31%) | 12 (36%) | 17 (35%) | 17 (100%) |
| Hospitalisation | 0 (0%) | 3 (9%) | 3 (6%) | 3 (100%) |
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lippi, G.; Sanchis-Gomar, F.; Cervellin, G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int. J. Stroke Off. J. Int. Stroke Soc. 2021, 16, 217–221. [Google Scholar] [CrossRef]
- Krijthe, B.P.; Kunst, A.; Benjamin, E.J.; Lip, G.Y.; Franco, O.H.; Hofman, A.; Witteman, J.C.; Stricker, B.H.; Heeringa, J. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur. Heart J. 2013, 34, 2746–2751. [Google Scholar] [CrossRef]
- Heart Disease and Stroke Statistics—2019 Update: A Report from the American Heart Association. Available online: https://www.ahajournals.org/doi/epub/10.1161/CIR.0000000000000659 (accessed on 17 April 2024).
- Pisters, R.; Lane, D.A.; Marin, F.; Camm, A.J.; Lip, G.Y.H. Stroke and Thromboembolism in Atrial Fibrillation: Systematic Review of Stroke Risk Factors and Risk Stratification Schema. Circ. J. 2012, 76, 2289–2304. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.G.; Pearce, L.A.; Aguilar, M.I. Meta-analysis: Antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann. Intern. Med. 2007, 146, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Andrade, J.G.; Aguilar, M.; Atzema, C.; Bell, A.; Cairns, J.A.; Cheung, C.C.; Cox, J.L.; Dorian, P.; Gladstone, D.J.; Healey, J.S.; et al. The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society Comprehensive Guidelines for the Management of Atrial Fibrillation. Can. J. Cardiol. 2020, 36, 1847–1948. [Google Scholar] [PubMed]
- Lip, G.Y.H.; Freedman, B.; De Caterina, R.; Potpara, T.S. Stroke prevention in atrial fibrillation: Past, present and future: Comparing the guidelines and practical decision-making. Thromb. Haemost. 2017, 117, 1230–1239. [Google Scholar] [CrossRef]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef]
- Steffel, J.; Verhamme, P.; Potpara, T.S.; Albaladejo, P.; Antz, M.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur. Heart J. 2018, 39, 1330–1393. [Google Scholar] [CrossRef]
- Kotecha, D.; Chua, W.W.L.; Fabritz, L.; Hendriks, J.; Casadei, B.; Schotten, U.; Vardas, P.; Heidbuchel, H.; Dean, V.; Kirchhof, P. European Society of Cardiology smartphone and tablet applications for patients with atrial fibrillation and their health care providers. EP Eur. 2018, 20, 225–233. [Google Scholar] [CrossRef]
- Brieger, D.; Amerena, J.; Attia, J.; Bajorek, B.; Chan, K.H.; Connell, C.; Freedman, B.; Ferguson, C.; Hall, T.; Haqqani, H.; et al. National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand: Australian Clinical Guidelines for the Diagnosis and Management of Atrial Fibrillation 2018. Heart Lung Circ. 2018, 27, 1209–1266. [Google Scholar] [CrossRef]
- Lowres, N.; Neubeck, L.; Salkeld, G.; Krass, I.; McLachlan, A.J.; Redfern, J.; Bennett, A.A.; Briffa, T.; Bauman, A.; Martinez, C.; et al. Feasibility and cost-effectiveness of stroke prevention through community screening for atrial fibrillation using iPhone ECG in pharmacies. The SEARCH-AF study. Thromb. Haemost. 2014, 111, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Hickey, K.T.; Hauser, N.R.; Valente, L.E.; Riga, T.C.; Frulla, A.P.; Creber, R.M.; Whang, W.; Garan, H.; Jia, H.; Sciacca, R.R.; et al. A single-center randomised, controlled trial investigating the efficacy of a mHealth ECG technology intervention to improve the detection of atrial fibrillation: The iHEART study protocol. BMC Cardiovasc. Disord. 2016, 16, 152. [Google Scholar] [CrossRef]
