Understanding LOT-CRT: Current Insights, Limitations, and Our Center’s Experience
Abstract
:1. Introduction
2. CRT Indications
3. Conduction System Pacing (CSP) Indications
4. LBBB Definitions
5. Technical Challenges on LBBAP
6. Current Studies and Clinical Significance
7. Advantages
8. Disadvantages
9. Our Center’s Experience—Proposed Implantation Algorithm
10. Patient’s Criteria Indicating Need for LOT-CRT
11. Limitations
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Normand, C.; Linde, C.; Blomström-Lundqvist, C.; Stellbrink, C.; Gasparini, M.; Anker, S.D.; Plummer, C.; Sarigul, N.U.; Papiashvili, G.; Iovev, S.; et al. Adherence to ESC cardiac resynchronization therapy guidelines: Findings from the ESC CRT Survey II. Europace 2020, 22, 932–938. [Google Scholar] [CrossRef]
- Herweg, B.; Ali, R.; Ilercil, A.; Madramootoo, C.; Cutro, R.; Weston, M.W.; Barold, S.S. Site-specific differences in latency intervals during biventricular pacing: Impact on paced QRS morphology and echo-optimized V-V interval. Pacing Clin. Electrophysiol. 2010, 33, 1382–1391. [Google Scholar] [CrossRef]
- Jastrzebski, M.; Wiliński, J.; Fijorek, K.; Sondej, T.; Czarnecka, D. Mortality and morbidity in cardiac resynchronization patients: Impact of lead position, paced left ventricular QRS morphology and other characteristics on long-term outcome. Europace 2013, 15, 258–265. [Google Scholar] [CrossRef]
- Hsu, J.C.; Solomon, S.D.; Bourgoun, M.; McNitt, S.; Goldenberg, I.; Klein, H.; Moss, A.J.; Foster, E.; MADIT-CRT Executive Committee. Predictors of super-response to cardiac resynchronization therapy and associated improvement in clinical outcome: The MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy) study. J. Am. Coll. Cardiol. 2012, 59, 2366–2373. [Google Scholar] [CrossRef] [PubMed]
- Proclemer, A.; Muser, D.; Facchin, D. What We Can Learn from “Super-Responders”. Heart Fail. Clin. 2017, 13, 225–232. [Google Scholar] [CrossRef]
- Su, L.; Wang, S.; Wu, S.; Xu, L.; Huang, Z.; Chen, X.; Zheng, R.; Jiang, L.; Ellenbogen, K.A.; Whinnett, Z.I.; et al. Long-Term Safety and Feasibility of Left Bundle Branch Pacing in a Large Single-Center Study. Circ. Arrhythmia Electrophysiol. 2021, 14, e009261. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraman, P.; Ponnusamy, S.; Cano, Ó.; Sharma, P.S.; Naperkowski, A.; Subsposh, F.A.; Moskal, P.; Bednarek, A.; Dal Forno, A.R.; Young, W.; et al. Left Bundle Branch Area Pacing for Cardiac Resynchronization Therapy: Results from the International LBBAP Collaborative Study Group. JACC Clin. Electrophysiol. 2021, 7, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qiu, C.; Xie, R.; Ma, W.; Wang, Z.; Li, H.; Wang, H.; Hua, W.; Zhang, S.; Yao, Y.; et al. Left bundle branch area pacing delivery of cardiac resynchronization therapy and comparison with biventricular pacing. ESC Heart Fail. 2020, 7, 1711–1722. [Google Scholar] [CrossRef]
- Vijayaraman, P. Left Bundle Branch Pacing Optimized Cardiac Resynchronization Therapy: A Novel Approach. JACC Clin. Electrophysiol. 2021, 7, 1076–1078. [Google Scholar] [CrossRef]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on Cardiac Pacing and Cardiac Resynchronization Therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef]
