Age-Related Electroencephalographic Delta and Alpha Oscillations During Sedation with Target-Controlled Propofol Infusion
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
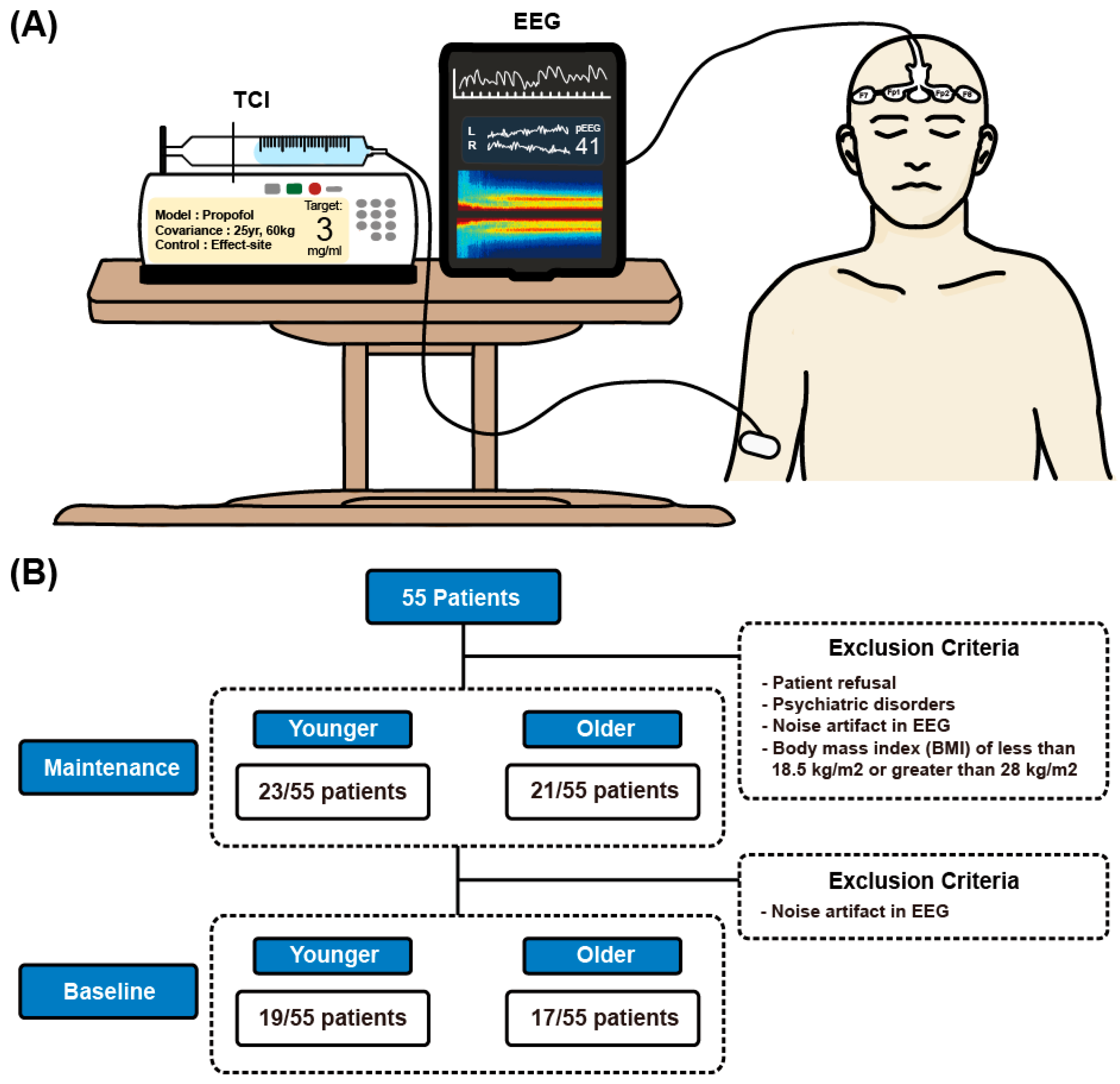
2.2. Patient Population
2.3. Anesthetic Management
2.4. Data Acquisition
2.5. EEG Analysis
2.5.1. Data Preprocessing
2.5.2. Spectral Analysis
2.5.3. Coherence Analysis
2.5.4. Functional Connectivity Analysis
2.6. Statistical Analysis
3. Results
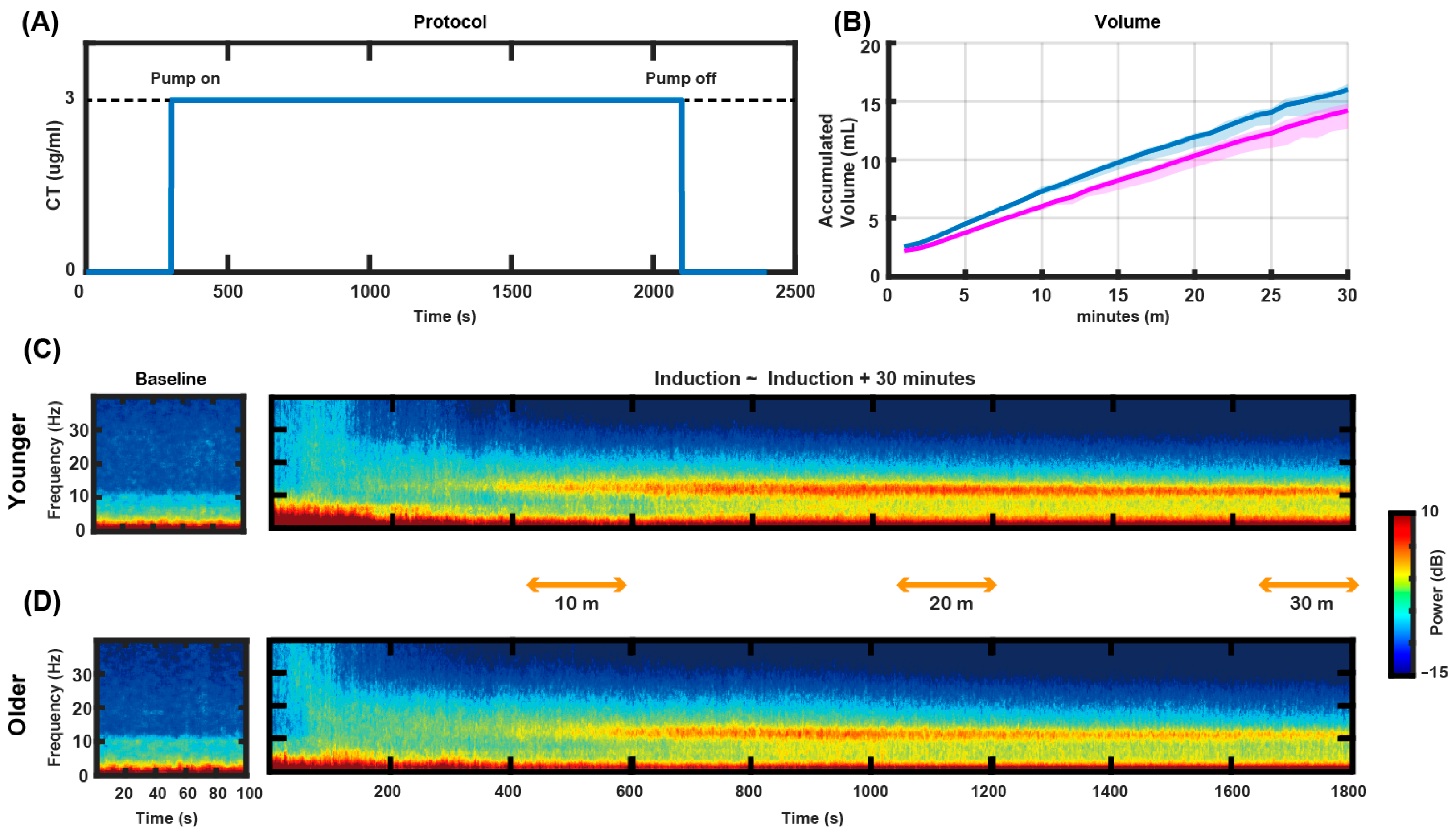
3.1. Study Design and TCI Management
3.2. Frontal Topographical Spectral Power Analysis
3.3. Frontal Connectivity Analysis
4. Discussion
4.1. Frontal Alpha Band Power and Age
4.2. Thalamocortical Circuit and Frontal Alpha Connectivity
4.3. Frontal Delta Band Power and Age
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BIS | Bispectral index |
| PSI | Patient state index |
| TCI | Target-controlled infusion |
| wPLI | Weighted phase lag index |
| BPB | Brachial plexus block |
Appendix A
Appendix A.1
| Alpha Power | ||
| Time Point | F Value | p Value |
| 10 min | 0.001 | 0.971 |
| 20 min | 0.820 | 0.367 |
| 30 min | 4.451 | 0.037 |
| Delta Power | ||
| Time Point | F Value | p Value |
| 10 min | 4.086 | 0.126 |
| 20 min | 14.544 | 0.002 |
| 30 min | 18.158 | <0.001 |
| Alpha Power | ||
| Time Point | F Value | p Value |
| 10 min | 0.001 | 0.907 |
| 20 min | 1.837 | 0.177 |
| 30 min | 3.842 | 0.052 |
| Delta Power | ||
| Time Point | F Value | p Value |
| 10 min | 4.534 | 0.035 |
| 20 min | 15.42 | <0.001 |
| 30 min | 18.622 | <0.001 |
Appendix A.2
References
- Rampil, I.J. A primer for EEG signal processing in anesthesia. Anesthesiology 1998, 89, 980–1002. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.N.; Lydic, R.; Schiff, N.D. General anesthesia, sleep, and coma. N. Engl. J. Med. 2010, 363, 2638–2650. [Google Scholar] [CrossRef] [PubMed]
- Purdon, P.L.; Sampson, A.; Pavone, K.J.; Brown, E.N. Clinical electroencephalography for anesthesiologists: Part I: Background and basic signatures. Anesthesiology 2015, 123, 937–960. [Google Scholar] [CrossRef]
- Bruhn, J.; Myles, P.S.; Sneyd, R.; Struys, M.M. Depth of anaesthesia monitoring: What’s available, what’s validated and what’s next? Br. J. Anaesth 2006, 97, 85–94. [Google Scholar] [CrossRef]
