Regulation of BDNF-TrkB Signaling and Potential Therapeutic Strategies for Parkinson’s Disease
Abstract
:1. Introduction
2. The General Function of BDNF/TrkB Signaling in Neuron
3. Correlation between BDNF/TrkB Signaling and PD
4. Correlation between BDNF/TrkB and α-Synuclein
5. The Function of TrkB Isoform in PD
6. BDNF/TrkB Signaling and Therapy for Parkinson’s Disease
6.1. Levodopa
6.2. Neupro (Rotigotine)
6.3. Selegiline (Deprenyl)
6.4. Azilect (Rasagiline)
6.5. Memantine
6.6. Amantadine
6.7. Cell Replacement Therapy
6.8. Other Agents
7. BDNF and TrkB Mutation and PD
8. Conclusions
Funding
Conflicts of Interest
References
- Arevalo, J.C.; Wu, S.H. Neurotrophin signaling: Many exciting surprises! Cell. Mol. Life Sci. 2006, 63, 1523–1537. [Google Scholar] [CrossRef] [PubMed]
- Zaccaro, M.C.; Ivanisevic, L.; Perez, P.; Meakin, S.O.; Saragovi, H.U. p75 co-receptors regulate ligand-dependent and ligand-independent Trk receptor activation, in part by altering Trk docking subdomains. J. Biol. Chem. 2001, 276, 31023–31029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castren, E.; Zafra, F.; Thoenen, H.; Lindholm, D. Light Regulates Expression of Brain-Derived Neurotrophic Factor Messenger-Rna in Rat Visual-Cortex. Proc. Natl. Acad. Sci. USA 1992, 89, 9444–9448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bracken, B.K.; Turrigiano, G.G. Experience-Dependent Regulation of TrkB Isoforms in Rodent Visual Cortex. Dev. Neurobiol. 2009, 69, 267–278. [Google Scholar] [CrossRef] [Green Version]
- Patterson, S.L.; Abel, T.; Deuel, T.A.S.; MArtin, K.C.; Rose, J.C.; Kandel, E.R. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron 1996, 16, 1137–1145. [Google Scholar] [CrossRef] [Green Version]
- Choo, M.; Miyazaki, T.; Yamazaki, M.; Kawamura, M.; Nakazawa, T.; Zhang, J.L.; Tanimura, A.; Uesaka, N.; Watanabe, M.; Sakimura, K.; et al. Retrograde BDNF to TrkB signaling promotes synapse elimination in the developing cerebellum. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Seroogy, K.B.; Lundgren, K.H.; Tran, T.M.D.; Guthrie, K.M.; Isackson, P.J.; Gall, C.M. Dopaminergic-Neurons in Rat Ventral Midbrain Express Brain-Derived Neurotrophic Factor and Neurotrophin-3 Messenger-Rnas. J. Comp. Neurol. 1994, 342, 321–334. [Google Scholar] [CrossRef]
- Ferrer, I.; Marin, C.; Rey, M.J.; Ribalta, T.; Goutan, E.; Blanco, R.; Tolosa, E.; Marti, E. BDNF and full-length and truncated TrkB expression in Alzheimer disease. Implications in therapeutic strategies. J. Neuropathol. Exp. Neurol. 1999, 58, 729–739. [Google Scholar] [CrossRef]
- Ohira, K.; Hayashi, M. Expression of TrkB subtypes in the adult monkey cerebellar cortex. J. Chem. Neuroanat. 2003, 25, 175–183. [Google Scholar] [CrossRef]
- Tang, S.; Machaalani, R.; Waters, K.A. Immunolocalization of pro- and mature-brain derived neurotrophic factor (BDNF) and receptor TrkB in the human brainstem and hippocampus. Brain Res. 2010, 1354, 1–14. [Google Scholar] [CrossRef]
- Liao, G.Y.; Kinney, C.E.; An, J.J.; Xu, B.J. TrkB-expressing neurons in the dorsomedial hypothalamus are necessary and sufficient to suppress homeostatic feeding. Proc. Natl. Acad. Sci. USA 2019, 116, 3256–3261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindsay, R.M.; Alderson, R.F.; Friedman, B.; Hyman, C.; Furth, M.E.; Maisonpierre, P.C.; Squinto, S.P.; Yancopoulos, G.D. The Neurotrophin Family of Ngf-Related Neurotrophic Factors. Restor. Neurol. Neurosci. 1991, 2, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.J.; Reichardt, L.F. Trk receptors: Roles in neuronal signal transduction. Annu. Rev. Biochem. 2003, 72, 609–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.J.; Welcher, A.A.; Shelton, D.; Schuman, E.M. Neurotrophins and time: Different roles for TrkB signaling in hippocampal long-term potentiation. Neuron 1997, 19, 653–664. [Google Scholar] [CrossRef] [Green Version]
- Numakawa, T.; Kumamaru, E.; Adachi, N.; Yagasaki, Y.; Izumi, A.; Kunugi, H. Glucocorticoid receptor interaction with TrkB promotes BDNF-triggered PLC-gamma signaling for glutamate release via a glutamate transporter. Proc. Natl. Acad. Sci. USA 2009, 106, 647–652. [Google Scholar] [CrossRef] [Green Version]
- Mattson, M.P. Glutamate and neurotrophic factors in neuronal plasticity and disease. Ann. N. Y. Acad. Sci. 2008, 1144, 97–112. [Google Scholar] [CrossRef] [Green Version]