- Mauriello, A.; Ascrizzi, A.; Roma, A.S.; Molinari, R.; Caturano, A.; Imbalzano, E.; D’Andrea, A.; Russo, V. Effects of Heart Failure Therapies on Atrial Fibrillation: Biological and Clinical Perspectives. Antioxidants 2024, 13, 806. [Google Scholar] [CrossRef] [PubMed]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef]
- Chung, M.K.; Patton, K.K.; Lau, C.P.; Dal Forno, A.R.J.; Al-Khatib, S.M.; Arora, V.; Birgersdotter-Green, U.M.; Cha, Y.M.; Chung, E.H.; Cronin, E.M.; et al. 2023 HRS/APHRS/LAHRS guideline on cardiac physiologic pacing for the avoidance and mitigation of heart failure. Heart Rhythm 2023, 20, e17–e91. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, S.S.; Vijayaraman, P. Left Bundle Branch Block–Induced Cardiomyopathy: Insights from Left Bundle Branch Pacing. JACC Clin. Electrophysiol. 2021, 7, 1155–1165. [Google Scholar] [CrossRef]
- Huang, W.; Wu, S.; Vijayaraman, P.; Su, L.; Chen, X.; Cai, B.; Zou, J.; Lan, R.; Fu, G.; Mao, G.; et al. Cardiac Resynchronization Therapy in Patients with Nonischemic Cardiomyopathy Using Left Bundle Branch Pacing. JACC Clin. Electrophysiol. 2020, 6, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Su, L.; Vijayaraman, P.; Zheng, R.; Cai, M.; Xu, L.; Shi, R.; Huang, Z.; Whinnett, Z.I.; Huang, W. Left Bundle Branch Pacing for Cardiac Resynchronization Therapy: Nonrandomized On-Treatment Comparison with His Bundle Pacing and Biventricular Pacing. Can. J. Cardiol. 2021, 37, 319–328. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, J.; Qi, Y.; Wang, F.; Guo, L.; Shi, X.; Wu, W.; Zhou, X.; Li, R. Cardiac resynchronization therapy by left bundle branch area pacing in patients with heart failure and left bundle branch block. Heart Rhythm 2019, 16, 1783–1790. [Google Scholar] [CrossRef]
- Glikson, M.; Nielsen, J.C.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Brignole, M.; Burri, H.; Camm, A.J.; Daubert, J.C.; et al. European Society of Cardiology (ESC) clinical consensus statement on indications for conduction system pacing, with special contribution of the European Heart Rhythm Association of the ESC and endorsed by the Asia Pacific Heart Rhythm Society, the Canadian Heart Rhythm Society, the Heart Rhythm Society, and the Latin American Heart Rhythm Society. Europace 2025, 27, euaf050. [Google Scholar] [CrossRef]
- Surawicz, B.; Childers, R.; Deal, B.J.; Gettes, L.S. AHA/ACCF/HRS Recommendations for the Standardization and Interpretation of the Electrocardiogram. Part III: Intraventricular Conduction Disturbances A Scientific Statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Circulation 2009, 119, e235–e240. [Google Scholar] [CrossRef]
- Beela, A.S.; Rijks, J.H.J.; Manetti, C.A.; Vernooy, K.; van Stipdonk, A.M.W.; Prinzen, F.W.; Delhaas, T.; Herbots, L.; Lumens, J. Left bundle branch block criteria in the 2021 ESC guidelines on CRT: A step back in identifying CRT candidates? Eur. Heart J. Cardiovasc. Imaging 2024, 25, e213–e215. [Google Scholar] [CrossRef]
- Strauss, D.G.; Selvester, R.H.; Wagner, G.S. Defining left bundle branch block in the era of cardiac resynchronization therapy. Am. J. Cardiol. 2011, 107, 927–934. [Google Scholar] [CrossRef]