- Haberman, Z.C.; Jahn, R.T.; Bose, R.; Tun, H.; Shinbane, J.S.; Doshi, R.N.; Chang, P.M.; Saxon, L.A. Wireless Smartphone ECG Enables Large-Scale Screening in Diverse Populations. J. Cardiovasc. Electrophysiol. 2015, 26, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Lowres, N.; Mulcahy, G.; Gallagher, R.; Ben Freedman, S.; Marshman, D.; Kirkness, A.; Orchard, J.; Neubeck, L. Self-monitoring for atrial fibrillation recurrence in the discharge period post-cardiac surgery using an iPhone electrocardiogram. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2016, 50, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Ricci, R.P.; Morichelli, L.; Santini, M. Remote control of implanted devices through Home Monitoring technology improves detection and clinical management of atrial fibrillation. EP Eur. 2009, 11, 54–61. [Google Scholar]
- Bajorek, B.; Magin, P.; Hilmer, S.; Krass, I. A cluster-randomised controlled trial of a computerised antithrombotic risk assessment tool to optimise stroke prevention in general practice: A study protocol. BMC Health Serv. Res. 2014, 14, 55. [Google Scholar] [CrossRef]
- Wang, Y.; Bajorek, B. Safe use of antithrombotics for stroke prevention in atrial fibrillation: Consideration of risk assessment tools to support decision-making. Ther. Adv. Drug Saf. 2014, 5, 21–37. [Google Scholar] [CrossRef]
- Eckman, M.H.; Wise, R.E.; Naylor, K.; Arduser, L.; Lip, G.Y.; Kissela, B.; Flaherty, M.; Kleindorfer, D.; Khan, F.; Schauer, D.P.; et al. Developing an Atrial Fibrillation Guideline Support Tool (AFGuST) for shared decision making. Curr. Med. Res. Opin. 2015, 31, 603–614. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, Y.; Lane, D.A.; Liu, L.; Wang, Y.; Lip, G.Y.H. Mobile Health Technology for Atrial Fibrillation Management Integrating Decision Support, Education, and Patient Involvement: mAF App Trial. Am. J. Med. 2017, 130, 1388–1396.e6. [Google Scholar] [CrossRef]
- Bubner, T.K.; Laurence, C.O.; Gialamas, A.; Yelland, L.N.; Ryan, P.; Willson, K.J.; Tideman, P.; Worley, P.; Beilby, J.J. Effectiveness of point-of-care testing for therapeutic control of chronic conditions: Results from the PoCT in General Practice Trial. Med. J. Aust. 2009, 190, 624–626. [Google Scholar] [CrossRef]
- Bereznicki, L.R.E.; Jackson, S.L.; Peterson, G.M. Supervised patient self-testing of warfarin therapy using an online system. J. Med. Internet Res. 2013, 15, e138. [Google Scholar] [CrossRef]
- Stafford, L.; Peterson, G.M.; Bereznicki, L.R.; Jackson, S.L.; van Tienen, E.C.; Angley, M.T.; Bajorek, B.V.; McLachlan, A.J.; Mullan, J.R.; Misan, G.M.; et al. Clinical Outcomes of a Collaborative, Home-Based Postdischarge Warfarin Management Service. Ann. Pharmacother. 2011, 45, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Toscos, T.; Coupe, A.; Wagner, S.; Ahmed, R.; Roebuck, A.; Flanagan, M.; Drouin, M.; Mirro, M. Engaging Patients in Atrial Fibrillation Management via Digital Health Technology: The Impact of Tailored Messaging. J. Innov. Card. Rhythm. Manag. 2020, 11, 4209–4217. [Google Scholar] [CrossRef]
- Desteghe, L.; Kluts, K.; Vijgen, J.; Koopman, P.; Dilling-Boer, D.; Schurmans, J.; Dendale, P.; Heidbuchel, H. The Health Buddies App as a Novel Tool to Improve Adherence and Knowledge in Atrial Fibrillation Patients: A Pilot Study. JMIR MHealth UHealth. 2017, 5, e7420. [Google Scholar] [CrossRef] [PubMed]
- Klimis, H.; Thakkar, J.; Chow, C.K. Breaking Barriers: Mobile Health Interventions for Cardiovascular Disease. Can. J. Cardiol. 2018, 34, 905–913. [Google Scholar] [CrossRef]
- Piette, J.D.; List, J.; Rana, G.K.; Townsend, W.; Striplin, D.; Heisler, M. Mobile Health Devices as Tools for Worldwide Cardiovascular Risk Reduction and Disease Management. Circulation 2015, 132, 2012–2027. [Google Scholar] [CrossRef] [PubMed]
- Pfaeffli Dale, L.; Dobson, R.; Whittaker, R.; Maddison, R. The effectiveness of mobile-health behaviour change interventions for cardiovascular disease self-management: A systematic review. Eur. J. Prev. Cardiol. 2016, 23, 801–817. [Google Scholar] [CrossRef]