- Sebel, P.S.; Lang, E.; Rampil, I.J.; White, P.F.; Cork, R.; Jopling, M.; Smith, N.T.; Glass, P.S.A.; Manberg, P. A multicenter study of bispectral electroencephalogram analysis for monitoring anesthetic effect. Anesth. Analg. 1997, 84, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Soehle, M.; Ellerkmann, R.K.; Grube, M.; Kuech, M.; Wirz, S.; Hoeft, A.; Bruhn, J. Comparison between bispectral index and patient state index as measures of the electroencephalographic effects of sevoflurane. Anesthesiology 2008, 109, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Evered, L.A.; Chan, M.T.; Han, R.; Chu, M.H.; Cheng, B.P.; Scott, D.A.; Pryor, K.O.; Sessler, D.I.; Veselis, R.; Frampton, C.; et al. Anaesthetic depth and delirium after major surgery: A randomised clinical trial. Br. J. Anaesth. 2021, 127, 704–712. [Google Scholar] [CrossRef]
- Purdon, P.L.; Pierce, E.T.; Mukamel, E.A.; Prerau, M.J.; Walsh, J.L.; Wong, K.F.K.; Salazar-Gomez, A.F.; Harrell, P.G.; Sampson, A.L.; Cimenser, A.; et al. Electroencephalogram signatures of loss and recovery of consciousness from propofol. Proc. Natl. Acad. Sci. USA 2013, 110, E1142–E1151. [Google Scholar] [CrossRef]
- Lewis, L.D.; Weiner, V.S.; Mukamel, E.A.; Donoghue, J.A.; Eskandar, E.N.; Madsen, J.R.; Anderson, W.S.; Hochberg, L.R.; Cash, S.S.; Brown, E.N.; et al. Rapid fragmentation of neuronal networks at the onset of propofol-induced unconsciousness. Proc. Natl. Acad. Sci. USA 2012, 109, E3377–E3386. [Google Scholar] [CrossRef]
- Cornelissen, L.; Kim, S.-E.; Purdon, P.L.; Brown, E.N.; Berde, C.B. Age-dependent electroencephalogram (EEG) patterns during sevoflurane general anesthesia in infants. eLife 2015, 4, e06513. [Google Scholar] [CrossRef]
- Cornelissen, L.; Kim, S.; Lee, J.; Brown, E.; Purdon, P.; Berde, C. Electroencephalographic markers of brain development during sevoflurane anaesthesia in children up to 3 years old. Br. J. Anaesth. 2018, 120, 1274–1286. [Google Scholar] [CrossRef] [PubMed]
- Purdon, P.L.; Pavone, K.J.; Akeju, O.; Smith, A.C.; Sampson, A.L.; Lee, J.; Zhou, D.W.; Solt, K.; Brown, E.N. The ageing brain: Age-dependent changes in the electroencephalogram during propofol and sevoflurane general anaesthesia. Br. J. Anaesth. 2015, 115, i46–i57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, A.; Wang, S.; Wang, T.; Liu, T.; Wang, Y.; Fu, J.; Zhao, G.; Yang, Q.; Dong, H. Differences in the EEG power spectrum and cross-frequency coupling patterns between young and elderly patients during sevoflurane anesthesia. Brain Sci. 2023, 13, 1149. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, D.; Cartailler, J.; Touchard, C.; Joachim, J.; Mebazaa, A.; Vallée, F.; Gayat, É.; Gramfort, A.; Engemann, D.A. Repurposing electroencephalogram monitoring of general anaesthesia for building biomarkers of brain ageing: An exploratory study. BJA Open 2023, 7, 100145. [Google Scholar] [CrossRef]
- Leroy, S.; Major, S.; Bublitz, V.; Dreier, J.P.; Koch, S. Unveiling age-independent spectral markers of propofol-induced loss of consciousness by decomposing the electroencephalographic spectrum into its periodic and aperiodic components. Front. Aging Neurosci. 2023, 14, 1076393. [Google Scholar] [CrossRef]
- Biggs, D.; Boncompte, G.; Pedemonte, J.C.; Fuentes, C.; Cortinez, L.I. The effect of age on electroencephalogram measures of anesthesia hypnosis: A comparison of BIS, alpha power, Lempel-Ziv complexity and permutation entropy during propofol induction. Front. Aging Neurosci. 2022, 14, 910886. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Tai, B.C.; Remani, D.; Phua, J.; Cove, M.E.; Kowitlawakul, Y. Age related inverse dose relation of sedatives and analgesics in the intensive care unit. PLoS ONE 2017, 12, e0185212. [Google Scholar] [CrossRef]
- Schnider, T.W.; Minto, C.F.; Gambus, P.L.; Andresen, C.; Goodale, D.B.; Shafer, S.L.; Youngs, E.J. The influence of method of administration and covariates on the pharmacokinetics of propofol in adult volunteers. Anesthesiology 1998, 88, 1170–1182. [Google Scholar] [CrossRef]
- Schnider, T.W.; Minto, C.F.; Struys, M.M.; Absalom, A.R. The safety of target-controlled infusions. Anesth. Analg. 2016, 122, 79–85. [Google Scholar] [CrossRef]
- Lee, H.C.; Jung, C.W. Vital Recorder-a free research tool for automatic recording of high-resolution time-synchronised physiological data from multiple anaesthesia devices. Sci. Rep. 2018, 8, 1527. [Google Scholar] [CrossRef]
- Baillet, S.; Friston, K.; Oostenveld, R. Academic software applications for electromagnetic brain mapping using MEG and EEG. Comput. Intell. Neurosci. 2011, 2011, 972050. [Google Scholar] [CrossRef] [PubMed]
- Bokil, H.; Andrews, P.; Kulkarni, J.E.; Mehta, S.; Mitra, P.P. Chronux: A platform for analyzing neural signals. J. Neurosci. Methods 2010, 192, 146–151. [Google Scholar] [CrossRef]