- Gulyaeva, N.V. Interplay between Brain BDNF and Glutamatergic Systems: A Brief State of the Evidence and Association with the Pathogenesis of Depression. Biochem. (Mosc.) 2017, 82, 301–307. [Google Scholar] [CrossRef]
- Espinera, A.R.; Ogle, M.E.; Gu, X.; Wei, L. Citalopram Enhances Neurovascular Regeneration and Sensorimotor Functional Recovery after Ischemic Stroke in Mice. Neuroscience 2013, 247, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Mizuno, M.; Yamada, K.; Olariu, A.; Nawa, H.; Nabeshima, T. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. J. Neurosci. 2000, 20, 7116–7121. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.L.; Wang, H.L.; Wu, H.C.; Wei, C.L.; Lee, E.H.Y. Brain-derived neurotrophic factor antisense oligonucleotide impairs memory retention and inhibits long-term potentiation in rats. Neuroscience 1998, 82, 957–967. [Google Scholar] [CrossRef]
- Yamada, K.; Mizuno, M.; Nabeshima, T. Role for brain-derived neurotrophic factor in learning and memory. Life Sci. 2002, 70, 735–744. [Google Scholar] [CrossRef]
- Minichiello, L.; Korte, M.; Wolfer, D.; Kuhn, R.; Unsicker, K.; Cestari, V.; Rossi-Arnaud, C.; Lipp, H.P.; Bonhoeffer, T.; Klein, R. Essential role for TrkB receptors in hippocampus-mediated learning. Neuron 1999, 24, 401–414. [Google Scholar] [CrossRef] [Green Version]
- Elkouzi, A.; Vedam-Mai, V.; Eisinger, R.S.; Okun, M.S. Emerging therapies in Parkinson disease-repurposed drugs and new approaches. Nat. Rev. Neurol. 2019, 15, 204–223. [Google Scholar] [CrossRef] [PubMed]
- Lang, A.E.; Lozano, A.M. Parkinson’s disease-First of two parts. N. Engl. J. Med. 1998, 339, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Parain, K.; Murer, M.G.; Yan, Q.; Faucheux, B.; Agid, Y.; Hirsch, E.; Raisman-Vozari, R. Reduced expression of brain-derived neurotrophic factor protein in Parkinson’s disease substantia nigra. Neuroreport 1999, 10, 557–561. [Google Scholar] [CrossRef]
- Mogi, M.; Togari, A.; Kondo, T.; Mizuno, Y.; Komure, O.; Kuno, S.; Ichinose, H.; Nagatsu, T. Brain-derived growth factor and nerve growth factor concentrations are decreased in the substantia nigra in Parkinson’s disease. Neurosci. Lett. 1999, 270, 45–48. [Google Scholar] [CrossRef]
- Guillin, O.; Diaz, J.; Carroll, P.; Griffon, N.; Schwartz, J.C.; Sokoloff, P. BDNF controls dopamine D-3 receptor expression and triggers behavioural sensitization. Nature 2001, 411, 86–89. [Google Scholar] [CrossRef]
- Bruna, B.; Lobos, P.; Herrera-Molina, R.; Hidalgo, C.; Paula-Lima, A.; Adasme, T. The signaling pathways underlying BDNF-induced Nrf2 hippocampal nuclear translocation involve ROS, RyR-Mediated Ca2+ signals, ERK and PI3K. Biochem. Biophys. Res. Commun. 2018, 505, 201–207. [Google Scholar] [CrossRef]
- Nitti, M.; Piras, S.; Brondolo, L.; Marinari, U.M.; Pronzato, M.A.; Furfaro, A.L. Heme Oxygenase 1 in the Nervous System: Does It Favor Neuronal Cell Survival or Induce Neurodegeneration? Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [Green Version]
- Ryu, E.J.; Harding, H.P.; Angelastro, J.M.; Vitolo, O.V.; Ron, D.; Greene, L.A. Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson’s disease. J. Neurosci. 2002, 22, 10690–10698. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Bower, K.A.; Ma, C.L.; Fang, S.Y.; Thiele, C.J.; Luo, J. Glycogen synthase kinase 3 beta (GSK3 beta) mediates 6-hydroxydopamine-induced neuronal death. Faseb J. 2004, 18, 1162. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.W.; Ru, J.; Ma, W.; Gao, Y.; Liang, Z.; Liu, J.; Guo, J.H.; Li, L.Y. BDNF promotes the growth of human neurons through crosstalk with the Wnt/beta-catenin signaling pathway via GSK-3 beta. Neuropeptides 2015, 54, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.; Ohno, M. TrkB reduction exacerbates Alzheimer’s disease-like signaling aberrations and memory deficits without affecting beta-amyloidosis in 5XFAD mice. Trans. Psychiatry 2015, 5, e562. [Google Scholar] [CrossRef] [PubMed]
