The Added Value of High Dose Spinal Cord Stimulation in Patients with Failed Back Surgery Syndrome after Conversion from Standard Spinal Cord Stimulation
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Population
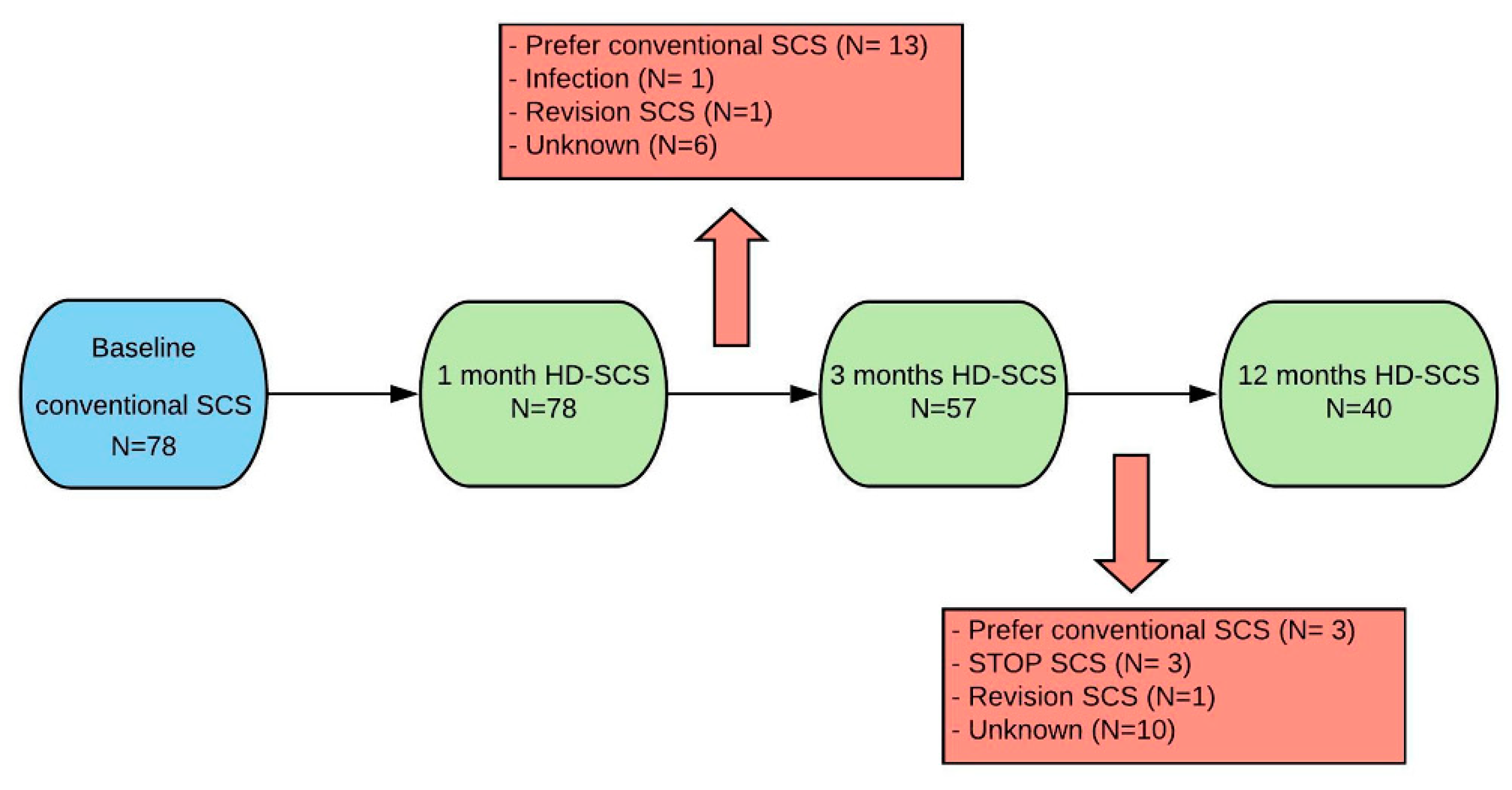
2.2. Study Protocol
2.3. Health Outcome Measures
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Health Related Quality of Life Outcome
3.3. Complete Case Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baber, Z.; Erdek, M.A. Failed back surgery syndrome: Current perspectives. J. Pain Res. 2016, 9, 979–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, S.; Kamiya, M.; Nishihara, M.; Arai, Y.C.P.; Ikemoto, T.; Ushida, T. Prevalence, characteristics, and burden of failed back surgery syndrome: The influence of various residual symptoms on patient satisfaction and quality of life as assessed by a nationwide Internet survey in Japan. J. Pain Res. 2017, 10, 811–823. [Google Scholar] [CrossRef] [Green Version]
- Thomson, S.; Jacques, L. Demographic characteristics of patients with severe neuropathic pain secondary to failed back surgery syndrome. Pain Pract. 2009, 9, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.S.; Taylor, R.J. The economic impact of failed back surgery syndrome. Br. J. Pain 2012, 6, 174–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doth, A.H.; Hansson, P.; Jensen, M.P.; Taylor, R.S. The burden of neuropathic pain: A systematic review and meta-analysis of health utilities. Pain 2010, 149, 338–344. [Google Scholar] [CrossRef]
- Kumar, K.; North, R.; Taylor, R.; Sculpher, M.; Abeele, C.D.; Gehring, M.; Jacques, L.; Eldabe, S.; Meglio, M.; Molet, J.; et al. Spinal cord stimulation vs. conventional medical management: A prospective, randomized, controlled, multicenter study of patients with failed back surgery syndrome (process study). Neuromodulation 2005, 8, 213–218. [Google Scholar] [CrossRef]
- Kumar, K.; Taylor, R.S.; Jacques, L.; Eldabe, S.; Meglio, M.; Molet, J.; Thomson, S.; O’Callaghan, J.; Eisenberg, E.; Milbouw, G.; et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: A multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain 2007, 132, 179–188. [Google Scholar] [CrossRef]
- Kumar, K.; Taylor, R.S.; Jacques, L.; Eldabe, S.; Meglio, M.; Molet, J.; Thomson, S.; O’Callaghan, J.; Eisenberg, E.; Milbouw, G.; et al. The effects of spinal cord stimulation in neuropathic pain are sustained: A 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery 2008, 63, 762–770. [Google Scholar] [CrossRef]