- Vinck, M.; Oostenveld, R.; van Wingerden, M.; Battaglia, F.; Pennartz, C.M. An improved index of phase-synchronization for electrophysiological data in the presence of volume-conduction, noise and sample-size bias. Neuroimage 2011, 55, 1548–1565. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, J.; Park, S.; Kim, K.N.; Ha, Y.; Yi, S.; Shin, D.A.; Kuh, S.U.; Lee, C.K.; Koo, B.-N.; et al. Differential effects of sevoflurane and desflurane on frontal intraoperative electroencephalogram dynamics associated with postoperative delirium. J. Clin. Anesth. 2024, 93, 111368. [Google Scholar] [CrossRef]
- Benjamini, Y.; Drai, D.; Elmer, G.; Kafkafi, N.; Golani, I. Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 2001, 125, 279–284. [Google Scholar] [CrossRef]
- Baayen, R.H.; Davidson, D.J.; Bates, D.M. Mixed-effects modeling with crossed random effects for subjects and items. J. Mem. Lang. 2008, 59, 390–412. [Google Scholar] [CrossRef]
- Akeju, O.; Pavone, K.J.; Westover, M.B.; Vazquez, R.; Prerau, M.J.; Harrell, P.G.; Hartnack, K.E.; Rhee, J.; Sampson, A.L.; Habeeb, K.; et al. A comparison of propofol- and dexmedetomidine-induced electroencephalogram dynamics using spectral and coherence analysis. Anesthesiology 2014, 121, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Ching, S.; Cimenser, A.; Purdon, P.L.; Brown, E.N.; Kopell, N.J. Thalamocortical model for a propofol-induced α-rhythm associated with loss of consciousness. Proc. Natl. Acad. Sci. USA 2010, 107, 22665–22670. [Google Scholar] [CrossRef] [PubMed]
- Flores, F.J.; Hartnack, K.E.; Fath, A.B.; Kim, S.-E.; Wilson, M.A.; Brown, E.N.; Purdon, P.L. Thalamocortical synchronization during induction and emergence from propofol-induced anesthesia. Proc. Natl. Acad. Sci. USA 2017, 114, E6660–E6668. [Google Scholar] [CrossRef]
- Steriade, M.; Gloor, P.; Llinás, R.R.; Lopes da Silva, F.H.; Mesulam, M.M. Basic mechanisms of cerebral rhythmic activities. Electroencephalogr. Clin. Neurophysiol. 1990, 76, 481–508. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Q.; Luo, J.; Hu, M.; Zuo, C. Effects of age and sex on subcortical volumes. Front. Aging. Neurosci. 2019, 11, 259. [Google Scholar] [CrossRef] [PubMed]
