Imaging in Primary Sjögren’s Syndrome
Abstract
:1. Introduction
2. Diagnosis and Classification of pSS
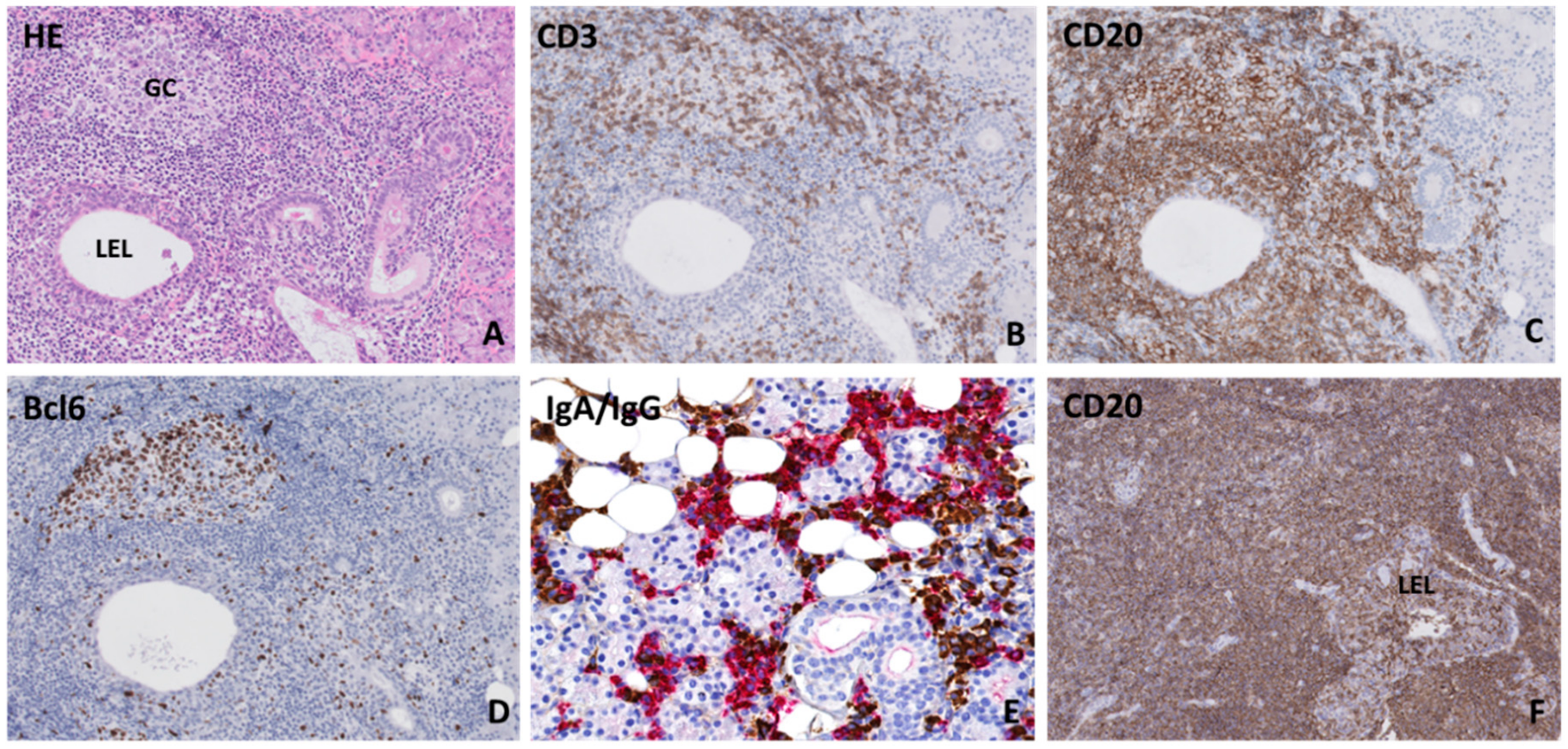
3. Histopathology of the Salivary Gland
4. Radiology Techniques
4.1. Sialography
4.2. Magnetic Resonance Imaging
4.3. Salivary Gland Ultrasonography
5. Sialendoscopy
6. Nuclear Medicine Techniques
6.1. Conventional Nuclear Medicine
6.1.1. 99mTc-Pertechnetate Scintigraphy
6.1.2. Future Promising Scintigraphic Tracers
6.2. Positron Emission Tomography/Computed Tomography
6.2.1. 18F-FDG-PET/CT
6.2.2. Future Promising PET Tracers
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ramos-Casals, M.; Brito-Zerón, P.; Solans, R.; Camps, M.T.; Casanovas, A.; Sopeña, B.; Díaz-López, B.; Rascón, F.-J.; Qanneta, R.; Fraile, G.; et al. Systemic involvement in primary Sjogren’s syndrome evaluated by the EULAR-SS disease activity index: Analysis of 921 Spanish patients (GEAS-SS Registry). Rheumatology 2014, 53, 321–331. [Google Scholar] [CrossRef] [Green Version]
- Greenspan, J.S.; Daniels, T.E.; Talal, N.; Sylvester, R.A. The histopathology of Sjögren’s syndrome in labial salivary gland biopsies. Oral Surg. Oral Med. Oral Pathol. 1974, 37, 217–229. [Google Scholar] [CrossRef]
- Baldini, C.; Zabotti, A.; Filipovic, N.; Vukicevic, A.; Luciano, N.; Ferro, F.; Lorenzon, M.; De Vita, S. Imaging in primary Sjögren’s syndrome: The “obsolete and the new.”. Clin. Exp. Rheumatol. 2018, 36, S215–S221. [Google Scholar]
- Signore, A.; Anzola, K.L.; Auletta, S.; Varani, M.; Petitti, A.; Pacilio, M.; Galli, F.; Lauri, C. Current status of molecular imaging in inflammatory and autoimmune disorders. Curr. Pharm. Des. 2018, 24, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Goules, A.V.; Tzioufas, A.G. Lymphomagenesis in Sjögren’s syndrome: Predictive biomarkers towards precision medicine. Autoimmun. Rev. 2019, 18, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Routsias, J.G.; Goules, J.D.; Charalampakis, G.; Tzima, S.; Papageorgiou, A.; Voulgarelis, M. Malignant lymphoma in primary Sjögren’s syndrome: An update on the pathogenesis and treatment. Semin. Arthritis Rheum. 2013, 43, 178–186. [Google Scholar] [CrossRef]
- Royer, B.; Cazals-Hatem, D.; Sibilia, J.; Agbalika, F.; Cayuela, J.M.; Soussi, T.; Maloisel, F.; Clauvel, J.P.; Brouet, J.C.; Mariette, X. Lymphomas in patients with Sjogren’s syndrome are marginal zone B-cell neoplasms, arise in diverse extranodal and nodal sites, and are not associated with viruses. Blood 1997, 90, 766–775. [Google Scholar] [CrossRef] [Green Version]
- Voulgarelis, M.; Dafni, U.G.; Isenberg, D.A.; Moutsopoulos, H.M. Malignant lymphoma in primary Sjögren’s syndrome: A multicenter, retrospective, clinical study by the European concerted action on Sjögren’s syndrome. Arthritis Rheum. 1999, 42, 1765–1772. [Google Scholar] [CrossRef]
- Johnson, S.A.; Kumar, A.; Matasar, M.J.; Schöder, H.; Rademaker, J. Imaging for staging and response assessment in lymphoma. Radiology 2015, 276, 323–338. [Google Scholar] [CrossRef] [Green Version]
- Barrington, S.F.; Mikhaeel, N.G.; Kostakoglu, L.; Meignan, M.; Hutchings, M.; Müeller, S.P.; Schwartz, L.H.; Zucca, E.; Fisher, R.I.; Trotman, J.; et al. Role of imaging in the staging and response assessment of lymphoma: Consensus of the international conference on malignant lymphomas imaging working group. J. Clin. Oncol. 2014, 32, 3048–3058. [Google Scholar] [CrossRef]
- Vitali, C.; Del Papa, N. Classification and diagnostic criteria in Sjögren’s syndrome: A long-standing and still open controversy. Ann. Rheum. Dis. 2017, 76, 1953–1954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bootsma, H.; Spijkervet, F.K.L.; Kroese, F.G.M.; Vissink, A. Toward new classification criteria for Sjögren’s syndrome? Arthritis Rheum. 2013, 65, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Van Nimwegen, J.F.; Van Ginkel, M.S.; Arends, S.; Haacke, E.A.; van der Vegt, B.; Sillevis Smitt-Kamminga, N.; Spijkervet, F.K.L.; Kroese, F.G.M.; Stel, A.J.; Brouwer, E.; et al. Validation of the ACR-EULAR criteria for primary Sjögren’s syndrome in a Dutch prospective diagnostic cohort. Rheumatology 2018, 57, 818–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome: A consensus and data-driven methodology involving three international patient cohorts. Ann. Rheum. Dis. 2017, 76, 9–16. [Google Scholar] [CrossRef]