- Langston, J.W. The MPTP Story. J. Parkinsons Dis. 2017, 7, S11–S19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meredith, G.E.; Rademacher, D.J. MPTP mouse models of Parkinson’s disease: An update. J. Parkinsons Dis. 2011, 1, 19–33. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.X.; Xia, Y.; Jiao, X.Y.; Duan, L.; Yu, J.; Wang, X.; Chen, L.W. The TrkB-Positive Dopaminergic Neurons are Less Sensitive to MPTP Insult in the Substantia Nigra of Adult C57/BL Mice. Neurochem. Res. 2011, 36, 1759–1766. [Google Scholar] [CrossRef]
- Galpern, W.R.; Frim, D.M.; Tatter, S.B.; Altar, C.A.; Beal, M.F.; Isacson, O. Cell-mediated delivery of brain-derived neurotrophic factor enhances dopamine levels in an MPP+ rat model of substantia nigra degeneration. Cell Transplant. 1996, 5, 225–232. [Google Scholar] [CrossRef]
- Rudow, G.; O’Brien, R.; Savonenko, A.V.; Resnick, S.M.; Zonderman, A.B.; Pletnikova, O.; Marsh, L.; Dawson, T.M.; Crain, B.J.; West, M.J.; et al. Morphometry of the human substantia nigra in ageing and Parkinson’s disease. Acta Neuropathol. 2008, 115, 461–470. [Google Scholar] [CrossRef] [Green Version]
- Parkinson, G.M.; Dayas, C.V.; Smith, D.W. Age-related gene expression changes in substantia nigra dopamine neurons of the rat. Mech. Ageing Dev. 2015, 149, 41–49. [Google Scholar] [CrossRef]
- Lin, D.; Wu, Q.; Lin, X.Y.; Borlongan, C.V.; He, Z.X.; Tan, J.; Cao, C.H.; Zhou, S.F. Brain-derived Neurotrophic Factor Signaling Pathway: Modulation by Acupuncture in Telomerase Knockout Mice. Altern. Ther. Health Med. 2015, 21, 36–46. [Google Scholar]
- Scheffold, A.; Holtman, I.R.; Dieni, S.; Brouwer, N.; Katz, S.F.; Jebaraj, B.M.; Kahle, P.J.; Hengerer, B.; Lechel, A.; Stilgenbauer, S.; et al. Telomere shortening leads to an acceleration of synucleinopathy and impaired microglia response in a genetic mouse model. Acta Neuropathol. Commun. 2016, 4, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vera, E.; Bosco, N.; Studer, L. Generating Late-Onset Human iPSC-Based Disease Models by Inducing Neuronal Age-Related Phenotypes through Telomerase Manipulation. Cell Rep. 2016, 17, 1184–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, J.D.; Ganat, Y.M.; Kishinevsky, S.; Bowman, R.L.; Liu, B.; Tu, E.Y.; Mandal, P.K.; Vera, E.; Shim, J.W.; Kriks, S.; et al. Human iPSC-Based Modeling of Late-Onset Disease via Progerin-Induced Aging. Cell Stem Cell 2013, 13, 691–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lotharius, J.; Brundin, P. Pathogenesis of Parkinson’s disease: Dopamine, vesicles and alpha-synuclein. Nat. Rev. Neurosci. 2002, 3, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Papapetropoulos, S.; Mash, D.C. Alpha-synuclein aggregation and its relation to neurodegenerative diseases. Ann. Neurol. 2005, 57, 605; author reply 605–606. [Google Scholar] [CrossRef] [PubMed]
- Kohno, R.; Sawada, H.; Kawamoto, Y.; Uemura, K.; Shibasaki, H.; Shimohama, S. BDNF is induced by wild-type alpha-synuclein but not by the two mutants, A30P or A53T, in glioma cell line. Biochem. Biophys. Res. Commun. 2004, 318, 113–118. [Google Scholar] [CrossRef] [Green Version]
- Szego, E.M.; Gerhardt, E.; Outeiro, T.F.; Kermer, P. Dopamine-depletion and increased alpha-synuclein load induce degeneration of cortical cholinergic fibers in mice. J. Neurol. Sci. 2011, 310, 90–95. [Google Scholar] [CrossRef]
- Zhou, B.; Cai, Q.; Xie, Y.X.; Sheng, Z.H. Snapin Recruits Dynein to BDNF-TrkB Signaling Endosomes for Retrograde Axonal Transport and Is Essential for Dendrite Growth of Cortical Neurons. Cell Rep. 2012, 2, 42–51. [Google Scholar] [CrossRef] [Green Version]
- Volpicelli-Daley, L.A.; Gamble, K.L.; Schultheiss, C.E.; Riddle, D.M.; West, A.B.; Lee, V.M. Formation of alpha-synuclein Lewy neurite-like aggregates in axons impedes the transport of distinct endosomes. Mol. Biol. Cell 2014, 25, 4010–4023. [Google Scholar] [CrossRef]
- Kang, S.S.; Zhang, Z.; Liu, X.; Manfredsson, F.P.; Benskey, M.J.; Cao, X.; Xu, J.; Sun, Y.E.; Ye, K. TrkB neurotrophic activities are blocked by alpha-synuclein, triggering dopaminergic cell death in Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2017, 114, 10773–10778. [Google Scholar] [CrossRef] [Green Version]
- von Bohlen und Halbach, O.; Minichiello, L.; Unsicker, K. Haploinsufficiency for trkB and trkC receptors induces cell loss and accumulation of alpha-synuclein in the substantia nigra. Faseb J. 2005, 19, 1740. [Google Scholar] [CrossRef]