- North, R.B.; Kidd, D.; Shipley, J.; Taylor, R.S. Spinal cord stimulation versus reoperation for failed back surgery syndrome: A cost effectiveness and cost utility analysis based on a randomized, controlled trial. Neurosurgery 2007, 61, 361–369, discussion 368–369. [Google Scholar] [CrossRef]
- Eldabe, S.; Kumar, K.; Buchser, E.; Taylor, R. An analysis of the components of pain, function, and health-related quality of life in patients with failed back surgery syndrome treated with spinal cord stimulation or conventional medical management. Neuromodulation 2010, 13, 201–209. [Google Scholar] [CrossRef]
- Farber, S.H.; Han, J.L.; Elsamadicy, A.A.; Hussaini, Q.; Yang, S.; Pagadala, P.; Parente, B.; Xie, J.; Lad, S.P. Long-term cost utility of spinal cord stimulation in patients with failed back surgery syndrome. Pain Physician 2017, 20, E797–E805. [Google Scholar] [PubMed]
- Perryman, L.T. Spinal cord stimulation costs and complications can be reduced by wireless nanotechnology. A review of traditional equipment expenses compared to wireless stimulation. Am. J. Anesth. Clin. Res. 2018, 4, 19–24. [Google Scholar]
- Camberlin, C.S.M.L.; Smit, Y.; Post, P.; Gerkens, S.; De Laet, C. Neuromodulation for the management of chronic pain: Implanted spinal cord stimulators and intrathecal analgesic delivery pumps. In Health Technology Assessment (HTA); Belgian KCE Report 189C; Health Care Knowledge Centre (KCE): Brussels, Belgium, 2012. [Google Scholar]
- Waszak, P.M.; Modrić, M.; Paturej, A.; Malyshev, S.M.; Przygocka, A.; Garnier, H.; Szmuda, T. Spinal cord stimulation in failed back surgery syndrome: Review of clinical use, quality of life and cost-effectiveness. Asian Spine J. 2016, 10. [Google Scholar] [CrossRef]
- Zhang, T.C.; Janik, J.J.; Grill, W.M. Mechanisms and models of spinal cord stimulation for the treatment of neuropathic pain. Brain Res. 2014, 1569, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Van Buyten, J.P.; Wille, F.; Smet, I.; Wensing, C.; Breel, J.; Karst, E.; Devos, M.; Pöggel-Krämer, K.; Vesper, J. Therapy-related explants after spinal cord stimulation: Results of an international retrospective chart review study. Neuromodulation 2017, 20, 642–649. [Google Scholar] [CrossRef] [Green Version]
- Pope, J.E.; Deer, T.R.; Falowski, S.; Provenzano, D.; Hanes, M.; Hayek, S.M.; Amrani, J.; Carlson, J.; Skaribas, I.; Parchuri, K.; et al. Multicenter retrospective study of neurostimulation with exit of therapy by explant. Neuromodulation 2017, 20, 543–552. [Google Scholar] [CrossRef]
- Wille, F.; Breel, J.S.; Bakker, E.W.; Hollmann, M.W. Altering conventional to high density spinal cord stimulation: An energy dose-response relationship in neuropathic pain therapy. Neuromodulation 2016, 20, 71–80. [Google Scholar] [CrossRef]