- Shiboski, S.C.; Shiboski, C.H.; Criswell, L.A.; Baer, A.N.; Challacombe, S.; Lanfranchi, H.; Schiødt, M.; Umehara, H.; Vivino, F.; Zhao, Y.; et al. American College of rheumatology classification criteria for Sjögren’s syndrome: A data-driven, expert consensus approach in the Sjögren’s International Collaborative Clinical Alliance cohort. Arthritis Care Res. 2012, 64, 475–487. [Google Scholar] [CrossRef]
- Vitali, C.; Bombardieri, S.; Jonsson, R.; Moutsopoulos, H.M.; Alexander, E.L.; Carsons, S.E.; Daniels, T.E.; Fox, P.C.; Fox, R.I.; Kassan, S.S.; et al. Classification criteria for Sjogren’s syndrome: A revised version of the European criteria proposed by the American-European Consensus Group. Ann. Rheum. Dis. 2002, 61, 554–558. [Google Scholar] [CrossRef] [Green Version]
- Chisholm, D.M.; Mason, D.K. Labial salivary gland biopsy in Sjögren’s disease. J. Clin. Pathol. 1968, 21, 656–660. [Google Scholar] [CrossRef]
- Bodeutsch, C.; De Wilde, P.C.M.; Kater, L.; Van Houwelingen, J.C.; Van Den Hoogen, F.H.J.; Kruize, A.A.; Hené, R.J.; Van De Putte, L.B.A.; Vooijs, G.P. Quantitative immunohistologic criteria are superior to the lymphocytic focus score criterion for the diagnosis of Sjögren’s syndrome. Arthritis Rheum. 1992, 35, 1075–1087. [Google Scholar] [CrossRef]
- Leroy, J.P.; Pennec, Y.L.; Letoux, G.; Youinou, P. Lymphocytic infiltration of salivary ducts: A histopathologic lesion specific for primary Sjögren’s syndrome? Arthritis Rheum. 1992, 35, 481–482. [Google Scholar] [CrossRef]
- Ihrler, S.; Zietz, C.; Sendelhofert, A.; Riederer, A.; Löhrs, U. Lymphoepithelial duct lesions in Sjogren-type sialadenitis. Virchows Arch. 1999, 434, 315–323. [Google Scholar] [CrossRef]
- Llamas-Gutierrez, F.J.; Reyes, E.; Martínez, B.; Hernández-Molina, G. Histopathological environment besides the focus score in Sjögren’s syndrome. Int. J. Rheum. Dis. 2014, 17, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Leehan, K.M.; Pezant, N.P.; Rasmussen, A.; Grundahl, K.; Moore, J.S.; Radfar, L.; Lewis, D.M.; Stone, D.U.; Lessard, C.J.; Rhodus, N.L.; et al. Minor salivary gland fibrosis in Sjögren’s syndrome is elevated, associated with focus score and not solely a consequence of aging. Clin. Exp. Rheumatol. 2018, 36, S80–S88. [Google Scholar]
- Leehan, K.M.; Pezant, N.P.; Rasmussen, A.; Grundahl, K.; Moore, J.S.; Radfar, L.; Lewis, D.M.; Stone, D.U.; Lessard, C.J.; Rhodus, N.L.; et al. Fatty infiltration of the minor salivary glands is a selective feature of aging but not Sjögren’s syndrome. Autoimmunity 2017, 50, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Skarstein, K.; Aqrawi, L.A.; Øijordsbakken, G.; Jonsson, R.; Jensen, J.L. Adipose tissue is prominent in salivary glands of Sjögren’s syndrome patients and appears to influence the microenvironment in these organs. Autoimmunity 2016, 49, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Risselada, A.P.; Kruize, A.A.; Goldschmeding, R.; Lafeber, F.P.J.G.; Bijlsma, J.W.J.; Van Roon, J.A.G. The prognostic value of routinely performed minor salivary gland assessments in primary Sjögren’s syndrome. Ann. Rheum. Dis. 2014, 73, 1537–1540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carubbi, F.; Alunno, A.; Cipriani, P.; Bartoloni, E.; Baldini, C.; Quartuccio, L.; Priori, R.; Valesini, G.; De Vita, S.; Bombardieri, S.; et al. A retrospective, multicenter study evaluating the prognostic value of minor salivary gland histology in a large cohort of patients with primary Sjögren’s syndrome. Lupus 2015, 24, 315–320. [Google Scholar] [CrossRef]
- De Re, V.; De Vita, S.; Carbone, A.; Ferraccioli, G.; Gloghini, A.; Marzotto, A.; Pivetta, B.; Dolcetti, R.; Boiocchi, M. The relevance of VDJ PCR protocols in detecting B-cell clonal expansion in lymphomas and other lymphoproliferative disorders. Tumori 1995, 81, 405–409. [Google Scholar] [CrossRef]
- Nakshbandi, U.; Haacke, E.A.; Bootsma, H.; Vissink, A.; Spijkervet, F.K.L.; Van Der Vegt, B.; Kroese, F.G.M. Bcl6 for identification of germinal centres in salivary gland biopsies in primary Sjögren’s syndrome. Oral Dis. 2020, 26, 707–710. [Google Scholar] [CrossRef]
- Rubin, P.; Holt, J. Secretory sialography in diseases of the major salivary glands. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1957, 77, 575–598. [Google Scholar]
- Wang, X.; Bootsma, H.; Terpstra, J.; Vissink, A.; Van Der Vegt, B.; Spijkervet, F.K.L.; Kroese, F.G.M.; Pringle, S. Progenitor cell niche senescence reflects pathology of the parotid salivary gland in primary Sjögren’s syndrome. Rheumatology 2020, in press. [Google Scholar] [CrossRef] [Green Version]