- Burri, H.; Jastrzebski, M.; Cano, Ó.; Čurila, K.; de Pooter, J.; Huang, W.; Israel, C.; Joza, J.; Romero, J.; Vernooy, K.; et al. EHRA clinical consensus statement on conduction system pacing implantation: Endorsed by the Asia Pacific Heart Rhythm Society (APHRS), Canadian Heart Rhythm Society (CHRS), and Latin American Heart Rhythm Society (LAHRS). Europace 2023, 25, 1208–1236. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Chen, X.; Wang, S.; Xu, L.; Xiao, F.; Huang, Z.; Zheng, R.; Jiang, L.; Vijayaraman, P.; Sharma, P.S.; et al. Evaluation of the Criteria to Distinguish Left Bundle Branch Pacing from Left Ventricular Septal Pacing. JACC Clin. Electrophysiol. 2021, 7, 1166–1177. [Google Scholar] [CrossRef] [PubMed]
- Jastrzębski, M.; Moskal, P.; Curila, K.; Wojdyła-Hordyńska, A.; Kiełbasa, G.; Bednarek, A.; Rajzer, M.; Vijayaraman, P.; Huang, W.; Heckman, L.I.; et al. Clinical Research Arrhythmias Left bundle branch area pacing outcomes: The multicentre European MELOS study. Eur. Heart J. 2022, 43, 4161–4173. [Google Scholar] [CrossRef]
- Jastrzębski, M.; Kiełbasa, G.; Curila, K.; Moskal, P.; Bednarek, A.; Rajzer, M.; Vijayaraman, P. Physiology-based electrocardiographic criteria for left bundle branch capture. Heart Rhythm 2021, 18, 935–943. [Google Scholar] [CrossRef]
- Diaz, J.C.; Tedrow, U.B.; Duque, M.; Aristizabal, J.; Braunstein, E.D.; Marin, J.; Niño, C.; Bastidas, O.; Lopez Cabanillas, N.; Koplan, B.A.; et al. Left Bundle Branch Pacing vs Left Ventricular Septal Pacing vs Biventricular Pacing for Cardiac Resynchronization Therapy. JACC Clin. Electrophysiol. 2024, 10, 295–305. [Google Scholar] [CrossRef] [PubMed]
- ujol-López, M.; Ferró, E.; Borràs, R.; Garre, P.; Guasch, E.; Jiménez-Arjona, R.; Garcia-Ribas, C.; Doltra, A.; Niebla, M.; Carro, E.; et al. Stepwise application of ECG and electrogram-based criteria to ensure electrical resynchronization with left bundle branch pacing. Europace 2023, 25, euad128. [Google Scholar] [CrossRef]
- Ali, N.; Arnold, A.D.; Miyazawa, A.A.; Keene, D.; Peters, N.S.; Kanagaratnam, P.; Qureshi, N.; Ng, F.S.; Linton, N.W.F.; Lefroy, D.C.; et al. Septal scar as a barrier to left bundle branch area pacing. Pacing Clin. Electrophysiol. 2023, 46, 1077–1084. [Google Scholar] [CrossRef]
- Upadhyay, G.A.; Cherian, T.; Shatz, D.Y.; Beaser, A.D.; Aziz, Z.; Ozcan, C.; Broman, M.T.; Nayak, H.M.; Tung, R. Intracardiac Delineation of Septal Conduction in Left Bundle-Branch Block Patterns: Mechanistic Evidence of Left Intrahisian Block Circumvented by His Bundle Pacing. Circulation 2019, 139, 1876–1888. [Google Scholar] [CrossRef]
- Jastrzębski, M.; Moskal, P.; Huybrechts, W.; Curila, K.; Sreekumar, P.; Rademakers, L.M.; Ponnusamy, S.S.; Herweg, B.; Sharma, P.S.; Bednarek, A.; et al. Left bundle branch–optimized cardiac resynchronization therapy (LOT-CRT): Results from an international LBBAP collaborative study group. Heart Rhythm 2022, 19, 13–21. [Google Scholar] [CrossRef]