- Hamm-Faber, T.E.; Gültuna, I.; Van Gorp, E.-J.; Aukes, H. High-dose spinal cord stimulation for treatment of chronic low back pain and leg pain in patients with FBSS, 12-month results: A prospective pilot study. Neuromodulation 2019, 23, 118–125. [Google Scholar] [CrossRef]
- Bala, M.M.; Riemsma, R.P.; Nixon, J.; Kleijnen, J. Systematic review of the (cost-)effectiveness of spinal cord stimulation for people with failed back surgery syndrome. Clin. J. Pain 2008, 24, 741–756. [Google Scholar] [CrossRef]
- Simpson, E.; Duenas, A.; Holmes, M.; Papaioannou, D.; Chilcott, J. Spinal cord stimulation for chronic pain of neuropathic or ischaemic origin: Systematic review and economic evaluation. Health Technol. Assess. 2009, 13, 1–154. [Google Scholar] [CrossRef]
- De Jaeger, M.; Van Hooff, R.J.; Goudman, L.; Espinoza, A.V.; Brouns, R.; Puylaert, M.; Duyvendak, W.; Moens, M. High-density in spinal cord stimulation: Virtual expert registry (DISCOVER): Study protocol for a prospective observational trial. Anesthesiol. Pain Med. 2017, 7, e13640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Follet, K.; Dirks, B. Etiology and evaluation of the failed back surgery syndrome. Neurosurg. Q. 1993, 3, 40–59. [Google Scholar]
- Agborsangaya, C.B.; Lau, D.; Lahtinen, M.; Cooke, T.; Johnson, J.A. Health-related quality of life and healthcare utilization in multimorbidity: Results of a cross-sectional survey. Qual. Life Res. 2013, 22, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, S.J.; Ali, S. Health outcomes in economic evaluation: The QALY and utilities. Br. Med. Bull. 2010, 96, 5–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Billingham, L.; Abrams, K.R.; Jones, D.R. Methods for the analysis of quality-of-life and survival data in health technology assessment. Health Technol. Assess. 1999, 3, 1–152. [Google Scholar] [CrossRef] [Green Version]
- Zhuo, L.; Xu, L.; Ye, J.; Sun, S.; Zhang, Y.; Burström, K.; Chen, J. Time trade-off value set for EQ-5D-3L based on a nationally representative chinese population survey. Value Health 2018, 21, 1330–1337. [Google Scholar] [CrossRef] [Green Version]
- Matthews, J.N.; Altman, D.G.; Campbell, M.J.; Royston, P. Analysis of serial measurements in medical research. BMJ 1990, 300, 230–235. [Google Scholar] [CrossRef] [Green Version]
- Streiner, D.L. The case of the missing data: Methods of dealing with dropouts and other research vagaries. Can. J. Psych. 2002, 47, 68–75. [Google Scholar] [CrossRef] [Green Version]
- North, J.M.; Hong, K.J.; Cho, P.Y. Clinical outcomes of 1 kHz subperception spinal cord stimulation in implanted patients with failed paresthesia-based stimulation: Results of a prospective randomized controlled trial. Neuromodulation 2016, 19, 731–737. [Google Scholar] [CrossRef]