- Swiecka, M.; Maślińska, M.; Paluch, L.; Zakrzewski, J.; Kwiatkowska, B. Imaging methods in primary Sjögren’s syndrome as potential tools of disease diagnostics and monitoring. Reumatologia 2019, 57, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Golder, W.; Stiller, M. Verteilungsmuster des Sjögren-Syndroms: Eine sialographische Studie. Z. Rheumatol. 2014, 73, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Keshet, N.; Aricha, A.; Friedlander-Barenboim, S.; Aframian, D.J.; Nadler, C. Novel parotid sialo-cone-beam computerized tomography features in patients with suspected Sjogren’s syndrome. Oral Dis. 2019, 25, 126–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kojima, I.; Sakamoto, M.; Iikubo, M.; Shimada, Y.; Nishioka, T.; Sasano, T. Relationship of MR imaging of submandibular glands to hyposalivation in Sjögren’s syndrome. Oral Dis. 2019, 25, 117–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niemelä, R.K.; Pääkkö, E.; Suramo, I.; Takalo, R.; Hakala, M. Magnetic Resonance Imaging and Magnetic Resonance Sialography of Parotid Glands in Primary Sjogren’s Syndrome. Arthritis Rheum. 2001, 45, 512–518. [Google Scholar] [CrossRef]
- Shimizu, M.; Okamura, K.; Kise, Y.; Takeshita, Y.; Furuhashi, H.; Weerawanich, W.; Moriyama, M.; Ohyama, Y.; Furukawa, S.; Nakamura, S.; et al. Effectiveness of imaging modalities for screening IgG4-related dacryoadenitis and sialadenitis (Mikulicz’s disease) and for differentiating it from Sjögren’s syndrome (SS), with an emphasis on sonography. Arthritis Res. 2015, 17, 223. [Google Scholar] [CrossRef] [Green Version]
- Su, G.Y.; Wang, C.B.; Hu, H.; Liu, J.; Ding, H.Y.; Xu, X.Q.; Wu, F.Y. Effect of laterality, gender, age and body mass index on the fat fraction of salivary glands in healthy volunteers: Assessed using iterative decomposition of water and fat with echo asymmetry and least-squares estimation method. Dentomaxillofac. Radiol. 2019, 48, 20180263. [Google Scholar] [CrossRef]
- Scott, J.; Flower, E.A.; Burns, J. A quantitative study of histological changes in the human parotid gland occurring with adult age. J. Oral Pathol. Med. 1987, 16, 505–510. [Google Scholar] [CrossRef]
- Izumi, M.; Eguchi, K.; Nakamura, H.; Nagataki, S.; Nakamura, T. Premature fat deposition in the salivary glands associated with Sjogren syndrome: MR and CT evidence. Am. J. Neuroradiol. 1997, 18, 951–958. [Google Scholar]
- Ren, Y.D.; Li, X.R.; Zhang, J.; Long, L.L.; Li, W.X.; Han, Y.Q. Conventional MRI techniques combined with MR sialography on T2-3D-DRIVE in Sjögren syndrome. Int. J. Clin. Exp. Med. 2015, 8, 3974–3982. [Google Scholar]
- Niemelä, R.K.; Takalo, R.; Pääkkö, E.; Suramo, I.; Päivänsalo, M.; Salo, T.; Hakala, M. Ultrasonography of salivary glands in primary Sjögren’s syndrome. A comparison with magnetic resonance imaging and magnetic resonance sialography of parotid glands. Rheumatology 2004, 43, 875–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izumi, M.; Eguchi, K.; Ohki, M.; Uetani, M.; Hayashi, K.; Kita, M.; Nagataki, S.; Nakamura, T. MR imaging of the parotid gland in Sjögren’s syndrome: A proposal for new diagnostic criteria. Am. J. Roentgenol. 1996, 166, 1483–1487. [Google Scholar] [CrossRef] [PubMed]
- El Miedany, Y.M.; Ahmed, I.; Mourad, H.G.; Mehanna, A.N.; Aty, S.A.; Gamal, H.M.; El Baddini, M.; Smith, P.; El Gafaary, M. Quantitative ultrasonography and magnetic resonance imaging of the parotid gland: Can they replace the histopathologic studies in patients with Sjogren’s syndrome? Jt. Bone Spine 2004, 71, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Pijpe, J.; Kalk, W.W.I.; Bootsma, H.; Spijkervet, F.K.L.; Kallenberg, C.G.M.; Vissink, A. Progression of salivary gland dysfunction in patients with Sjögren’s syndrome. Ann. Rheum. Dis. 2007, 66, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, J.; Travis, W.; Pillemer, S.; Bermudez, D.; Wolff, A.; Fox, P. Major salivary gland function in primary Sjögren’s syndrome and its relationship to clinical features. J. Rheumatol. 1990, 17, 318–322. [Google Scholar]
- Izumi, M.; Eguchi, K.; Uetani, M.; Nakamura, H.; Takagi, Y.; Hayashi, K.; Nakamura, T. MR features of the lacrimal gland in Sjogren’s syndrome. Am. J. Roentgenol. 1998, 170, 1661–1666. [Google Scholar] [CrossRef] [Green Version]
- Morgen, K.; McFarland, H.F.; Pillemer, S.R. Central nervous system disease in primary Sjögren’s syndrome: The role of magnetic resonance imaging. Semin. Arthritis Rheum. 2004, 34, 623–630. [Google Scholar] [CrossRef]