- Ndode-Ekane, X.E.; Puigferrat Pérez, M.D.M.; Di Sapia, R.; Lapinlampi, N.; Pitkänen, A. Reorganization of thalamic inputs to lesioned cortex following experimental traumatic brain injury. Int. J. Mol. Sci. 2021, 22, 6329. [Google Scholar] [CrossRef]
- Murty, D.V.P.S.; Manikandan, K.; Kumar, W.S.; Ramesh, R.G.; Purokayastha, S.; Javali, M.; Rao, N.P.; Ray, S. Gamma oscillations weaken with age in healthy elderly in human EEG. Neuroimage 2020, 215, 116826. [Google Scholar] [CrossRef] [PubMed]
- Merkin, A.; Sghirripa, S.; Graetz, L.; Smith, A.E.; Hordacre, B.; Harris, R.; Pitcher, J.; Semmler, J.; Rogasch, N.C.; Goldsworthy, M. Do age-related differences in aperiodic neural activity explain differences in resting EEG alpha? Neurobiol. Aging 2023, 121, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Kumar, W.S.; Ray, S. Healthy ageing and cognitive impairment alter EEG functional connectivity in distinct frequency bands. Eur. J. Neurosci. 2023, 58, 3432–3449. [Google Scholar] [CrossRef]
- Gutierrez, R.; Egaña, J.I.; Saez, I.; Reyes, F.; Briceño, C.; Venegas, M.; Lavado, I.; Penna, A. Intraoperative low alpha power in the electroencephalogram is associated with postoperative subsyndromal delirium. Front. Syst. Neurosci. 2019, 13, 56. [Google Scholar] [CrossRef]
- Zhang, H.; Geng, X.; Wang, Y.; Guo, Y.; Gao, Y.; Zhang, S.; Du, W.; Liu, L.; Sun, M.; Jiao, F.; et al. The significance of EEG alpha oscillation spectral power and beta oscillation phase synchronization for diagnosing probable Alzheimer disease. Front. Aging Neurosci. 2021, 13, 631587. [Google Scholar] [CrossRef]
- Buchmann, A.; Kurth, S.; Ringli, M.; Geiger, A.; Jenni, O.G.; Huber, R. Anatomical markers of sleep slow wave activity derived from structural magnetic resonance images. J. Sleep Res. 2011, 20, 506–513. [Google Scholar] [CrossRef]
- Piantoni, G.; Poil, S.S.; Linkenkaer-Hansen, K.; Verweij, I.M.; Ramautar, J.R.; Van Someren, E.J.; Van Der Werf, Y.D. Individual differences in white matter diffusion affect sleep oscillations. J. Neurosci. 2013, 33, 227–233. [Google Scholar] [CrossRef]
- Raz, N.; Ghisletta, P.; Rodrigue, K.M.; Kennedy, K.M.; Lindenberger, U. Trajectories of brain aging in middle-aged and older adults: Regional and individual differences. Neuroimage 2010, 51, 501–511. [Google Scholar] [CrossRef]