- Chen, X.; Ye, Y.; Wang, Z.; Jin, Q.; Qiu, Z.; Wang, J.; Qin, S.; Bai, J.; Wang, W.; Liang, Y.; et al. Cardiac resynchronization therapy via left bundle branch pacing vs. optimized biventricular pacing with adaptive algorithm in heart failure with left bundle branch block: A prospective, multi-centre, observational study. Europace 2022, 24, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.F.; Yang, L.C.; Zhao, Y.; Yu, Y.C.; Liu, B.; Li, Y.G. Effects of adaptive left bundle branch–optimized cardiac resynchronization therapy: A single centre experience. BMC Cardiovasc. Disord. 2022, 22, 360. [Google Scholar] [CrossRef]
- Parale, C.; Bootla, D.; Jain, A.; Satheesh, S.; Anantharaj, A.; Ahmed, A.S.; Sukumaran, S.K.; Balaguru, S.; Selvaraj, R. Comparison of electrocardiographic parameters between left bundle optimized cardiac resynchronization therapy (LOT-CRT) and left bundle branch pacing—Cardiac resynchronization therapy (LBBP-CRT). Pacing Clin. Electrophysiol. 2023, 46, 840–847. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Bai, Y.; Wang, J.; Qin, S.; Bai, J.; Wang, W.; Liang, Y.; Chen, H.; Su, Y.; et al. Electrical Resynchronization and Clinical Outcomes During Long-Term Follow-Up in Intraventricular Conduction Delay Patients Applied Left Bundle Branch Pacing-Optimized Cardiac Resynchronization Therapy. Circ. Arrhythmia Electrophysiol. 2023, 16, E011761. [Google Scholar] [CrossRef] [PubMed]
- Ezer, P.; Szűcs, K.; Lukács, R.; Bisztray, T.; Vilmányi, G.; Szokodi, I.; Komócsi, A.; Kónyi, A. The Suboptimal QLV Ratio May Indicate the Need for a Left Bundle Branch Area Pacing-Optimized Cardiac Resynchronization Therapy Upgrade. J. Clin. Med. 2024, 13, 5742. [Google Scholar] [CrossRef] [PubMed]
- Ribes, F.; Perez-Rosello, V.; Gunturiz-Beltran, C.; De La Cruz-Cereceda, S.; Bellver-Navarro, A. QRS Narrowing in Patients Undergoing Cardiac Resynchronization Therapy: LOT-CRT vs. LBBAP. Europace 2024, 26 (Suppl. S1), euae102.468. [Google Scholar] [CrossRef]
- Jastrzębski, M.; Foley, P.; Chandrasekaran, B.; Whinnett, Z.; Vijayaraman, P.; Upadhyay, G.A.; Schaller, R.D.; Gardas, R.; Richardson, T.; Kudlik, D.; et al. Multicenter Hemodynamic Assessment of the LOT-CRT Strategy: When Does Combining Left Bundle Branch Pacing and Coronary Venous Pacing Enhance Resynchronization? Primary Results of the CSPOT Study. Circ. Arrhythmia Electrophysiol. 2024, 17, e013059. [Google Scholar] [CrossRef]
- Zucchelli, G.; Soldati, E.; Di Cori, A.; De Lucia, R.; Segreti, L.; Solarino, G.; Borelli, G.; Di Bello, V.; Bongiorni, M.G. Role of intraoperative electrical parameters in predicting reverse remodelling after cardiac resynchronization therapy and correlation with interventricular mechanical dyssynchrony. Europace 2010, 12, 1453–1459. [Google Scholar] [CrossRef]
- Strisciuglio, T.; Ammirati, G.; Pergola, V.; Imparato, L.; Carella, C.; Koci, E.; Chiappetti, R. Contrast-induced nephropathy after cardiac resynchronization therapy implant impairs the recovery of ejection fraction in responders. ESC Heart Fail. 2019, 6, 1266–1273. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, H.; Li, X.; Yao, Y.; Liu, Z.; Fan, X. Comparison of Procedure and Fluoroscopy Time Between Left Bundle Branch Area Pacing and Right Ventricular Pacing for Bradycardia: The Learning Curve for the Novel Pacing Strategy. Front. Cardiovasc. Med. 2021, 8, 695531. [Google Scholar] [CrossRef]