- McCoy, S.S.; Baer, A.N. Neurological complications of Sjögren’s syndrome: Diagnosis and Management. Curr. Treat. Options Rheumatol. 2017, 3, 275–288. [Google Scholar] [CrossRef]
- Cassidy, D.T.; McKelvie, P.; Harris, G.J.; Rose, G.E.; McNab, A.A. Lacrimal gland orbital lobe cysts associated with MALT lymphoma and primary Sjögren’s syndrome. Orbit 2005, 24, 257–263. [Google Scholar] [CrossRef]
- Tonami, H.; Matoba, M.; Kuginuki, Y.; Yokota, H.; Higashi, K.; Yamamoto, I.; Sugai, S. Clinical and imaging findings of lymphoma in patients with Sjögren syndrome. J. Comput. Assist. Tomogr. 2003, 27, 517–524. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, P.; Yang, J.; Yu, Q. Non-Hodgkin lymphoma involving the parotid gland: CT and MR imaging findings. Dentomaxillofac. Radiol. 2013, 42, 20130046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonami, H.; Matoba, M.; Yokota, H.; Higashi, K.; Yamamoto, I.; Sugai, S. CT and MR findings of bilateral lacrimal gland enlargement in Sjögren syndrome. Clin. Imaging 2002, 26, 392–396. [Google Scholar] [CrossRef]
- Grevers, G.; Ihrler, S.; Vogl, T.J.; Weiss, M. A comparison of clinical, pathological and radiological findings with magnetic resonance imaging studies of lymphomas in patients with Sjögren’s syndrome. Eur. Arch. Oto-Rhino-Laryngol. 1994, 251, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Tonami, H.; Matoba, M.; Yokota, H.; Higashi, K.; Yamamoto, I.; Sugai, S. Mucosa-associated lymphoid tissue lymphoma in Sjögren’s syndrome: Initial and follow-up imaging features. Am. J. Roentgenol. 2002, 179, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Carotti, M.; Salaffi, F.; Di Carlo, M.; Barile, A.; Giovagnoni, A. Diagnostic value of major salivary gland ultrasonography in primary Sjögren’s syndrome: The role of grey-scale and colour/power Doppler sonography. Gland Surg. 2019, 8, S159–S167. [Google Scholar] [CrossRef]
- Devauchelle-Pensec, V.; Zabotti, A.; Carvajal-Alegria, G.; Filipovic, N.; Jousse-Joulin, S.; De Vita, S. Salivary gland ultrasonography in primary Sjögren’s syndrome: Opportunities and challenges. Rheumatology 2019, in press. [Google Scholar] [CrossRef]
- Le Goff, M.; Cornec, D.; Jousse-Joulin, S.; Guellec, D.; Costa, S.; Marhadour, T.; Le Berre, R.; Genestet, S.; Cochener, B.; Boisrame-Gastrin, S.; et al. Comparison of 2002 AECG and 2016 ACR/EULAR classification criteria and added value of salivary gland ultrasonography in a patient cohort with suspected primary Sjögren’s syndrome. Arthritis Res. 2017, 19, 269. [Google Scholar] [CrossRef] [Green Version]
- Jousse-Joulin, S.; Gatineau, F.; Baldini, C.; Baer, A.; Barone, F.; Bootsma, H.; Bowman, S.; Brito-Zerón, P.; Cornec, D.; Dorner, T.; et al. Weight of salivary gland ultrasonography compared to other items of the 2016 ACR/EULAR classification criteria for Primary Sjögren’s syndrome. J. Intern. Med. 2020, 287, 180–188. [Google Scholar] [CrossRef]
- Takagi, Y.; Nakamura, H.; Sumi, M.; Shimizu, T.; Hirai, Y.; Horai, Y.; Takatani, A.; Kawakami, A.; Eida, S.; Sasaki, M.; et al. Combined classification system based on ACR/EULAR and ultrasonographic scores for improving the diagnosis of Sjögren’s syndrome. PLoS ONE 2018, 13, e0195113. [Google Scholar] [CrossRef] [Green Version]
- Van Nimwegen, J.F.; Mossel, E.; Delli, K.; van Ginkel, M.S.; Stel, A.J.; Kroese, F.G.M.; Spijkervet, F.K.L.; Vissink, A.; Arends, S.; Bootsma, H. Incorporation of Salivary Gland Ultrasonography into the American College of Rheumatology/European League Against Rheumatism Criteria for Primary Sjögren’s Syndrome. Arthritis Care Res. 2020, 72, 583–590. [Google Scholar] [CrossRef] [Green Version]
- Jousse-Joulin, S.; D’Agostino, M.A.; Nicolas, C.; Naredo, E.; Ohrndorf, S.; Backhaus, M.; Tamborrini, G.; Chary-Valckenaere, I.; Terslev, L.; Iagnocco, A.; et al. Video clip assessment of a salivary gland ultrasound scoring system in Sjögren’s syndrome using consensual definitions: An OMERACT ultrasound working group reliability exercise. Ann. Rheum. Dis. 2019, 78, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Mossel, E.; Arends, S.; Van Nimwegen, J.F.; Delli, K.; Stel, A.J.; Kroese, F.G.M.; Spijkervet, F.K.L.; Vissink, A.; Bootsma, H. Scoring hypoechogenic areas in one parotid and one submandibular gland increases feasibility of ultrasound in primary Sjögren’s syndrome. Ann. Rheum. Dis. 2018, 77, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Mossel, E. Comparing ultrasound, histopathology and saliva production of the parotid gland in patients with primary Sjögren’s syndrome. Unpublished work.