- Giorgio, A.; Santelli, L.; Tomassini, V.; Bosnell, R.; Smith, S.; De Stefano, N.; Johansen-Berg, H. Age-related changes in grey and white matter structure throughout adulthood. Neuroimage 2010, 51, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Lebel, C.; Gee, M.; Camicioli, R.; Wieler, M.; Martin, W.; Beaulieu, C. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage 2012, 60, 340–352. [Google Scholar] [CrossRef] [PubMed]



| Younger Age Group (n = 23) | Older Age Group (n = 21) | p-Value | |
|---|---|---|---|
| Age (years), median (IQR) | 27 (22–34) | 60 (58–65) | - |
| Height (cm), mean (SD) | 170.9 (7.5) | 161.2 (8.9) | <0.001 |
| Weight (kg), mean (SD) | 68.7 (11.6) | 64.7 (12.2) | 0.279 |
| BMI (kg/m2), mean (SD) | 23.5 (3.3) | 24.7 (2.9) | 0.205 |
| Sex (male), N (%) | 17 (73.9) | 10 (47.6) | 0.121 |
| ASA I, N (%) | 0 (0) | 2 (0.09) | 1.0 |
| ASA II, N (%) | 20 (0.87) | 19 (0.91) | |
| ASA III, N (%) | 3 (0.13) | 0 (0) | |
| PSI, median (IQR) | 35 (31–49) | 36 (30–48) | 0.547 |
| Dose of propofol at 10 min (mL), median (IQR) | 7.3 (6.9–7.6) | 6.1 (5.8–6.5) | <0.001 |
| Dose of propofol at 20 min (mL), median (IQR) | 12.1 (11.7–12.9) | 10.4 (9.9–11.6) | <0.001 |
| Dose of propofol at 30 min (mL), median (IQR) | 16.0 (15.5–17.6) | 14.3 (13.6–16.3) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Park, J.; Chung, W.; Jo, Y.; Oh, C.; Hong, B.; Kim, S. Age-Related Electroencephalographic Delta and Alpha Oscillations During Sedation with Target-Controlled Propofol Infusion. J. Clin. Med. 2025, 14, 3024. https://doi.org/10.3390/jcm14093024
Kim Y, Park J, Chung W, Jo Y, Oh C, Hong B, Kim S. Age-Related Electroencephalographic Delta and Alpha Oscillations During Sedation with Target-Controlled Propofol Infusion. Journal of Clinical Medicine. 2025; 14(9):3024. https://doi.org/10.3390/jcm14093024
Chicago/Turabian StyleKim, Yeonsu, Jiho Park, Woosuk Chung, Yumin Jo, Chahyun Oh, Boohwi Hong, and Seongeun Kim. 2025. "Age-Related Electroencephalographic Delta and Alpha Oscillations During Sedation with Target-Controlled Propofol Infusion" Journal of Clinical Medicine 14, no. 9: 3024. https://doi.org/10.3390/jcm14093024
APA StyleKim, Y., Park, J., Chung, W., Jo, Y., Oh, C., Hong, B., & Kim, S. (2025). Age-Related Electroencephalographic Delta and Alpha Oscillations During Sedation with Target-Controlled Propofol Infusion. Journal of Clinical Medicine, 14(9), 3024. https://doi.org/10.3390/jcm14093024