- Imnadze, G.; Bocchini, Y.; Fink, T.; Eitz, T.; Sciacca, V.; Niehaus, C.; El Hamriti, M.; Khalaph, M.; Guckel, D.; Braun, M.; et al. Dual-Chamber CRT-D Using ICD Lead Implantation in the LBBAP Position. Eur. Heart J. 2024, 45 (Suppl. S1), ehae666.725. [Google Scholar] [CrossRef]
- Yoshida, K.; Yoshino, M.; Kawabata, T.; Tasaka, H.; Kadota, K. Cardiac resynchronization therapy-defibrillator implantation with shock lead placement in the left bundle branch area: A case report. Eur. Heart J. Case Rep. 2024, 8, ytae323. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Qin, C.; Du, A.; Wang, Q.; He, C.; Zou, F.; Li, X.; Tao, J.; Wang, C.; Liu, Z.; et al. Comparisons of long-term clinical outcomes with left bundle branch pacing, left ventricular septal pacing, and biventricular pacing for cardiac resynchronization therapy. Heart Rhythm 2024, 21, 1342–1353. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, S.S.; Ganesan, V.; Ramalingam, V.; Syed, T.; Mariappan, S.; Murugan, S.; Kumar, M.; Anand, V.; Murugan, M.; Vijayaraman, P. MAgnetic resonance imaging based DUal lead cardiac Resynchronization therapy: A prospectIve Left Bundle Branch Pacing study (MADURAI LBBP study). Heart Rhythm 2023, 20, 1119–1127. [Google Scholar] [CrossRef]
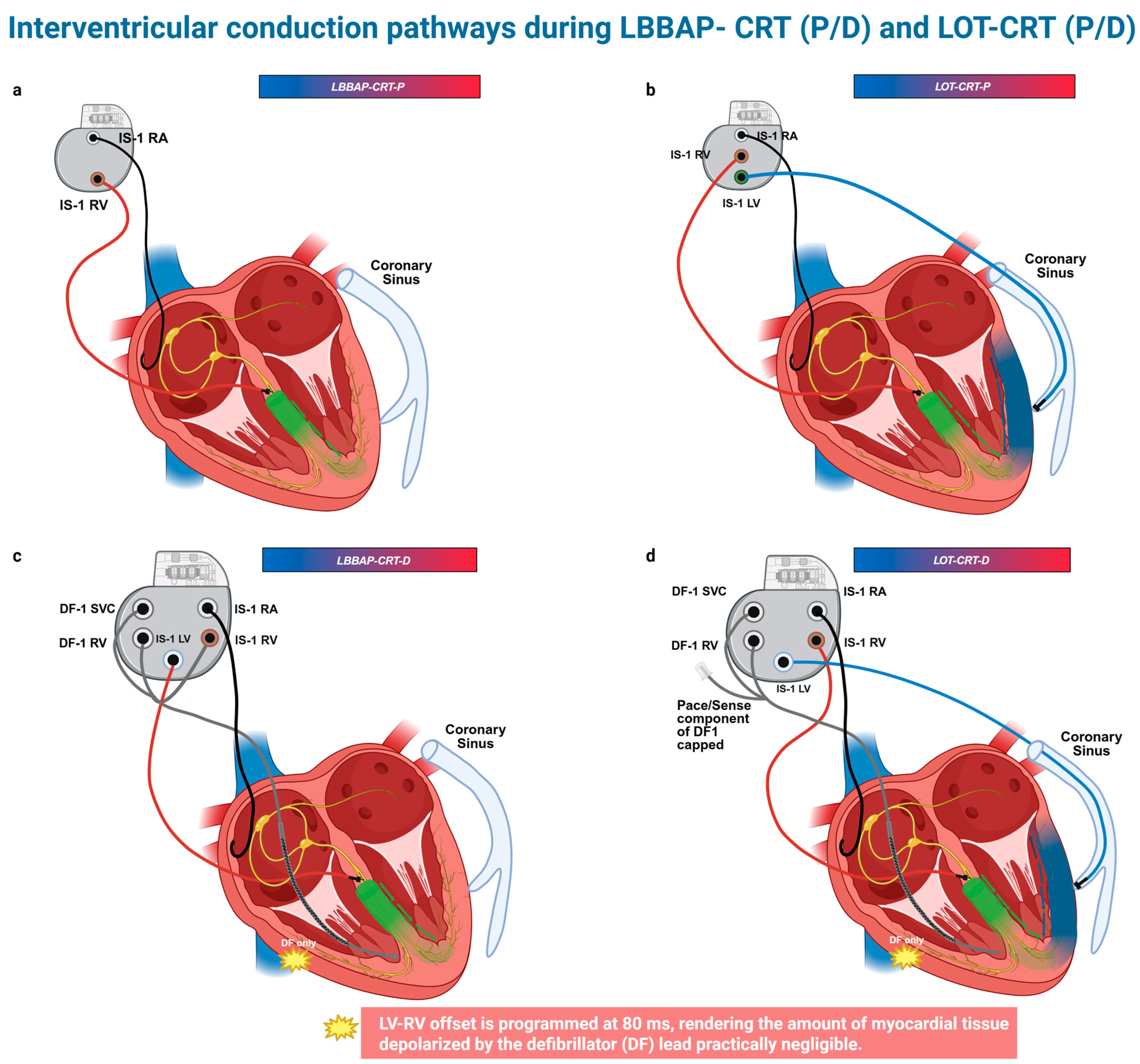

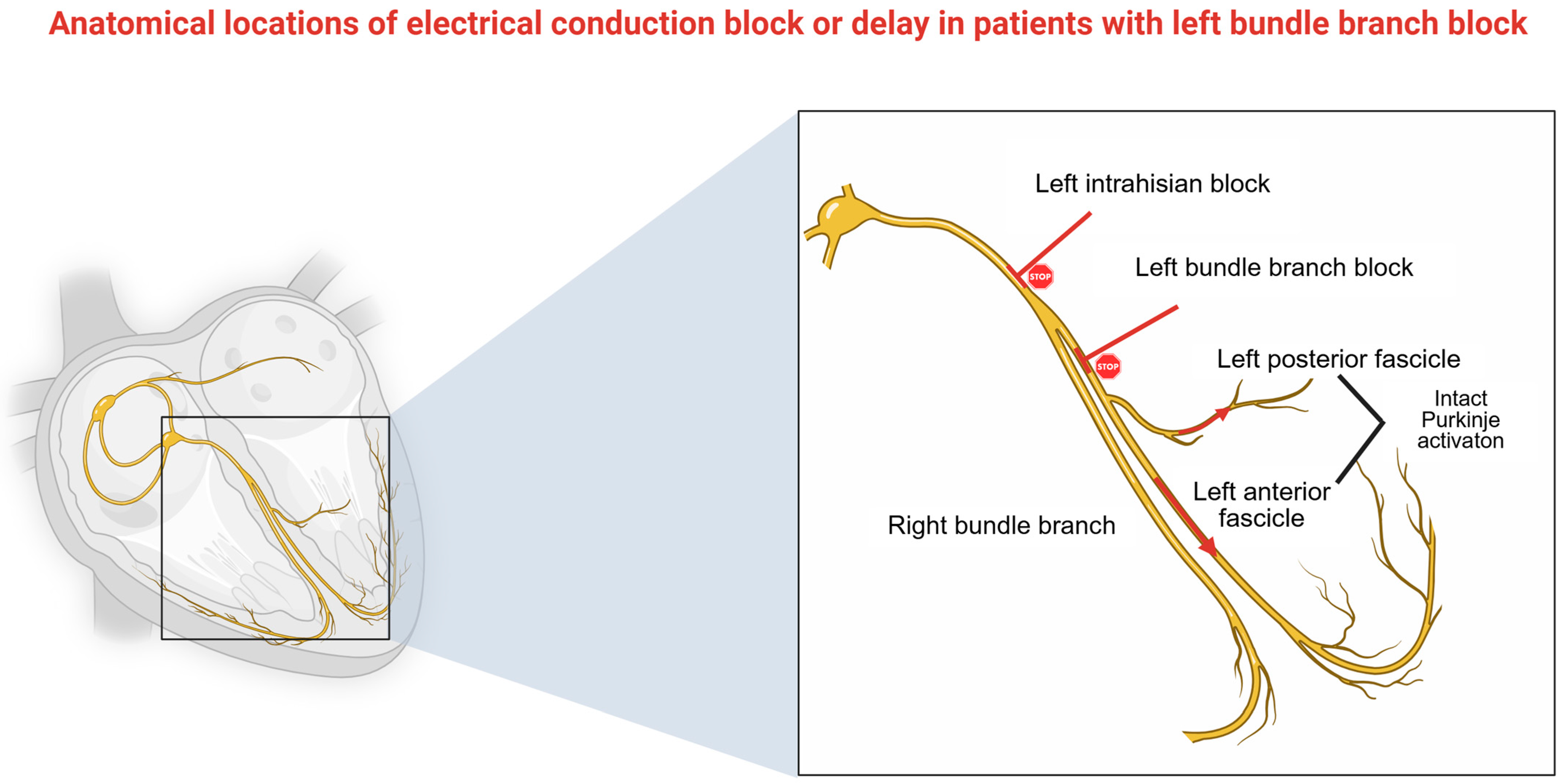

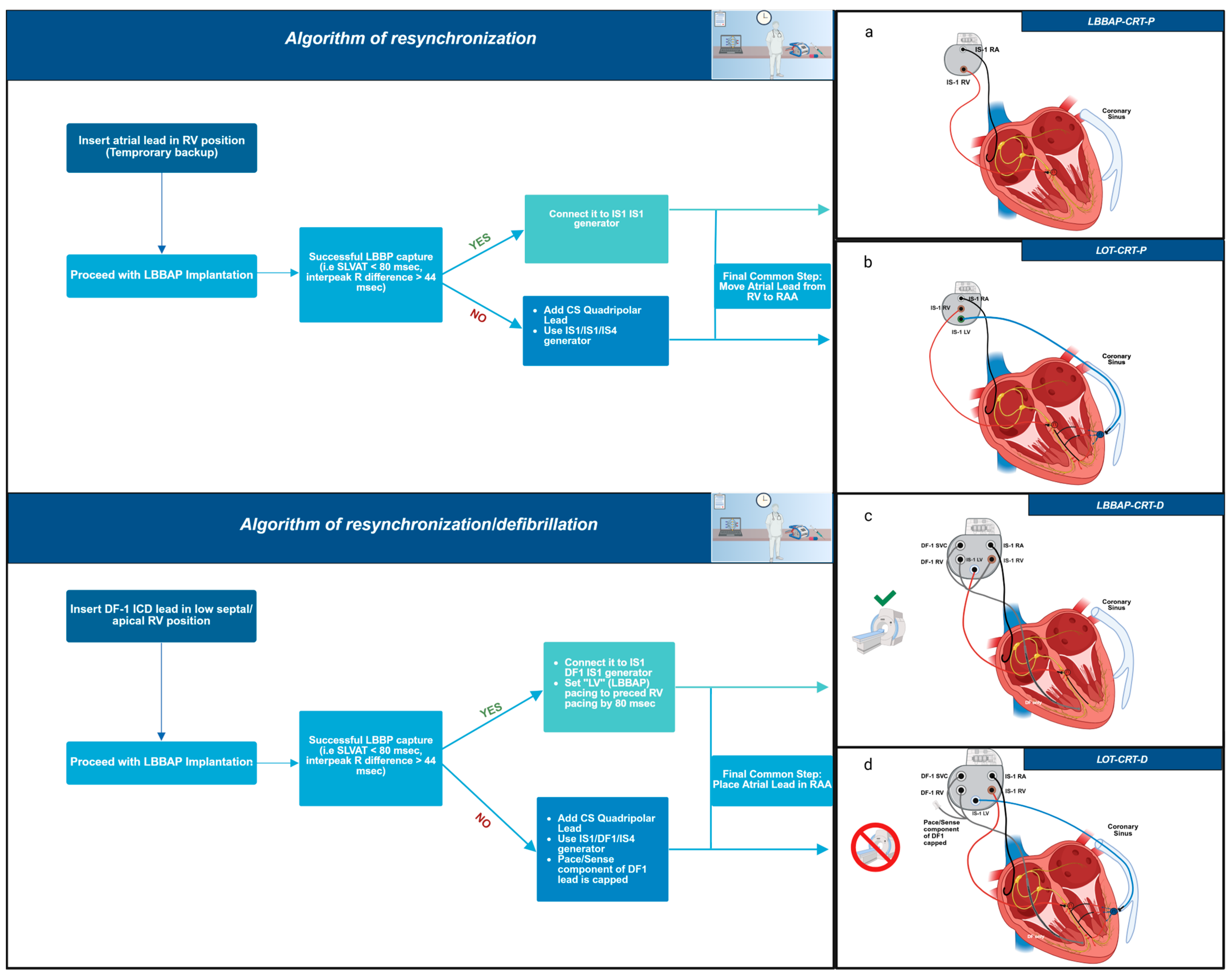
| Criteria | Strauss Criteria (2011) | ESC Guidelines 2021 | 2018 Bradycardia Clinical Practice Guidelines: Executive Summary 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients |
|---|---|---|---|
| QRS durations | ≥130 ms for women. ≥140 ms for men. | ≥120 ms | ≥120 ms |
| QRS morphology | Broad, notched or slurred R wave. Broad, notched or slurred mid-QRS ≥ 2 of V1, V2, V5, V6, I and aVL leads. | Notches or slurring in middle third of QRS in at least two of V1, V2, V5, V6, I, and aVL. Frontal Plane: R wave in I and aVL often with negative asymmetric T wave. Horizontal plane: unique Q wave in V6 with negative asymmetrical T wave. QRS axis is variable. | Broad, notched or slurred R wave in I, avL, V1, V2, V5, V6. Occasional RS pattern in V5, V6. |
| Q waves | QS or rS in V1 | Frontal plane: QS in aVR, with positive T wave. Horizontal plane: QS or rS in V1 with small ‘r’ with ST slightly elevated and positive asymmetrical T wave. | Absent Q wave in I, V5, V6, but in aVL a narrow Q wave may be present in the absence of myocardial pathology. |
| R peak time | N/A | Prolonged delayed peak in R in V5–V6 > 60 ms. | >60 ms in V5 and V6 but normal in V1, V2 and V3 with small initial R waves. |
| ST and T wave changes | N/A | When QRS < 140 ms, T wave in V6 may be positive. Frontal plane: ST depression. ST segment slightly opposed to QRS polarity, particularly when it is 140 ms at least and is rapidly followed by an asymmetrical T wave with also opposed polarity. | ST and T waves usually in opposite direction to QRS. |
| Study Writers or Name [Year, Patients (n)] | Study Design | Study Population (Indication, N, Type of Cardiomyopathy) | Success of Procedure and LBBAP Capture | Primary and Secondary Endpoints | Outcomes |
|---|---|---|---|---|---|