- Rahmani, G.; McCarthy, P.; Bergin, D. The diagnostic accuracy of ultrasonography for soft tissue lipomas: A systematic review. Acta Radiol. Open 2017, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baer, A.N.; Grader-Beck, T.; Antiochos, B.; Birnbaum, J.; Fradin, J.M. Ultrasound-guided biopsy of suspected salivary gland lymphoma in Sjögren’s syndrome. Arthritis Care Res. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Mossel, E.; Delli, K.; Arends, S.; Haacke, E.A.; Van Der Vegt, B.; Van Nimwegen, J.F.; Stel, A.J.; Spijkervet, F.K.L.; Vissink, A.; Kroese, F.G.M.; et al. Can ultrasound of the major salivary glands assess histopathological changes induced by treatment with rituximab in primary Sjögren’s syndrome? Ann. Rheum. Dis. 2019, 78, e27. [Google Scholar] [CrossRef] [Green Version]
- Radovic, M.; Vukicevic, A.; Zabotti, A.; Milic, V.; De Vita, S.; Filipovic, N. Deep learning based approach for assessment of primary Sjögren’s syndrome from salivary gland ultrasonography images. In Computational Bioengineering and Bioinformatics. Learning and Analytics in Intelligent Systems. Proceedings of the ICCB 2019 8th International Conference on Computational Bioengineering, Belgrade, Serbia, 4–6 September 2019; Filipovic, N., Ed.; Springer: Cham, Switzerland, 2020; Volume 11. [Google Scholar]
- Fidelix, T.; Czapkowski, A.; Azjen, S.; Andriolo, A.; Trevisani, V.F.M. Salivary gland ultrasonography as a predictor of clinical activity in Sjögren’s syndrome. PLoS ONE 2017, 12, e0182287. [Google Scholar] [CrossRef]
- Inanc, N.; Sahinkaya, Y.; Mumcu, G.; Özdemir, F.T.; Paksoy, A.; Ertürk, Z.; Direskeneli, H.; Bruyn, G.A. Evaluation of salivary gland ultrasonography in primary Sjögren’s syndrome: Does it reflect clinical activity and outcome of the disease? Clin. Exp. Rheumatol. 2019, 37, S140–S145. [Google Scholar]
- Cornec, D.; Jousse-Joulin, S.; Costa, S.; Marhadour, T.; Marcorelles, P.; Berthelot, J.M.; Hachulla, E.; Hatron, P.Y.; Goeb, V.; Vittecoq, O.; et al. High-grade salivary-gland involvement, assessed by histology or ultrasonography, is associated with a poor response to a single rituximab course in primary Sjögren’s syndrome: Data from the TEARS randomized trial. PLoS ONE 2016, 11, e0162787. [Google Scholar] [CrossRef]
- Zabotti, A.; Callegher, S.Z.; Gandolfo, S.; Valent, F.; Giovannini, I.; Cavallaro, E.; Lorenzon, M.; De Vita, S. Hyperechoic bands detected by salivary gland ultrasonography are related to salivary impairment in established Sjögren’s syndrome. Clin. Exp. Rheumatol. 2019, 37, S146–S152. [Google Scholar]
- Theander, E.; Mandl, T. Primary Sjögren’s syndrome: Diagnostic and prognostic value of salivary gland ultrasonography using a simplified scoring system. Arthritis Care Res. 2014, 66, 1102–1107. [Google Scholar] [CrossRef]
- Milic, V.; Colic, J.; Cirkovic, A.; Stanojlovic, S.; Damjanov, N. Disease activity and damage in patients with primary Sjogren’s syndrome: Prognostic value of salivary gland ultrasonography. PLoS ONE 2019, 14, e0226498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mossel, E.; van Nimwegen, J.F.; Stel, A.J.; Wijnsma, R.; Delli, K.; van Zuiden, G.S.; Olie, L.; Vehof, J.; Los, L.; Vissink, A.; et al. Clinical phenotyping of primary Sjögren’s patients using salivary gland ultrasonography—Data from the REgistry of Sjögren syndrome in Umcg LongiTudinal (RESULT) cohort. Unpublished work.