- Haider, N.; Ligham, D.; Quave, B.; Harum, K.E.; García, E.A.; Gilmore, C.A.; Miller, N.; Moore, G.A.; Bains, A.; Lechleiter, K.; et al. Spinal cord stimulation (SCS) trial outcomes after conversion to a multiple waveform SCS system. Neuromodulation 2018, 21, 504–507. [Google Scholar] [CrossRef]
- Scalone, L.; Zucco, F.; Lavano, A.; Costantini, A.; De Rose, M.; Poli, P.; Fortini, G.; DeMartini, L.; De Simone, E.; Menardo, V.; et al. Benefits in pain perception, ability function and health-related quality of life in patients with failed back surgery syndrome undergoing spinal cord stimulation in a clinical practice setting. Health Qual. Life Outcomes 2018, 16, 68. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, E.; Hansson-Hedblom, A.; Kirketeig, T.; Fritzell, P.; Hägg, O.; Borgström, F. Cost and health outcomes patterns in patients treated with spinal cord stimulation following spine surgery—A register-based study. Neuromodulation 2019, 23, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Manca, A.; Kumar, K.; Taylor, R.; Jacques, L.; Eldabe, S.; Meglio, M.; Molet, J.; Thomson, S.; O’Callaghan, J.; Eisenberg, E.; et al. Quality of life, resource consumption and costs of spinal cord stimulation versus conventional medical management in neuropathic pain patients with failed back surgery syndrome (PROCESS trial). Eur. J. Pain 2008, 12, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Zucco, F.; Ciampichini, R.; Lavano, A.; Costantini, A.; De Rose, M.; Poli, P.; Fortini, G.; DeMartini, L.; De Simone, E.; Menardo, V.; et al. Cost-effectiveness and cost-utility analysis of spinal cord stimulation in patients with failed back surgery syndrome: Results from the PRECISE study. Neuromodulation 2015, 18, 266–276, discussion 276. [Google Scholar] [CrossRef] [Green Version]
- Kemler, M.A.; Furnee, C.A.; Meola, G. Economic evaluation of spinal cord stimulation for chronic reflex sympathetic dystrophy. Neurology 2002, 59, 1203–1209. [Google Scholar] [CrossRef]
- Moens, M.; Goudman, L.; Brouns, R.; Msc, A.V.E.; De Jaeger, M.; Huysmans, E.; Putman, K.; Verlooy, J. Return to work of patients treated with spinal cord stimulation for chronic pain: A systematic review and meta-analysis. Neuromodulation 2019, 22, 253–261. [Google Scholar] [CrossRef]
- Dyster-Aas, J.; Kildal, M.; Willebrand, M. Return to work and health-related quality of life after burn injury. J. Rehabil. Med. 2007, 39, 49–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shmagel, A.; Foley, R.; Ibrahim, H. Epidemiology of chronic low back pain in US adults: Data from the 2009–2010 national health and nutrition examination survey. Arthritis Care Res. 2016, 68, 1688–1694. [Google Scholar] [CrossRef] [Green Version]