- Slaugenhaupt, S.A.; Blumenfeld, A.; Liebert, C.B.; Mull, J.; Lucente, D.E.; Monahan, M.; Breakefield, X.O.; Maayan, C.; Parada, L.; Axelrod, F.B.; et al. The Human Gene for Neurotrophic Tyrosine Kinase Receptor-Type-2 (Ntrk2) Is Located on Chromosome-9 but Is Not the Familial Dysautonomia Gene. Genomics 1995, 25, 730–732. [Google Scholar] [CrossRef]
- Haniu, M.; Talvenheimo, J.; Le, J.; Katta, V.; Welcher, A.; Rohde, M.F. Extracellular Domain of Neurotrophin Receptor Trkb-Disulfide Structure, N-Glycosylation Sites, and Ligand-Binding. Arch. Biochem. Biophys. 1995, 322, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Luberg, K.; Wong, J.; Weickert, C.S.; Timmusk, T. Human TrkB gene: Novel alternative transcripts, protein isoforms and expression pattern in the prefrontal cerebral cortex during postnatal development. J. Neurochem. 2010, 113, 952–964. [Google Scholar] [CrossRef] [PubMed]
- Fenner, M.E.; Achim, C.L.; Fenner, B.M. Expression of full-length and truncated trkB in human striatum and substantia nigra neurons: Implications for Parkinson’s disease. J. Mol. Histol. 2014, 45, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Fogerson, S.M.; van Brummen, A.J.; Busch, D.J.; Allen, S.R.; Roychaudhuri, R.; Banks, S.M.; Klarner, F.G.; Schrader, T.; Bitan, G.; Morgan, J.R. Reducing synuclein accumulation improves neuronal survival after spinal cord injury. Exp. Neurol. 2016, 278, 105–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, T.S.; Huang, Y.P.; Wang, H.I.; Pan, S.L. Spinal cord injury and Parkinson’s disease: A population-based, propensity score-matched, longitudinal follow-up study. Spinal Cord 2016, 54, 1215–1219. [Google Scholar] [CrossRef]
- King, V.R.; Bradbury, E.J.; McMahon, S.B.; Priestley, J.V. Changes in truncated trkB and p75 receptor expression in the rat spinal cord following spinal cord hemisection and spinal cord hemisection plus neurotrophin treatment. Exp. Neurol. 2000, 165, 327–341. [Google Scholar] [CrossRef]
- Liebl, D.J.; Huang, W.; Young, W.; Parada, L.F. Regulation of Trk receptors following contusion of the rat spinal cord. Exp. Neurol. 2001, 167, 15–26. [Google Scholar] [CrossRef]
- Wu, J.F.; Renn, C.L.; Faden, A.I.; Dorsey, S.G. TrkB.T1 Contributes to Neuropathic Pain after Spinal Cord Injury through Regulation of Cell Cycle Pathways. J. Neurosci. 2013, 33, 12447–12463. [Google Scholar] [CrossRef] [Green Version]
- Matyas, J.J.; O’Driscoll, C.M.; Yu, L.N.; Coll-Miro, M.; Daugherty, S.; Renn, C.L.; Faden, A.I.; Dorsey, S.G.; Wu, J.F. Truncated TrkB.T1-Mediated Astrocyte Dysfunction Contributes to Impaired Motor Function and Neuropathic Pain after Spinal Cord Injury. J. Neurosci. 2017, 37, 3956–3971. [Google Scholar] [CrossRef]
- Holt, L.M.; Hernandez, R.D.; Pacheco, N.L.; Ceja, B.T.; Hossain, M.; Olsen, M.L. Astrocyte morphogenesis is dependent on BDNF signaling via astrocytic TrkB.T1. Elife 2019, 8. [Google Scholar] [CrossRef]
- Ninkina, N.; Grashchuck, M.; Buchman, V.L.; Davies, A.M. TrkB variants with deletions in the leucine-rich motifs of the extracellular domain. J. Biol. Chem. 1997, 272, 13019–13025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baxter, G.T.; Radeke, M.J.; Kuo, R.C.; Makrides, V.; Hinkle, B.; Hoang, R.; MedinaSelby, A.; Coit, D.; Valenzuela, P.; Feinstein, S.C. Signal transduction mediated by the truncated trkB receptor isoforms, trkB.T1 and trkB.T2. J. Neurosci. 1997, 17, 2683–2690. [Google Scholar] [CrossRef] [PubMed]
- Stoilov, P.; Castren, E.; Stamm, S. Analysis of the human TrkB gene genomic organization reveals novel TrkB isoforms, unusual gene length, and splicing mechanism. Biochem. Biophys. Res. Commun. 2002, 290, 1054–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pramanik, S.; Sulistio, Y.A.; Heese, K. Neurotrophin Signaling and Stem Cells-Implications for Neurodegenerative Diseases and Stem Cell Therapy. Mol. Neurobiol. 2017, 54, 7401–7459. [Google Scholar] [CrossRef] [PubMed]
- Stojkovska, I.; Wagner, B.M.; Morrison, B.E. Parkinson’s disease and enhanced inflammatory response. Exp. Biol. Med. 2015, 240, 1387–1395. [Google Scholar] [CrossRef] [Green Version]
- Corrigan, F.; Vink, R.; Turner, R.J. Inflammation in acute CNS injury: A focus on the role of substance P. Br. J. Pharmacol. 2016, 173, 703–715. [Google Scholar] [CrossRef] [Green Version]