- Kim, J.W.; Lee, H.; Park, S.H.; Kim, S.K.; Choe, J.Y.; Kim, J.K. Salivary gland ultrasonography findings are associated with clinical, histological, and serologic features of Sjögren’s syndrome. Scand. J. Rheumatol. 2018, 47, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.A.; Lee, S.H.; Kim, H.R. Diagnostic and predictive evaluation using salivary gland ultrasonography in primary Sjögren’s syndrome. Clin. Exp. Rheumatol. 2018, 36, S165–S172. [Google Scholar]
- Coiffier, G.; Martel, A.; Albert, J.D.; Lescoat, A.; Bleuzen, A.; Perdriger, A.; De Bandt, M.; Maillot, F. Ultrasonographic damages of major salivary glands are associated with cryoglobulinemic vasculitis and lymphoma in primary Sjogren’s syndrome: Are the ultrasonographic features of the salivary glands new prognostic markers in Sjogren’s syndrome? Ann. Rheum. Dis 2019, in press. [Google Scholar] [CrossRef] [Green Version]
- Jousse-Joulin, S.; D’agostino, M.A.; Hočevar, A.; Naredo, E.; Terslev, L.; Ohrndorf, S.; Iagnocco, A.; Schmidt, W.A.; Finzel, S.; Alavi, Z.; et al. Could we use salivary gland ultrasonography as a prognostic marker in Sjogren’s syndrome? Response to: Ultrasonographic damages of major salivary glands are associated with cryoglobulinemic vasculitis and lymphoma in primary Sjogren’s syndrome: Are the ultrasonographic features of the salivary glands new prognostic markers in Sjogren’s syndrome? Ann. Rheum. Dis 2019, in press. [Google Scholar]
- De Luca, R.; Trodella, M.; Vicidomini, A.; Colella, G.; Tartaro, G. Endoscopic management of salivary gland obstructive diseases in patients with Sjögren’s syndrome. J. Cranio-Maxillofac. Surg. 2015, 43, 1643–1649. [Google Scholar] [CrossRef]
- Hakki Karagozoglu, K.; Vissink, A.; Forouzanfar, T.; Brand, H.S.; Maarse, F.; Jan Jager, D.H. Sialendoscopy enhances salivary gland function in Sjögren’s syndrome: A 6-month follow-up, randomised and controlled, single blind study. Ann. Rheum. Dis. 2018, 77, 1025–1031. [Google Scholar] [CrossRef]
- Gallo, A.; Martellucci, S.; Fusconi, M.; Pagliuca, G.; Greco, A.; De Virgilio, A.; De Vincentiis, M. Sialendoscopic management of autoimmune sialadenitis: A review of literature. Acta Otorhinolaryngol. Ital. 2017, 37, 148–154. [Google Scholar]
- Jager, D.J.; Karagozoglu, K.H.; Maarse, F.; Brand, H.S.; Forouzanfar, T. Sialendoscopy of salivary glands affected by Sjögren syndrome: A randomized controlled pilot study. J. Oral Maxillofac. Surg. 2016, 74, 1167–1174. [Google Scholar] [CrossRef]
- Shacham, R.; Puterman, M.B.; Ohana, N.; Nahlieli, O. Endoscopic treatment of salivary glands affected by autoimmune diseases. J. Oral Maxillofac. Surg. 2011, 69, 476–481. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, N.; Wu, C.; Xue, L.; Zhou, Q. Sialendoscopy-assisted treatment for chronic obstructive parotitis related to Sjogren syndrome. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 123, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Capaccio, P.; Canzi, P.; Torretta, S.; Rossi, V.; Benazzo, M.; Bossi, A.; Vitali, C.; Cavagna, L.; Pignataro, L. Combined interventional sialendoscopy and intraductal steroid therapy for recurrent sialadenitis in Sjögren’s syndrome: Results of a pilot monocentric trial. Clin. Otolaryngol. 2018, 43, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Karagozoglu, K.H.; De Visscher, J.G.; Forouzanfar, T.; van der Meij, E.H.; Jager, D.J. Complications of Sialendoscopy in Patients with Sjögren Syndrome. J. Oral Maxillofac. Surg. 2017, 75, 978–983. [Google Scholar] [CrossRef]
- Nakayama, M.; Okizaki, A.; Nakajima, K.; Takahashi, K. Approach to Diagnosis of Salivary Gland Disease from Nuclear Medicine Images, Salivary Glands—New Approaches in Diagnostics and Treatment, Işıl Adadan Güvenç, IntechOpen. Available online: https://www.intechopen.com/books/salivary-glands-new-approaches-in-diagnostics-and-treatment/approach-to-diagnosis-of-salivary-gland-disease-from-nuclear-medicine-images (accessed on 3 July 2020).
- Soto-Rojas, A.E.; Kraus, A. The oral side of Sjögren syndrome. Diagnosis and treatment. A review. Arch. Med. Res. 2002, 33, 95–106. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brito-Zerón, P.; Perez-De-Lis, M.; Diaz-Lagares, C.; Bove, A.; Soto, M.J.; Jimenez, I.; Belenguer, R.; Siso, A.; Muxí, A.; et al. Clinical and prognostic significance of parotid scintigraphy in 405 patients with primary Sjögren’s syndrome. J. Rheumatol. 2010, 37, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Brito-Zero, P.; Ramos-Casals, M.; Bove, A.; Sentis, J.; Font, J. Predicting adverse outcomes in primary Sjogren’s syndrome: Identification of prognostic factors. Rheumatology 2007, 46, 1359–1362. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Wu, J.; Zhao, L.; Liu, W.; Wei, J.; Hu, Z.; Hao, B.; Wu, H.; Sun, L.; Chen, H. Quantitative evaluation of salivary gland scintigraphy in Sjögren’s syndrome: Comparison of diagnostic efficacy and relationship with pathological features of the salivary glands. Ann. Nucl. Med. 2020, 34, 289–298. [Google Scholar] [CrossRef]
- Aung, W.; Murata, Y.; Ishida, R.; Takahashi, Y.; Okada, N.; Shibuya, H. Study of Quantitative Oral Radioactivity in Salivary Gland Scintigraphy and Determination of the Clinical Stage of Sjögren’s Syndrome. J. Nucl. Med. 2001, 42, 38–43. [Google Scholar]
- Aksoy, T.; Kiratli, P.O.; Erbas, B. Correlations between histopathologic and scintigraphic parameters of salivary glands in patients with Sjögren’s syndrome. Clin. Rheumatol. 2012, 31, 1365–1370. [Google Scholar] [CrossRef]
- Ferone, D.; Lombardi, G.; Colao, A. Somatostatin Receptors in Immune System Cells. Minerva Endocrinol. 2001, 26, 165–173. [Google Scholar]
- Duet, M.; Lioté, F. Somatostatin and somatostatin analog scintigraphy: Any benefits for rheumatology patients? Jt. Bone Spine 2004, 71, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Ferone, D.; Van Hagen, P.M.; Semino, C.; Dalm, V.A.; Barreca, A.; Colao, A.; Lamberts, S.W.J.; Minuto, F.; Hofland, L.J. Somatostatin receptor distribution and function in immune system. Dig. Liver Dis. 2004, 36, S68–S77. [Google Scholar] [CrossRef] [PubMed]
- Anzola, L.K.; Rivera, J.N.; Dierckx, R.A.; Lauri, C.; Valabrega, S.; Galli, F.; Moreno Lopez, S.; Glaudemans, A.W.J.M.; Signore, A. Value of Somatostatin Receptor Scintigraphy with 99mTc-HYNIC-TOC in Patients with Primary Sjögren Syndrome. J. Clin. Med. 2019, 8, 763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malviya, G.; Anzola, K.L.; Podestà, E.; Laganà, B.; Del Mastro, C.; Dierckx, R.A.; Scopinaro, F.; Signore, A. 99mTc-labeled rituximab for imaging B lymphocyte infiltration in inflammatory autoimmune disease patients. Mol. Imaging Biol. 2012, 14, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Jamar, F.; Buscombe, J.; Chiti, A.; Christian, P.E.; Delbeke, D.; Donohoe, K.J.; Israel, O.; Martin-Comin, J.; Signore, A. EANM/SNMMI guideline for 18F-FDG use in inflammation and infection. J. Nucl. Med. 2013, 54, 647–658. [Google Scholar] [CrossRef] [Green Version]
- EARL. An EANM Initiative. Available online: http://earl.eanm.org/cms/website.php (accessed on 29 June 2020).