- Palmer, N.; Guan, Z.; Chai, N.C. Spinal cord stimulation for failed back surgery syndrome—Patient selection considerations. Transl. Perioper. Pain Med. 2019, 6, 81–90. [Google Scholar]

| Patient Characteristics at Baseline (n = 78) | |||
|---|---|---|---|
| Age (years) | 56 ± 11.1 | ||
| Sex | Male | 25 (32.1%) | |
| Female | 53 (67.9%) | ||
| Paresthesia coverage | Back | Full | 32 (41.0%) |
| Partial | 46 (59.0%) | ||
| Leg | Full | 41 (52.6%) | |
| Partial | 37 (47.4%) | ||
| Type of SCS | RestoreSensor | 7 (9.0%) | |
| PrimeAdvanced | 71 (91.0%) | ||
| NRS (/10) | Low back pain | 7.0 [5.0–9.0] | |
| Leg pain | 7.0 [5.0–9.0] | ||
| ODI | 52.8 ± 16.7 | ||
| PSQI | 12.3 ± 4.3 | ||
| EQ-5D-3L | 0.28 ± 0.21 | ||
| Baseline | 1 Month | 3 Months | 12 Months | ||
|---|---|---|---|---|---|
| Domain | Problems | n = 78 | n = 78 | n = 57 | n = 40 |
| Mobility | No | 10 (12.8%) | 17 (21.8%) | 17 (29.8%) | 15 (37.5%) |
| Moderate | 68 (87.2%) | 61 (78.2%) | 39 (68.4%) | 25 (62.5%) | |
| Extreme | / | / | 1 (1.8%) | / | |
| Selfcare | No | 36 (46.2%) | 43 (55.1%) | 35 (61.4%) | 27 (67.5%) |
| Moderate | 39 (50.0%) | 34 (43.6%) | 21 (36.8%) | 13 (32.5%) | |
| Extreme | 3 (3.8%) | 1 (1.3%) | 1 (1.8%) | / | |
| Usual activities | No | 7 (9.0%) | 17 (21.8%) | 15 (26.3%) | 15 (37.5%) |
| Moderate | 45 (57.7%) | 51 (65.4%) | 36 (63.2%) | 24 (60.0%) | |
| Extreme | 27 (33.3%) | 10 (12.8%) | 6 (10.5%) | 1 (2.5%) | |
| Pain | No | 1 (1.3%) | 6 (7.7%) | 6 (10.5%) | 9 (22.5%) |
| Moderate | 25 (32.1%) | 40 (51.3%) | 30 (52.6%) | 23 (57.5%) | |
| Extreme | 52 (66.7%) | 32 (41.0%) | 21 (36.8%) | 8 (20.0%) | |
| Anxiety | No | 31 (39.7%) | 41 (52.6%) | 29 (50.9%) | 24 (60.0%) |
| Moderate | 32 (41.0%) | 25 (32.1%) | 20 (35.1%) | 13 (32.5%) | |
| Extreme | 15 (19.2%) | 12 (15.4%) | 8 (14.0%) | 3 (7.5%) | |
| Utility Values | ||||||
|---|---|---|---|---|---|---|
| Baseline | 1 Month | 3 Months | 12 Months | |||
| All patients (n = 78 with last observation carried forward for n = 22) | Utility | Mean | 0.283 (±0.21) | 0.439 (±0.28) | 0.437 (±0.30) | 0.452 (±0.29) |
| Median | 0.236 [0.133–0.473] | 0.551 [0.181–0.659] | 0.473 [0.133–0.659] | 0.515 [0.186–0.659] | ||
| VAS | Mean | 51.1 (±20.3) | 53.4 (±20.7) | 54.6 (±21.4) | 54.9 (±20.6) | |
| Median | 50.0 [40.0–65.0] | 56.5 [38.5–70.0] | 54.5 [37.0–70.0] | 58.5 [40.0–70.0] | ||
| Baseline | 1 Month | 3 Months | 12 Months | ||
|---|---|---|---|---|---|
| Domain | Problems | n = 40 | n = 40 | n = 40 | n = 40 |
| Mobility | No | 7 (17.5%) | 10 (25.0%) | 14 (35.0%) | 15 (37.5%) |
| Moderate | 33 (82.5%) | 30 (75.0%) | 26 (65.0%) | 25 (62.5%) | |