| Jastrzębski et al. (2021, 112) [30] | -Prospective observational multicenter, non-randomized, single-arm -LBBAP-optimized CRT (LOT-CRT) | -Patients qualified for CRT and patients with CRT that were not responders -112 -ICM: 68/112 (61%) NICM: 44/112 (39%) | -91/112 had successful implantation -LBB capture: 68/91 (75%) LVS:23/91 (25%) | Primary endpoint: ECG, echocardiographic and clinical response at 3 months | -QRS duration: from 188 ± 26 ms to 144 ± 22 ms (p < 0.0001) -LVEF: from 28.5% ± 9.9% to 37.2% ± 6.12 (p < 0.0001). -LVEDD: from 62.0 ± 8.9 to 59.1 ± 9.1 (p = 0.0442) -LVEDV and LVESV: from 209.8 ± 99 to 171.4 ± 83 (p < 0.0001), from 149.5 ± 84 to 110.6 ± 69 (p < 0.0001), respectively. -NYHA: 2.9 ± 0.6 to 1.9 ± 0.6 (p < 0.0001) -NT-pro-BNP: from 5668 ± 8249 pg/mL to 2561 ± 3555 pg/mL (p < 0.0001) |
| Chen et al. (2022, 100) [31] | -Non-randomized, prospective observational study multicenter -LBBAP-CRT (LOT) vs. BVP-aCRT | -HFrEF with LVEF < 35% and LBBB -LBBAP-CRT: 49 BVP-aCRT: 51 -LBBAP-CRT: DCM: 36/49 (73.47%) BVP-aCRT: DCM: 41/51 (80.39%) | LBBAP-CRT: 49 -1 failed and 4 were added from BVP-aCRT | Comparison of electromechanical effects and clinical efficacy of LOT-CRT at 6 months and 1 year | -QRS duration: Fused LBBAP vs. -BVP-aCRT (102.61 ± 9.66 ms vs. 126.54 ± 11.67, p < 0.001) -LVEF: LBBAP vs. BiV-aCRT (47.58 ± 12.02% vs. 41.24 ± 10.56% p = 0.008 at 6 months and 49.10 ± 10.43% vs. 43.62 ± 11.33%, p = 0.021 at 1 year). In super responders LVEF had a great increase in LBBAP-CRT vs. -BVP-aCRT (from 53.06% to 61.22% vs. 36.59% to 38.22%, respectively, p < 0.001) -LVEDD: LBBAP vs. BiV-aCRT (57.30 ± 8.00 mm vs. 62.40 ± 10.07 ms, p = 0.015 at 6 months and at 1 year 54.50 ± 6.13 vs. 60.99 ± 10.68 mm, p = 0.001) -LVSDD: LBBAP vs. BiV-aCRT (43.40 ± 9.66 ms vs. 49.44 ± 11.51 mm, p = 0.014 at 6 months and at one year 41.78 ± 9.05 vs. 48.33 ± 12.63 mm, p = 0.007) -NYHA: LBBAP-CRT group vs. BiV-aCRT, (4.08% vs. 19.61% p = 0.028) at 1 year |
| BVP-aCRT: 51/55 (5 were converted in LBBAP-CRT) | |||||
| Feng et al. (2022) [32] | -Prospective, observational, single center. -LOT-aCRT vs. BiV-aCRT | -HF with LVEF ≤ 35% and LBBB -LOT-aCRT: 10 BiV-aCRT: 11 -LOT-aCRT: ICM: 6/10 (60%) BiV-aCRT: ICM: 5/11 (45.5%) | LBB capture: 7/10 (70%) | Feasibility, safety and efficacy of LOT-aCRT, 9-month follow-up | -QRS duration: LOT-CRT group (unipolar LBBAP-LOT-BiV-CRT) vs. BiV-CRT group (from 168.1 ± 18.9 to 123.0 ± 5.7 ms with unipolar LBBAP, p = 0.01, to 121 ± 3.8 ms with LOT, p > 0.05 and from 158.0 ± 13.0 ms to 132.0 ± 4.5 ms p = 0.019 with BiV pacing vs. from 176.7 ± 19.7 ms to 133.3 ± 8.2 ms in the BiV group, p = 0.011) -LVEF: LOT-aCRT vs. BiV-aCRT (32.0 ± 4.2 to 45.0 ± 5.1%, p = 0.011 vs. 34.0 ± 1.3% to 45.8 ± 12.0% p = 0.143, respectively) -NT-pro-BNP: LOT-aCRT vs. BiV-aCRT (from 3240 ± 2258 pg/mL to 1151 ± 1774 pg/mL, p = 0.04) vs. 2684 ± 1083 pg/mL to 2066 ± 1444, p = 0.219 -NYHA: LOT-aCRT vs. BiV-aCRT (3.4 ± 0.55 to 2.4 ± 0.55 at 9 months, p = 0.032 vs. 3.3 ± 0.52 to 2.5 ± 0.55, p = 0.024) |