- Pelletier-Galarneau, M.; Ruddy, T.D. PET/CT for Diagnosis and Management of Large-Vessel Vasculitis. Curr. Cardiol. Rep. 2019, 21, 34. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; Ma, Y.; Xiao, Y.; Niu, N.; Lin, W.; Wang, X.; Liang, Z.; Zhang, F.; Li, F.; et al. Characterizing IgG4-related disease with 18F-FDG PET/CT: A prospective cohort study. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1624–1634. [Google Scholar] [CrossRef] [Green Version]
- Jadvar, H.; Bonyadlou, S.; Iagaru, A.; Colletti, P.M. FDG PET-CT demonstration of Sjogren’s sialoadenitis. Clin. Nucl. Med. 2005, 30, 698–699. [Google Scholar] [CrossRef]
- Kumar, P.; Jaco, M.J.; Pandit, A.G.; Shanmughanandan, K.; Jain, A.; Rajeev; Ravina, M. Miliary sarcoidosis with secondary sjogren’s syndrome. J. Assoc. Physicians India 2013, 61, 505–507. [Google Scholar]
- Sharma, P.; Chatterjee, P. 18F-FDG PET/CT in multisystem Sjögren Syndrome. Clin. Nucl. Med. 2015, 40, e293-4. [Google Scholar] [CrossRef]
- Nakamoto, Y.; Tatsumi, M.; Hammoud, D.; Cohade, C.; Osman, M.M.; Wahl, R.L. Normal FDG distribution patterns in the head and neck: PET/CT evaluation. Radiology 2005, 234, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Zincirkeser, S.; Sahin, E.; Halac, M.; Sager, S. Standardized uptake values of normal organs on 18F-fluorodeoxyglucose positron emission tomography and computed tomography imaging. J. Int. Med. Res. 2007, 35, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Carter, K.R.; Kotlyarov, E. Common causes of false positive F18 FDG PET/CT scans in oncology. Braz. Arch. Biol. Technol. 2007, 50, 29–35. [Google Scholar] [CrossRef]
- Nicolau, J.; Sassaki, K.T. Metabolism of carbohydrate in the major salivary glands of rats. Arch. Oral Biol. 1976, 21, 659–661. [Google Scholar] [CrossRef]
- Jurysta, C.; Nicaise, C.; Cetik, S.; Louchami, K.; Malaisse, W.J.; Sener, A. Glucose Transport by Acinar Cells in Rat Parotid Glands. Cell. Physiol. Biochem. 2012, 29, 325–330. [Google Scholar] [CrossRef]
- Cetik, S.; Hupkens, E.; Malaisse, W.J.; Sener, A.; Popescu, I.R. Expression and Localization of Glucose Transporters in Rodent Submandibular Salivary Glands. Cell. Physiol. Biochem. 2014, 33, 1149–1161. [Google Scholar] [CrossRef]
- Cohen, C.; Mekinian, A.; Uzunhan, Y.; Fauchais, A.L.; Dhote, R.; Pop, G.; Eder, V.; Nunes, H.; Brillet, P.Y.; Valeyre, D.; et al. 18F-fluorodeoxyglucose positron emission tomography/computer tomography as an objective tool for assessing disease activity in Sjögren’s syndrome. Autoimmun. Rev. 2013, 12, 1109–1114. [Google Scholar] [CrossRef]
- Keraen, J.; Blanc, E.; Besson, F.L.; Leguern, V.; Meyer, C.; Henry, J.; Belkhir, R.; Nocturne, G.; Mariette, X.; Seror, R. Usefulness of 18F-Labeled Fluorodeoxyglucose–Positron Emission Tomography for the Diagnosis of Lymphoma in Primary Sjögren’s Syndrome. Arthritis Rheumatol. 2019, 71, 1147–1157. [Google Scholar] [CrossRef]
- Serizawa, I.; Inubushi, M.; Kanegae, K.; Morita, K.; Inoue, T.; Shiga, T.; Itoh, T.; Fukae, J.; Koike, T.; Tamaki, N. Lymphadenopathy due to amyloidosis secondary to Sjögren syndrome and systemic lupus erythematosus detected by F-18 FDG PET. Clin. Nucl. Med. 2007, 32, 881–882. [Google Scholar] [CrossRef]
- Ma, D.; Lu, H.; Qu, Y.; Wang, S.; Ying, Y.; Xiao, W. Primary Sjögren’s syndrome accompanied by pleural effusion: A case report and literature review. Int. J. Clin. Exp. Pathol. 2015, 8, 15322–15327. [Google Scholar]
- Perry, C.; Herishanu, Y.; Metzer, U.; Bairey, O.; Ruchlemer, R.; Trejo, L.; Naparstek, E.; Sapir, E.E.; Polliack, A. Diagnostic accuracy of PET/CT in patients with extranodal marginal zone MALT lymphoma. Eur. J. Haematol. 2007, 79, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Durmo, R.; Treglia, G.; Giubbini, R.; Bertagna, F. 18F-FDG PET/CT or PET Role in MALT Lymphoma: An Open Issue not Yet Solved—A Critical Review. Clin. Lymphoma Myeloma Leuk. 2020, 20, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Bural, G. Rare case of Primary Pulmonary Extranodal Non-Hodgkin’s Lymphoma in a Patient with Sjogrens Syndrome: Role of FDG-PET/CT in the Initial Staging and Evaluating Response to Treatment. Mol. Imaging Radionucl. Ther. 2012, 21, 117–120. [Google Scholar] [PubMed]
- Shih, W.J.; Ghesani, N.; Hongming, Z.; Alavi, A.; Schusper, S.; Mozley, D. F-18 FDG positron emission tomography demonstrates resolution of non-Hodgkin’s lymphoma of the parotid gland in a patient with Sjogren’s syndrome before and after anti-CD20 antibody rituximab therapy. Clin. Nucl. Med. 2002, 27, 142–143. [Google Scholar] [CrossRef]