- Gandhi, S.; Chen, S.; Hong, L.; Sun, K.; Gong, E.; Li, C.; Yan, L.L.; Schwalm, J.-D. Effect of Mobile Health Interventions on the Secondary Prevention of Cardiovascular Disease: Systematic Review and Meta-analysis. Can. J. Cardiol. 2017, 33, 219–231. [Google Scholar] [CrossRef]
- Cevasco, K.E.; Morrison Brown, R.E.; Woldeselassie, R.; Kaplan, S. Patient Engagement with Conversational Agents in Health Applications 2016–2022: A Systematic Review and Meta-Analysis. J. Med. Syst. 2024, 48, 40. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Posadzki, P.; Mastellos, N.; Ryan, R.; Gunn, L.H.; Felix, L.M.; Pappas, Y.; Gagnon, M.-P.; Julious, S.A.; Xiang, L.; Oldenburg, B.; et al. Automated telephone communication systems for preventive healthcare and management of long-term conditions. Cochrane Database Syst. Rev. 2016, 2016, CD009921. [Google Scholar] [CrossRef]
- Kassavou, A.; Sutton, S. Automated telecommunication interventions to promote adherence to cardio-metabolic medications: Meta-analysis of effectiveness and meta-regression of behaviour change techniques. Health Psychol. Rev. 2018, 12, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Tsoli, S.; Sutton, S.; Kassavou, A. Interactive voice response interventions targeting behaviour change: A systematic literature review with meta-analysis and meta-regression. BMJ Open 2018, 8, e018974. [Google Scholar] [CrossRef] [PubMed]
- Tudor Car, L.; Dhinagaran, D.; Kyaw, B.; Kowatsch, T.; Joty, S.; Theng, Y.; Atun, R. Conversational Agents in Health Care: Scoping Review and Conceptual Analysis. J. Med. Internet Res. 2020, 22, e17158. [Google Scholar] [CrossRef] [PubMed]
- Milne-Ives, M.; de Cock, C.; Lim, E.; Shehadeh, M.; de Pennington, N.; Mole, G.; Normando, E.; Meinert, E. The Effectiveness of Artificial Intelligence Conversational Agents in Health Care: Systematic Review. J. Med. Internet Res. 2020, 22, e20346. [Google Scholar] [CrossRef]
- de Cock, C.; Milne-Ives, M.; van Velthoven, M.H.; Alturkistani, A.; Lam, C.; Meinert, E. Effectiveness of Conversational Agents (Virtual Assistants) in Health Care: Protocol for a Systematic Review. JMIR Res. Protoc. 2020, 9, e16934. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McTear, M.F. Spoken dialogue technology: Enabling the conversational user interface. ACM Comput. Surv. 2002, 34, 90–169. [Google Scholar] [CrossRef]
- Lobo, J.; Ferreira, L.; Ferreira, A.J. CARMIE. Int. J. E-Health Med. Commun. 2017, 8, 21–37. [Google Scholar] [CrossRef]
- Cheng, A.; Raghavaraju, V.; Kanugo, J.; Handrianto, Y.P.; Shang, Y. Development and evaluation of a healthy coping voice interface application using the Google home for elderly patients with type 2 diabetes. In Proceedings of the 2018 15th IEEE Annual Consumer Communications & Networking Conference (CCNC), Las Vegas, NV, USA, 12–15 January 2018; pp. 1–5. [Google Scholar] [CrossRef]
- García Bermúdez, I.; González Manso, M.; Sánchez Sánchez, E.; Rodríguez Hita, A.; Rubio Rubio, M.; Suárez Fernández, C. Utilidad y aceptación del seguimiento telefónico de un asistente virtual a pacientes COVID-19 tras el alta [Usefulness and acceptance of telephone monitoring by a virtual assistant for patients with COVID-19 following discharge]. Rev. Clin. Esp. 2021, 221, 464–467. (In Spanish) [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dorado-Díaz, P.I.; Sampedro-Gómez, J.; Vicente-Palacios, V.; Sánchez, P.L. Applications of Artificial Intelligence in Cardiology. The Future is Already Here. Rev. Esp. Cardiol. 2019, 72, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.; Mahadevappa, M.; Chilakamarri, S. The Emergence of Artificial Intelligence in Cardiology: Current and Future Applications. Curr. Cardiol. Rev. 2022, 18, e191121198124. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ledziński, Ł.; Grześk, G. Artificial Intelligence Technologies in Cardiology. J. Cardiovasc. Dev. Dis. 2023, 10, 202. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pegoraro, V.; Bidoli, C.; Mas, F.D.; Bert, F.; Cobianchi, L.; Zantedeschi, M.; Campostrini, S.; Migliore, F.; Boriani, G. Cardiology in a Digital Age: Opportunities and Challenges for e-Health: A Literature Review. J. Clin. Med. 2023, 12, 4278. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, A.; Antón Maldonado, C.; Sánchez-Quiñones, B.; Ibarra Vega, N.; Ayo González, M.; Gonzalez Cabezas, P.; Carrasco Moreno, R. Implementing Telemedicine in Clinical Practice in the First Digital Hematology Unit: Feasibility Study. JMIR Form Res. 2023, 7, e48987. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barrios, V.; Cinza-Sanjurjo, S.; García-Alegría, J.; Freixa-Pamias, R.; Llordachs-Marques, F.; Molina, C.A.; Santamaría, A.; Vivas, D.; Suárez Fernandez, C. Role of telemedicine in the management of oral anticoagulation in atrial fibrillation: A practical clinical approach. Future Cardiol. 2022, 18, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Aquero, A. Net Promoter Score (NPS) and Customer Satisfaction: Relationship and Efficient Management. Sustainability 2022, 14, 2011. [Google Scholar] [CrossRef]
- Rotella, P.; Chulani, S. Analysis of customer satisfaction survey data. In Proceedings of the 2012 9th IEEE Working Conference on Mining Software Repositories (MSR), Zurich, Switzerland, 2–3 June 2012; pp. 88–97. [Google Scholar] [CrossRef]
- Fatima, B.; Mohan, A.; Altaie, I.; Abughosh, S. Predictors of adherence to direct oral anticoagulants after cardiovascular or bleeding events in Medicare Advantage Plan enrollers with atrial fibrillation. J. Manag. Care Spec. Pharm. 2024, 30, 408–419. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Questionnaire | Result | Explanation |
|---|---|---|
| CSAT | 4.63/5 | All patients, except 1, indicated being satisfied or very satisfied with LOLA; 1 patient answered with a rating of 3 (i.e., neutral). |
| NPS | 44.73% | 38 patients answered this question, and only 5 of them provided a rating lower than 7. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santamaría, A.; Antón-Maldonado, C.; Sánchez-Quiñones, B.; Ibarra Vega, N.; González, P.; Carrasco, R. LOLATAO—An Artificial-Intelligence-Based Virtual Assistant for Clinical Follow-Up of Patients with Non-Valvular Atrial Fibrillation (AF) Undergoing Oral Anticoagulant Therapy (OAT): A Feasibility Study. J. Clin. Med. 2025, 14, 3023. https://doi.org/10.3390/jcm14093023
Santamaría A, Antón-Maldonado C, Sánchez-Quiñones B, Ibarra Vega N, González P, Carrasco R. LOLATAO—An Artificial-Intelligence-Based Virtual Assistant for Clinical Follow-Up of Patients with Non-Valvular Atrial Fibrillation (AF) Undergoing Oral Anticoagulant Therapy (OAT): A Feasibility Study. Journal of Clinical Medicine. 2025; 14(9):3023. https://doi.org/10.3390/jcm14093023
Chicago/Turabian StyleSantamaría, Amparo, Cristina Antón-Maldonado, Beatriz Sánchez-Quiñones, Nataly Ibarra Vega, Pedro González, and Rafael Carrasco. 2025. "LOLATAO—An Artificial-Intelligence-Based Virtual Assistant for Clinical Follow-Up of Patients with Non-Valvular Atrial Fibrillation (AF) Undergoing Oral Anticoagulant Therapy (OAT): A Feasibility Study" Journal of Clinical Medicine 14, no. 9: 3023. https://doi.org/10.3390/jcm14093023
APA StyleSantamaría, A., Antón-Maldonado, C., Sánchez-Quiñones, B., Ibarra Vega, N., González, P., & Carrasco, R. (2025). LOLATAO—An Artificial-Intelligence-Based Virtual Assistant for Clinical Follow-Up of Patients with Non-Valvular Atrial Fibrillation (AF) Undergoing Oral Anticoagulant Therapy (OAT): A Feasibility Study. Journal of Clinical Medicine, 14(9), 3023. https://doi.org/10.3390/jcm14093023