| Extreme | / | / | / | / | |
| Self-care | No | 21 (52.5%) | 26 (65.0%) | 27 (67.5%) | 27 (67.5%) |
| Moderate | 18 (45.0%) | 14 (35.0%) | 13 (32.5%) | 13 (32.5%) | |
| Extreme | 1 (2.5%) | / | / | / | |
| Usual activities | No | 5 (12.5%) | 14 (35.0%) | 14 (35.0%) | 15 (37.5%) |
| Moderate | 21 (52.5%) | 23 (57.5%) | 23 (57.5%) | 24 (60.0%) | |
| Extreme | 14 (35.0%) | 3 (7.5%) | 3 (7.5%) | 1 (2.5%) | |
| Pain | No | 1 (2.5%) | 5 (12.5%) | 6 (15.0%) | 9 (22.5%) |
| Moderate | 15 (37.5%) | 26 (65.0%) | 26 (65.0%) | 23 (57.5%) | |
| Extreme | 24 (60.0%) | 9 (22.5%) | 8 (20.0%) | 8 (20.0%) | |
| Anxiety | No | 16 (40.0%) | 24 (60.0%) | 23 (57.5%) | 24 (60.0%) |
| Moderate | 15 (37.5%) | 12 (30.0%) | 11 (27.5%) | 13 (32.5%) | |
| Extreme | 9 (22.5%) | 4 (10.0%) | 6 (15.0%) | 3 (7.5%) | |
| Utility Values | ||||||
|---|---|---|---|---|---|---|
| Baseline | 1 Month | 3 Months | 12 Months | |||
| All patients (n = 40) | Utility | Mean | 0.297 (±0.22) | 0.521 (±0.26) | 0.542 (±0.30) | 0.564 (±0.28) |
| Median | 0.236 [0.139–0.473] | 0.644 [0.249–0.690] | 0.618 [0.221–0.733] | 0.577 [0.388–0.722] | ||
| VAS | Mean | 56.6 (±17.9) | 62.6 (±15.5) | 62.5 (±18.3) | 62.6 (±16.6) | |
| Median | 55.0 [49.0–70.0] | 65.0 [50.0–71.0] | 60.0 [50.0–80.0] | 70.0 [50.0–70.0] | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Jaeger, M.; Goudman, L.; Putman, K.; De Smedt, A.; Rigoard, P.; Geens, W.; Moens, M. The Added Value of High Dose Spinal Cord Stimulation in Patients with Failed Back Surgery Syndrome after Conversion from Standard Spinal Cord Stimulation. J. Clin. Med. 2020, 9, 3126. https://doi.org/10.3390/jcm9103126
De Jaeger M, Goudman L, Putman K, De Smedt A, Rigoard P, Geens W, Moens M. The Added Value of High Dose Spinal Cord Stimulation in Patients with Failed Back Surgery Syndrome after Conversion from Standard Spinal Cord Stimulation. Journal of Clinical Medicine. 2020; 9(10):3126. https://doi.org/10.3390/jcm9103126
Chicago/Turabian StyleDe Jaeger, Mats, Lisa Goudman, Koen Putman, Ann De Smedt, Philippe Rigoard, Wietse Geens, and Maarten Moens. 2020. "The Added Value of High Dose Spinal Cord Stimulation in Patients with Failed Back Surgery Syndrome after Conversion from Standard Spinal Cord Stimulation" Journal of Clinical Medicine 9, no. 10: 3126. https://doi.org/10.3390/jcm9103126
APA StyleDe Jaeger, M., Goudman, L., Putman, K., De Smedt, A., Rigoard, P., Geens, W., & Moens, M. (2020). The Added Value of High Dose Spinal Cord Stimulation in Patients with Failed Back Surgery Syndrome after Conversion from Standard Spinal Cord Stimulation. Journal of Clinical Medicine, 9(10), 3126. https://doi.org/10.3390/jcm9103126