| Parale et al. (2023) [33] | -Prospective, Cross-sectional, single center. - LOT-CRT vs. LBBAP-CRT on the same patient population with three pacing modalities (AAI, DDD from LBBAP and DDD for LBB and LV pacing) | -NICMP with LVEF < 35% and LBBB -24 -N/A | LBB capture: 21/24 had successful LBB pacing | Primary: QRS duration Secondary: TpTe, QT interval | -QRS duration: LOT-CRT vs. LBBAP-CRT (from 167 ± 21.2 ms to 129.5 ± 18.6 ms, p = 0.01 vs. from 167 ± 21.2 ms to 134.5 ± 23.6 ms, p < 0.001, respectively) -QRS area: LOT-CRT vs. LBBAP-CRT (from 148.3 ± 80.5 μVs to 63.8 ± 34.9 μVs p < 0.0001 vs. from 148.3 ± 80.5 μVs to 94.2 ± 61.6 μVs, p < 0.001) -TpTe: LOT-CRT vs. LBBAP-CRT (from 94.6 ± 22.3 ms to 66.2 ± 21.9 ms, p < 0.001 vs. 94.6 ± 22.3 ms to 75.8 ± 21.2 ms, p < 0.001, respectively) -TpTe/QT ratio: LOT-CRT vs. LBBAP-CRT (0.22 ± 0.04 to 0.16 ± 0.04, p < 0.001 vs. 0.22 ± 0.04 to 0.19 ± 0.04, p = 0.05) -IVMD: LOT-CRT vs. LBBAP-CRT (78.3 ± 22 ms to 17.6 ± 13.8 ms, p < 0.001 vs. 78.3 ± 22 ms to 17.5 ± 13.9 ms, p < 0.001) |
| Chen et al. (2023, 85) [34] | -Prospective, observational. -LOT-CRT vs. BiV-CRT | -IVCD with LVEF ≤ 35% -LOT-CRT: 30 BiV-CRT: 55 -LOT-CRT: ICM: 10/30 (33.3%) BiV-CRT: ICM: 12/55 (21.8%) | LOT-CRT: 30/31 (crossover to BiV-CRT) BiV-CRT: 55/56 (one crossover from the LOT-CRT group, one failure of CS lead) | Echocardiographic and clinical characteristics at 2-year follow-up | -QRS duration: LOT-CRT vs. BiV-CRT (183.6 ± 20.3 ms to 140.9 ± 17.6 ms vs. 176 ± 19.9 ms to 154.1 ± 20.2 ms, respectively, p < 0.005) -LVEF: LOT-CRT vs. BiV-CRT (30 ± 7.3% to 36.7 ± 9.8% at 6 months, p < 0.01 and to 37 ± 9.5% at 24 months vs. 29.1 ± 6.8% to 30.5 ± 7% at 24 months) -LVESV: LOT-CRT vs. BiV-CRT (198.4 ± 74.6 mL to 161.1 ± 68.9 mL, p = 0.0018 vs. 203.91 ± 70.7 to 203.8 ± 68.5 mL) -LVEDV: LOT-CRT vs. BiV-CRT (275 ± 73.5 to 252.3 ± 80, p = 0.0494 vs. 280.4 ± 81.5 to 289.5 ± 81) -NYHA: LOT-CRT vs. BiV-CRT (2.9 to 2.2, p = 0.0006 vs. 2.9 to 2.6, p = 0.0067) -NT-pro-BNP: LOT-CRT vs. BiV-CRT (4073 to 1794 μg/mL, p = 0.0048 vs. 4431 to 3036, p = 0.0027, respectively) |
| Ezer et al. (2024, 68) [35] | -Prospective, observational, single center -LOT-CRT vs. BiV-CRT based on the QLV ratio | -HFrEF with IVCD (LBBB or ns-IVCD) -LOT-CRT with QLV ratio < 70%: 28 BiV-CRT with QLV ratio > 80%: 12 BiV-CRT with QLV ratio 70–80%: 28 -LOT-CRT: Ns-IVCD: 10/28 (34%) BiV-CRT: Ns-IVCD: 8/40 (20%) | LBB capture: 20/28 (71%) | ECG and echocardiographic characteristics at 12-month follow-up | -QRS duration: LOT-CRT vs. BiV-CRT (a reduction of 40.4 ± 14 ms vs. 32 ± 13 ms, p = 0.024, respectively) -LVEF: LOT-CRT vs. BiV-CRT (increase of 14.9 ± 8% vs. 10.3 ± 7.4% p = 0.001, respectively). -NYHA: LOT-CRT vs. BiV-CRT (decrease to 1.2 ± 0.5 vs. 0.8 ± 0.4, p = 0.031, respectively) -NT-pro-BNP: LOT-CRT vs. BiV-CRT (1863 ± 380 pg/mL vs. 1238 ± 412 pg/mL, p = 0.012, respectively) |
| LVS: 8/28 (28%) | |||||
| Ribes et al. (2024, 38) [36] | -Prospective, observational. -LOT-CRT-P vs. LBBAP | -HF with LVEF ≤ 35% and LBBB -38 -55.3% idiopathic cardiomyopathy, 21.1% ICM | N/A | Determinations of the predictors of better QRS narrowing, 1-year follow-up | -QRS duration: LOT-CRT vs. LBBAP-CRT (from 180 ± 22 ms to 132 ± 16 ms vs. from 180 ± 22 to 152 ± 16 ms, respectively, p < 0.001). |