- Gardner, R.C.; Burke, J.F.; Nettiksimmons, J.; Goldman, S.; Tanner, C.M.; Yaffe, K. Traumatic brain injury in later life increases risk for Parkinson disease. Ann. Neurol. 2015, 77, 987–995. [Google Scholar] [CrossRef]
- Joyce, J.M.; Monchi, O.; Ismail, Z.; Kibreab, M.; Cheetham, J.; Kathol, I.; Sarna, J.; Martino, D.; Debert, C.T. The impact of traumatic brain injury on cognitive and neuropsychiatric symptoms of Parkinson’s disease. Int. Rev. Psychiatry 2019. [Google Scholar] [CrossRef]
- Irwin, D.J.; Lee, V.M.Y.; Trojanowski, J.Q. Parkinson’s disease dementia: Convergence of alpha-synuclein, tau and amyloid-beta pathologies. Nat. Rev. Neurosci. 2013, 14, 626–636. [Google Scholar] [CrossRef]
- Araki, K.; Yagi, N.; Aoyama, K.; Choong, C.J.; Hayakawa, H.; Fujimura, H.; Nagai, Y.; Goto, Y.; Mochizuki, H. Parkinson’s disease is a type of amyloidosis featuring accumulation of amyloid fibrils of alpha-synuclein. Proc. Natl. Acad. Sci. USA 2019, 116, 17963–17969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeronimo-Santos, A.; Vaz, S.H.; Parreira, S.; Rapaz-Lerias, S.; Caetano, A.P.; Buee-Scherrer, V.; Castren, E.; Valente, C.A.; Blum, D.; Sebastiao, A.M.; et al. Dysregulation of TrkB Receptors and BDNF Function by Amyloid-beta Peptide is Mediated by Calpain. Cereb Cortex 2015, 25, 3107–3121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Luo, D.; Ning, Z.; Rong, J.; Lao, L. Electro-Acupuncture Ameliorated MPTP-Induced Parkinsonism in Mice via TrkB Neurotrophic Signaling. Front. Neurosci. 2019, 13, 496. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Goke, J.; Cukuroglu, E.; Dranias, M.R.; VanDongen, A.M.J.; Stanton, L.W. Molecular Features Underlying Neurodegeneration Identified through In Vitro Modeling of Genetically Diverse Parkinson’s Disease Patients. Cell Rep. 2016, 15, 2411–2426. [Google Scholar] [CrossRef] [Green Version]
- Tomassoni-Ardori, F.; Fulgenzi, G.; Becker, J.; Barrick, C.; Palko, M.E.; Kuhn, S.; Koparde, V.; Cam, M.; Yanpallewar, S.; Oberdoerffer, S.; et al. Rbfox1 up-regulation impairs BDNF-dependent hippocampal LTP by dysregulating TrkB isoform expression levels. Elife 2019, 8. [Google Scholar] [CrossRef]
- Gandhi, K.R.; Saadabadi, A. Levodopa (L-Dopa); StatPearls: Treasure Island, FL, USA, 2019. [Google Scholar]
- Zhang, X.; Andren, P.E.; Svenningsson, P. Repeated l-DOPA treatment increases c-fos and BDNF mRNAs in the subthalamic nucleus in the 6-OHDA rat model of Parkinson’s disease. Brain Res. 2006, 1095, 207–210. [Google Scholar] [CrossRef]
- Rylander, D.; Parent, M.; O’Sullivan, S.S.; Dovero, S.; Lees, A.J.; Bezard, E.; Descarries, L.; Cenci, M.A. Maladaptive plasticity of serotonin axon terminals in levodopa-induced dyskinesia. Ann. Neurol. 2010, 68, 619–628. [Google Scholar] [CrossRef]
- Iderberg, H.; McCreary, A.C.; Varney, M.A.; Cenci, M.A.; Newman-Tancredi, A. Activity of serotonin 5-HT1A receptor ‘biased agonists’ in rat models of Parkinson’s disease and L-DOPA-induced dyskinesia. Neuropharmacology 2015, 93, 52–67. [Google Scholar] [CrossRef]
- Tronci, E.; Napolitano, F.; Munoz, A.; Fidalgo, C.; Rossi, F.; Bjorklund, A.; Usiello, A.; Carta, M. BDNF over-expression induces striatal serotonin fiber sprouting and increases the susceptibility to L-DOPA-induced dyskinesia in 6-OHDA-lesioned rats. Exp. Neurol. 2017, 297, 73–81. [Google Scholar] [CrossRef]
- Samadi, P.; Morisette, M.; Levesque, D.; Di Paolo, T. BDNF Levels Are Not Related with Levodopa-Induced Dyskinesias in MPTP Monkeys. Mov. Disord. 2010, 25, 116–121. [Google Scholar] [CrossRef]
- Scheller, D.; Ullmer, C.; Berkels, R.; Gwarek, M.; Lubbert, H. The in vitro receptor profile of rotigotine: A new agent for the treatment of Parkinson’s disease. Naunyn Schmiedebergs Arch. Pharm. 2009, 379, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.; Dubois, V.; Scheller, D.; Gillard, M. Rotigotine is a potent agonist at dopamine D-1 receptors as well as at dopamine D-2 and D-3 receptors. Br. J. Pharmacol. 2015, 172, 1124–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunten, S.; Happe, S. Rotigotine transdermal system: A short review. Neuropsychiatr. Dis. Treat. 2006, 2, 421–426. [Google Scholar] [CrossRef] [Green Version]