- Cytawa, W.; Kircher, S.; Schirbel, A.; Shirai, T.; Fukushima, K.; Buck, A.K.; Wester, H.J.; Lapa, C. Chemokine Receptor 4 Expression in Primary Sjögren’s Syndrome. Clin. Nucl. Med. 2018, 43, 835–836. [Google Scholar] [CrossRef]
- Ambrosini, V.; Nanni, C.; Fanti, S. The use of gallium-68 labeled somatostatin receptors in PET/CT imaging. PET Clin. 2014, 9, 323–329. [Google Scholar] [CrossRef]
- Delli, K.; Haacke, E.A.; Kroese, F.G.M.; Pollard, R.P.; Ihrler, S.; van der Vegt, B.; Vissink, A.; Bootsma, H.; Spijkervet, F.K.L. Towards personalised treatment in primary Sjögren’s syndrome: Baseline parotid histopathology predicts responsiveness to rituximab treatment. Ann. Rheum. Dis. 2016, 75, 1933–1938. [Google Scholar] [CrossRef]
- Laban, K.G.; Kalmann, R.; Leguit, R.J.; De Keizer, B. Zirconium-89-labelled rituximab PET-CT in orbital inflammatory disease. EJNMMI Res. 2019, 9, 69. [Google Scholar] [CrossRef] [Green Version]
- Bruijnen, S.; Tsang-A-Sjoe, M.; Raterman, H.; Ramwadhdoebe, T.; Vugts, D.; van Dongen, G.; Huisman, M.; Hoekstra, O.; Tak, P.P.; Voskuyl, A.; et al. B-cell imaging with zirconium-89 labelled rituximab PET-CT at baseline is associated with therapeutic response 24weeks after initiation of rituximab treatment in rheumatoid arthritis patients. Arthritis Res. Ther. 2016, 18, 266. [Google Scholar] [CrossRef] [Green Version]
- Voulgarelis, M.; Tzioufas, A.G. Pathogenetic mechanisms in the initiation and perpetuation of Sjogren’s syndrome. Nat. Rev. 2010, 6, 529–537. [Google Scholar] [CrossRef]
- Di Gialleonardo, V.; Signore, A.; Glaudemans, A.W.J.M.; Dierckx, R.A.J.O.; De Vries, E.F.J. N-(4-18F-fluorobenzoyl)interleukin-2 for PET of human-activated T lymphocytes. J. Nucl. Med. 2012, 53, 679–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| 2016-ACR-EULAR [14] | 2012-ACR [15] | 2002-AECG [16] | |
|---|---|---|---|
| ESSDAI ≥ 1 | + (Entry Criterion) | − | − |
| Sicca Symptoms | + (Entry Criterion) | − | + |
| Salivary Gland Biopsy | + | + | + |
| Serology | |||
| Anti-Ro/SSA | + | + | + |
| Anti-La/SSB | − | + | + |
| Antinuclear Antibodies | − | + | − |
| Rheumatoid Factor | − | + | − |
| Oral Signs | |||
| UWS ≤ 0.1mL/Min | + | − | + |
| Sialography | − | − | + |
| Scintigraphy | − | − | + |
| Ocular Signs | |||
| Schirmer’s Test ≤ 5 | + | − | + |
| Ocular Staining (OSS or vBv) | + | + | + |
| Contribution To: | Advantages | Disadvantages | ||||
|---|---|---|---|---|---|---|
| Diagnosing pSS | Assessing Disease Activity/Disease Progression | Diagnosing pSS -Associated Lymphoma | Staging pSS-Associated Lymphoma | |||
| Salivary Gland Biopsy | +++ | + | +++ | - | -Gold Standard of Salivary and Lacrimal Gland MALT Lymphoma Diagnosis | -Invasive -Risk of Sampling Error |
| Sialography | + | + | − | − | -Moderate to High Sensitivity and Specificity | -Invasive -Contrast Medium |
| MRI | + | + | + | + | -High Spatial Resolution -Useful in Local Staging of PSS-Associated Lymphomas of Salivary and Lacrimal Glands | -Expensive -Moderate Differentiation Between Benign and Malignant Lesions of Salivary and Lacrimal Glands |
| Ultrasound | ++ | + | − | − | -Noninvasive -Widely Available | -No Consensus Scoring System |
| Sialendoscopy | − | − | − | − | -Possible Therapeutic Effect of Rinsing the Ductal System | -Invasive -No Added Value in Diagnostic Work-Up |
| Scintigraphy with 99mTc-Pertechnetate | + | + | − | − | -Possibility of Whole-Body Imaging | -Low Specificity -Low Spatial Resolution |
| 18F-FDG-PET/CT | + | ++ | + | +++ | -Whole-Body Imaging -Useful in Assessing Treatment Response -Objective Quantification Possible | -Expensive -No Exact Interpretation Criteria for pSS Available |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Ginkel, M.S.; Glaudemans, A.W.J.M.; van der Vegt, B.; Mossel, E.; Kroese, F.G.M.; Bootsma, H.; Vissink, A. Imaging in Primary Sjögren’s Syndrome. J. Clin. Med. 2020, 9, 2492. https://doi.org/10.3390/jcm9082492
van Ginkel MS, Glaudemans AWJM, van der Vegt B, Mossel E, Kroese FGM, Bootsma H, Vissink A. Imaging in Primary Sjögren’s Syndrome. Journal of Clinical Medicine. 2020; 9(8):2492. https://doi.org/10.3390/jcm9082492
Chicago/Turabian Stylevan Ginkel, Martha S., Andor W.J.M. Glaudemans, Bert van der Vegt, Esther Mossel, Frans G.M. Kroese, Hendrika Bootsma, and Arjan Vissink. 2020. "Imaging in Primary Sjögren’s Syndrome" Journal of Clinical Medicine 9, no. 8: 2492. https://doi.org/10.3390/jcm9082492
APA Stylevan Ginkel, M. S., Glaudemans, A. W. J. M., van der Vegt, B., Mossel, E., Kroese, F. G. M., Bootsma, H., & Vissink, A. (2020). Imaging in Primary Sjögren’s Syndrome. Journal of Clinical Medicine, 9(8), 2492. https://doi.org/10.3390/jcm9082492