| CSPOT (2024, 48) [37] | -Prospective, multicenter -LOT-CRT vs. BiV-CRT vs. unipolar LBBAP/bipolar LBBAP | -IVCD and non-LBBB -48 -ICM: 14/48 (29%) | LBBAP: 27/48 (56%) DSP: 21/48 (44%) | Hemodynamic and electrocardiographic effect in CRT eligible patients | -LV dP/dtmax: LOT-CRT increase of (25.8% [95% CI, 20.9–30.7%] and BiV-CRT 26.4% [ 95% CI, 20.2–32.6%]), unipolar LBBAP (19.3% [95% CI, 15.0–23.7%]) or bipolar LBBAP (16.4% [95% CI, 12.7–20.0%]) -QRS shortening: LOT-CRT (29.5 [95% CI, 23.4–35.6] ms) from a baseline of 171 ± 21 ms, BiV-CRT (18.5 [95% CI, 11.0–25.9] ms, p = 0.005) bipolar LBBAP (11.7 [95% CI, 6.4–17.0] ms, p < 0.001) unipolar LBBAP (11.9 [95% CI, 6.1–17.7] ms, p < 0.01). |
| BATTLE NCT 06061627 (ONGOING 2023–2027 estimated time completion) | -Prospective, multicenter, randomized controlled study -LOT-CRT vs. BiV-CRT | -HF with NICD -83 -N/A | N/A | LV systolic function in 6 months | N/A |
| RESCUE NCT 06148571 (ONGOING 2023–2026 estimated time completion) | -Prospective, observational, cohort study -LBBAP or LOT-CRT as a rescue therapy for BiV-CRT | -LBBB and HF -200 N/A | N/A | Acute complications at 1 year and successful LBBP | N/A |
| Study or Writer (Year) | Fluoroscopy Time (min) | Procedure’s Duration (min) |
|---|---|---|
| Jastrzębski et al. (2021) [30] | 27.3 ± 22 | N/A |
| Chen et al. (2022) [31] | LOT-CRT: 9.50 ± 1.99 | N/A |
| BiV-CRT: 13.84 ± 5.47 | ||
| Feng et al. (2022) [32] | LOT-aCRT: 29.2 ± 8.8 | LOT-aCRT: 152 ± 31 |
| BiV-aCRT: 21.8 ± 3.1 | BiV-aCRT: 122 ± 10 | |
| Parale et al. (2023) [33] | 20.8 ± 6.5 | 121.8 ± 22.9 |
| Chen et al. (2023) [34] | N/A | LOT-CRT: 126.5 ± 22.6 |
| BiV-CRT: 105.8 ± 18.1 | ||
| Ezer et al. (2024) [35] | LOT-CRT: 28 ± 15 | LOT-CRT: 119.5 ± 59.5 |
| BiV-CRT: 21 ± 8 | BiV-CRT: 89 ± 56 | |
| Ribes et al. (2024) [36] | N/A | N/A |
| CSPOT (2024) [37] | N/A | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leventopoulos, G.; Nastouli, K.-M.; Bozika, M.; Papastavrou, E.; Apostolos, A.; Koros, R.; Perperis, A.; Koniari, I.; Vlassopoulou, N.; Chronopoulos, P.; et al. Understanding LOT-CRT: Current Insights, Limitations, and Our Center’s Experience. J. Clin. Med. 2025, 14, 3025. https://doi.org/10.3390/jcm14093025
Leventopoulos G, Nastouli K-M, Bozika M, Papastavrou E, Apostolos A, Koros R, Perperis A, Koniari I, Vlassopoulou N, Chronopoulos P, et al. Understanding LOT-CRT: Current Insights, Limitations, and Our Center’s Experience. Journal of Clinical Medicine. 2025; 14(9):3025. https://doi.org/10.3390/jcm14093025
Chicago/Turabian StyleLeventopoulos, Georgios, Kassiani-Maria Nastouli, Maria Bozika, Eleni Papastavrou, Anastasios Apostolos, Rafail Koros, Angelos Perperis, Ioanna Koniari, Niki Vlassopoulou, Panagiotis Chronopoulos, and et al. 2025. "Understanding LOT-CRT: Current Insights, Limitations, and Our Center’s Experience" Journal of Clinical Medicine 14, no. 9: 3025. https://doi.org/10.3390/jcm14093025
APA StyleLeventopoulos, G., Nastouli, K.-M., Bozika, M., Papastavrou, E., Apostolos, A., Koros, R., Perperis, A., Koniari, I., Vlassopoulou, N., Chronopoulos, P., Travlos, C. K., Moulias, A., & Davlouros, P. (2025). Understanding LOT-CRT: Current Insights, Limitations, and Our Center’s Experience. Journal of Clinical Medicine, 14(9), 3025. https://doi.org/10.3390/jcm14093025