- Adachi, N.; Yoshimura, A.; Chiba, S.; Ogawa, S.; Kunugi, H. Rotigotine, a dopamine receptor agonist, increased BDNF protein levels in the rat cortex and hippocampus. Neurosci. Lett. 2018, 662, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Cai, D.; Bai, Y. Selegiline rescues gait deficits and the loss of dopaminergic neurons in a subacute MPTP mouse model of Parkinson’s disease. Int. J. Mol. Med. 2013, 32, 883–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishna, R.; Ali, M.; Moustafa, A.A. Effects of combined MAO-B inhibitors and levodopa vs. monotherapy in Parkinson’s disease. Front. Aging Neurosci. 2014, 6, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, D.Q.; Li, M.X.; Jiang, L.L.; Chen, X.B.; Zhou, X.W. Comparison of selegiline and levodopa combination therapy versus levodopa monotherapy in the treatment of Parkinson’s disease: A meta-analysis. Aging Clin. Exp. Res. 2019. [Google Scholar] [CrossRef]
- Nakaso, K.; Nakamura, C.; Sato, H.; Imamura, K.; Takeshima, T.; Nakashima, K. Novel cytoprotective mechanism of anti-parkinsonian drug deprenyl: PI3K and Nrf2-derived induction of antioxidative proteins. Biochem. Biophys. Res. Commun. 2006, 339, 915–922. [Google Scholar] [CrossRef]
- Mandel, S.A.; Sagi, Y.; Amit, T. Rasagiline promotes regeneration of substantia nigra dopaminergic neurons in Post-MPTP-induced parkinsonism via activation of tyrosine kinase receptor signaling pathway. Neurochem. Res. 2007, 32, 1694–1699. [Google Scholar] [CrossRef]
- Aarsland, D.; Ballard, C.; Walker, Z.; Bostrom, F.; Alves, G.; Kossakowski, K.; Leroi, I.; Pozo-Rodriguez, F.; Minthon, L.; Londos, E. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: A double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2009, 8, 613–618. [Google Scholar] [CrossRef]
- Emre, M.; Tsolaki, M.; Bonuccelli, U.; Destee, A.; Tolosa, E.; Kutzelnigg, A.; Ceballos-Baumann, A.; Zdravkovic, S.; Bladstrom, A.; Jones, R.; et al. Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: A randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010, 9, 969–977. [Google Scholar] [CrossRef]
- Zhu, G.Q.; Li, J.Y.; He, L.; Wang, X.C.; Hong, X.Q. MPTP-induced changes in hippocampal synaptic plasticity and memory are prevented by memantine through the BDNF-TrkB pathway. Br. J. Pharmacol. 2015, 172, 2354–2368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ossola, B.; Schendzielorz, N.; Chen, S.H.; Bird, G.S.; Tuominen, R.K.; Mannisto, P.T.; Hong, J.S. Amantadine protects dopamine neurons by a dual action: Reducing activation of microglia and inducing expression of GNDF in astroglia (vol 61, pg 574, 2011). Neuropharmacology 2012, 62, 1162–1162. [Google Scholar] [CrossRef]
- Pajo, A.T.; Espiritu, A.I.; Jamora, R.D.G. Efficacy and safety of extended-release amantadine in levodopa-induced dyskinesias: A meta-analysis. Neurodegener. Dis. Manag. 2019, 9, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Rogoz, Z.; Skuza, G.; Daniel, W.A.; Wojcikowski, J.; Dudek, D.; Wrobel, A. Amantadine as an additive treatment in patients suffering from drug-resistant unipolar depression. Pharmacol. Rep. 2007, 59, 778–784. [Google Scholar] [PubMed]
- Raupp-Barcaro, I.F.; Vital, M.A.; Galduroz, J.C.; Andreatini, R. Potential antidepressant effect of amantadine: A review of preclinical studies and clinical trials. Rev. Bras. Psiquiatr. 2018, 40, 449–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobkin, R.D.; Interian, A. Improved understanding, detection, and management of neuropsychiatric complications: Essential components to the optimal treatment of Parkinson’s disease. Int. Psychogeriatr. 2019, 31, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Bjorklund, A.; Lindvall, O. Cell replacement therapies for central nervous system disorders. Nat. Neurosci. 2000, 3, 537–544. [Google Scholar] [CrossRef]
- Emerich, D.F.; Hemendinger, R.; Halberstadt, C.R. The testicular-derived Sertoli cell: Cellular immunoscience to enable transplantation. Cell Transplant. 2003, 12, 335–349. [Google Scholar] [CrossRef]
- Wernig, M.; Zhao, J.P.; Pruszak, J.; Hedlund, E.; Fu, D.D.; Soldner, F.; Broccoli, V.; Constantine-Paton, M.; Isacson, O.; Jaenisch, R. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2008, 105, 5856–5861. [Google Scholar] [CrossRef] [Green Version]
- Soldner, F.; Hockemeyer, D.; Beard, C.; Gao, Q.; Bell, G.W.; Cook, E.G.; Hargus, G.; Blak, A.; Cooper, O.; Mitalipova, M.; et al. Parkinson’s Disease Patient-Derived Induced Pluripotent Stem Cells Free of Viral Reprogramming Factors (vol 136, pg 964, 2009). Cell 2009, 137, 1356–1356. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.L.; Yang, M.; Poremsky, E.; Kidd, S.; Schneider, J.S.; Iacovitti, L. Dopaminergic Neurons Derived from Human Induced Pluripotent Stem Cells Survive and Integrate into 6-OHDA-Lesioned Rats. Stem Cells Dev. 2010, 19, 1017–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grudina, C.G.; Nonaka, T.; Hasegawa, M.; Matsas, R.; Zurzolo, C. Human NPCs can degrade α-syn fibrils and transfer them preferentially in a cell contact-dependent manner possibly through TNT-like structures. Neurobiol. Dis. 2019. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Loria, F.; Vargas, J.Y.; Bousset, L.; Syan, S.; Salles, A.; Melki, R.; Zurzolo, C. alpha-Synuclein transfer between neurons and astrocytes indicates that astrocytes play a role in degradation rather than in spreading. Acta Neuropathol. 2017, 134, 789–808. [Google Scholar] [CrossRef] [PubMed]
- Galieva, L.R.; Mukhamedshina, Y.O.; Arkhipova, S.S.; Rizvanov, A.A. Human Umbilical Cord Blood Cell Transplantation in Neuroregenerative Strategies. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Sun, H.M.; Yan, J.H.; Xue, H.; Wu, B.; Dong, F.; Li, W.S.; Ji, F.Q.; Zhou, D.S. Conditioned medium from human amniotic epithelial cells may induce the differentiation of human umbilical cord blood mesenchymal stem cells into dopaminergic neuron-like cells. J. Neurosci. Res. 2013, 91, 978–986. [Google Scholar] [CrossRef]
- Kang, E.J.; Lee, Y.H.; Kim, M.J.; Lee, Y.M.; Kumar, B.M.; Jeon, B.G.; Ock, S.A.; Kim, H.J.; Rho, G.J. Transplantation of porcine umbilical cord matrix mesenchymal stem cells in a mouse model of Parkinson’s disease. J. Tissue Eng. Regen. Med. 2013, 7, 169–182. [Google Scholar] [CrossRef]
- Calon, F.; Lim, G.P.; Morihara, T.; Yang, F.S.; Ubeda, O.; Salem, N.; Frautschy, S.A.; Cole, G.M. Dietary n-3 polyunsaturated fatty acid depletion activates caspases and decreases NMDA receptors in the brain of a transgenic mouse model of Alzheimer’s disease. Eur. J. Neurosci. 2005, 22, 617–626. [Google Scholar] [CrossRef]
- Lim, G.P.; Calon, F.; Morihara, T.; Yang, F.S.; Teter, B.; Ubeda, O.; Salem, N.; Frautschy, S.A.; Cole, G.M. A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J. Neurosci. 2005, 25, 3032–3040. [Google Scholar] [CrossRef] [Green Version]
- Bousquet, M.; Gibrat, C.; Saint-Pierre, M.; Julien, C.; Calon, F.; Cicchetti, F. Modulation of brain-derived neurotrophic factor as a potential neuroprotective mechanism of action of omega-3 fatty acids in a parkinsonian animal model. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2009, 33, 1401–1408. [Google Scholar] [CrossRef]
- Liu, C.Y.; Chan, C.B.; Ye, K.Q. 7,8-dihydroxyflavone, a small molecular TrkB agonist, is useful for treating various BDNF-implicated human disorders. Transl. Neurodegener. 2016, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Chua, K.W.; Chua, C.C.; Yu, H.; Pei, A.; Chua, B.H.; Hamdy, R.C.; Xu, X.; Liu, C.F. Antioxidant activity of 7,8-dihydroxyflavone provides neuroprotection against glutamate-induced toxicity. Neurosci. Lett. 2011, 499, 181–185. [Google Scholar] [CrossRef]
- Sconce, M.D.; Churchill, M.J.; Moore, C.; Meshul, C.K. Intervention with 7,8-Dihydroxyflavone Blocks Further Striatal Terminal Loss and Restores Motor Deficits in a Progressive Mouse Model of Parkinson’s Disease. Neuroscience 2015, 290, 454–471. [Google Scholar] [CrossRef]
- Liu, X.; Obianyo, O.; Chan, C.B.; Huang, J.; Xue, S.; Yang, J.J.; Zeng, F.; Goodman, M.; Ye, K. Biochemical and biophysical investigation of the brain-derived neurotrophic factor mimetic 7,8-dihydroxyflavone in the binding and activation of the TrkB receptor. J. Biol. Chem. 2014, 289, 27571–27584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.H.; Dai, C.F.; Chen, L.; Zhou, W.T.; Han, H.L.; Dong, Z.F. 7,8-dihydroxyflavone Ameliorates Motor Deficits Via Suppressing alpha-synuclein Expression and Oxidative Stress in the MPTP-induced Mouse Model of Parkinson’s Disease. CNS Neurosci. Ther. 2016, 22, 617–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Xiang, Z.; Zhu, X.; Ai, Z.; Shen, J.; Huang, T.; Liu, L.; Ji, W.; Li, T. Neuroprotective Effects of 7, 8-dihydroxyflavone on Midbrain Dopaminergic Neurons in MPP(+)-treated Monkeys. Sci. Rep. 2016, 6, 34339. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chan, C.B.; Jang, S.W.; Pradoldej, S.; Huang, J.; He, K.; Phun, L.H.; France, S.; Xiao, G.; Jia, Y.; et al. A synthetic 7,8-dihydroxyflavone derivative promotes neurogenesis and exhibits potent antidepressant effect. J. Med. Chem. 2010, 53, 8274–8286. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Wang, Z.; Zhang, Z.; Liu, X.; Kang, S.S.; Zhang, Y.; Ye, K. The prodrug of 7,8-dihydroxyflavone development and therapeutic efficacy for treating Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2018, 115, 578–583. [Google Scholar] [CrossRef] [Green Version]
- Cannon, J.R.; Tapias, V.; Na, H.M.; Honick, A.S.; Drolet, R.E.; Greenamyre, J.T. A highly reproducible rotenone model of Parkinson’s disease. Neurobiol. Dis. 2009, 34, 279–290. [Google Scholar] [CrossRef] [Green Version]
- Nie, S.K.; Ma, K.; Sun, M.K.; Lee, M.; Tan, Y.; Chen, G.Q.; Zhang, Z.T.; Zhang, Z.H.; Cao, X.B. 7,8-Dihydroxyflavone Protects Nigrostriatal Dopaminergic Neurons from Rotenone-Induced Neurotoxicity in Rodents. Parkinsons Dis. 2019. [Google Scholar] [CrossRef]
- Botsakis, K.; Mourtzi, T.; Panagiotakopoulou, V.; Vreka, M.; Stathopoulos, G.T.; Pediaditakis, I.; Charalampopoulos, I.; Gravanis, A.; Delis, F.; Antoniou, K.; et al. BNN-20, a synthetic microneurotrophin, strongly protects dopaminergic neurons in the “weaver” mouse, a genetic model of dopamine-denervation, acting through the TrkB neurotrophin receptor. Neuropharmacology 2017, 121, 140–157. [Google Scholar] [CrossRef] [PubMed]
- Foltynie, T.; Cheeran, B.; Williams-Gray, C.H.; Edwards, M.J.; Schneider, S.A.; Weinberger, D.; Rothwell, J.C.; Barker, R.A.; Bhatia, K.P. BDNF val66met influences time to onset of levodopa induced dyskinesia in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2009, 80, 141–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusters, C.D.J.; Paul, K.C.; Guella, I.; Bronstein, J.M.; Sinsheimer, J.S.; Farrer, M.J.; Ritz, B.R. Dopamine receptors and BDNF-haplotypes predict dyskinesia in Parkinson’s disease. Parkinsonism Relat. Disord. 2018, 47, 39–44. [Google Scholar] [CrossRef] [PubMed]



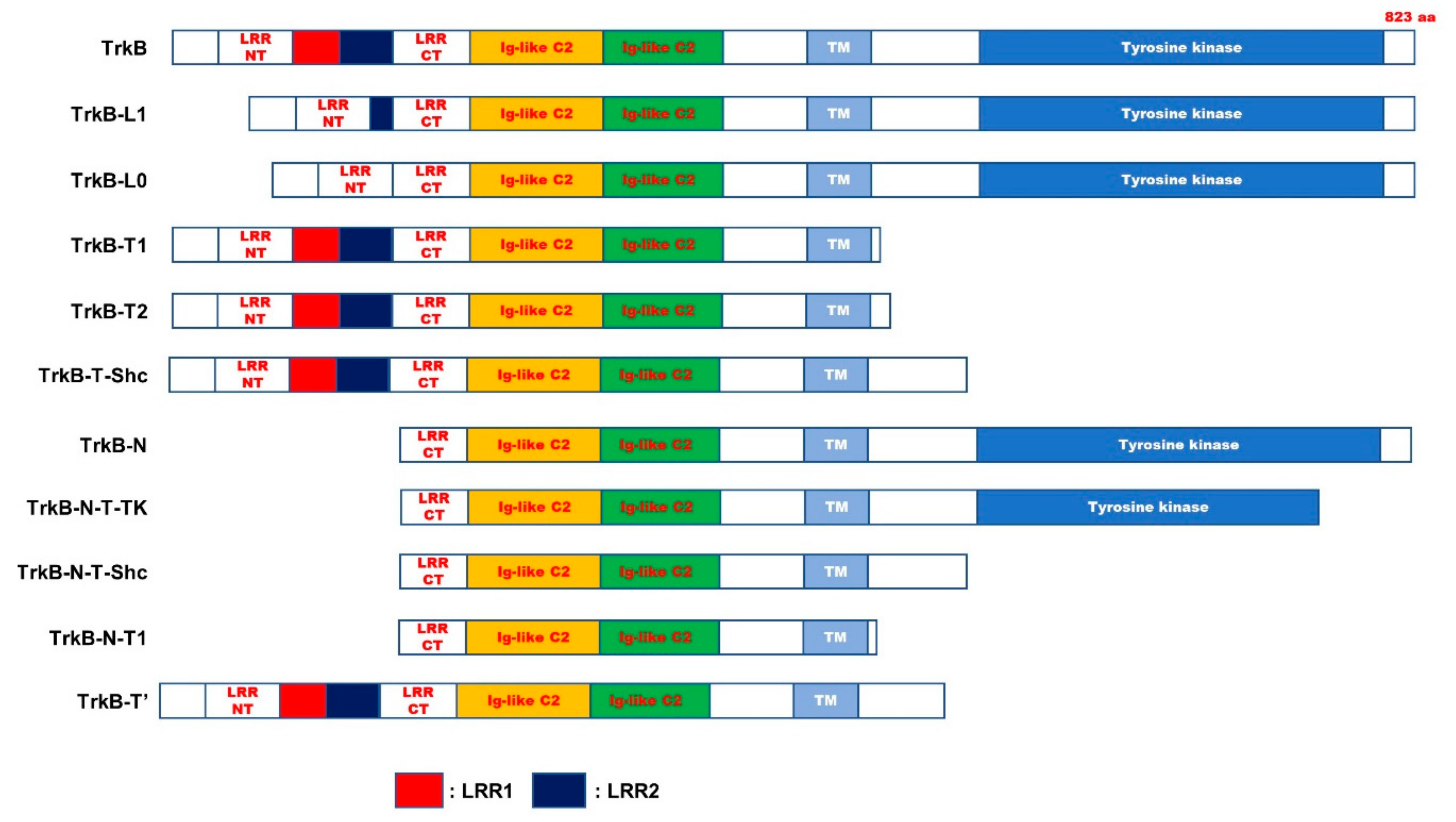
| Name | Characterization | Relevance of PD | |
|---|---|---|---|
| TrkB-L1 | lacked the first two LRMs of three leucine-rich motifs (LRMs) in the ECD | ND | [63] |
| TrkB-L0 | lacked the all of three leucine-rich motifs (LRMs) in the ECD | ND | [63] |
| TrkB.T1 | 467-477 AA: PASVISNDDDS → FVLFHKIPLDG 478-822 AA: Missing | Involved | [64] |
| TrkB.T2 | Contain only 23 amino acids of the ICD. | Involved | [64] |
| TrkB-T-Shc | 529-537 AA: FVQHIKRHN → WPRGSPKTA 538-822 AA: Missing. | ND | [54,65] |
| TrkB-T-TK | 710-735 AA: GGHTMLPIRWMPPESIMYRKFTTESD → SSCADQRPQGPLSLRDPCCICLLRLS 736-822 AA: Missing. | ND | [54,65] |
| TrkB-N | lack of N-terminal signal sequence | ND | [65] |
| TrkB-N-T-TK | 710-735 AA: GGHTMLPIRWMPPESIMYRKFTTESD → SSCADQRPQGPLSLRDPCCICLLRLS 736-822 AA: Missing. | ND | [54,65] |
| TrkB-N-Shc | 529-537 AA: FVQHIKRHN → WPRGSPKTA 538-822 AA: Missing. | ND | [54,65] |
| TrkB-N-T1 | 1-156 AA: Missing. 467-477 AA: PASVISNDDDS → FVLFHKIPLDG 478-822 AA: Missing. | ND | [54,65] |
| TrkB.Kin | Additional six-AA insertion between the Ig2 and the TM | ND | [66] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, W. Regulation of BDNF-TrkB Signaling and Potential Therapeutic Strategies for Parkinson’s Disease. J. Clin. Med. 2020, 9, 257. https://doi.org/10.3390/jcm9010257
Jin W. Regulation of BDNF-TrkB Signaling and Potential Therapeutic Strategies for Parkinson’s Disease. Journal of Clinical Medicine. 2020; 9(1):257. https://doi.org/10.3390/jcm9010257
Chicago/Turabian StyleJin, Wook. 2020. "Regulation of BDNF-TrkB Signaling and Potential Therapeutic Strategies for Parkinson’s Disease" Journal of Clinical Medicine 9, no. 1: 257. https://doi.org/10.3390/jcm9010257
APA StyleJin, W. (2020). Regulation of BDNF-TrkB Signaling and Potential Therapeutic Strategies for Parkinson’s Disease. Journal of Clinical Medicine, 9(1), 257. https://doi.org/10.3390/jcm9010257




