Optimal Harvest Time for Preventing Hot Pepper Seed Browning during Cold Storage Is Associated with Seed Maturity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Storage Conditions
2.2. Fruit Quality Evaluation
2.3. Antioxidant, Total Phenol Analysis
2.4. Gene Expression Analysis
2.5. Anatomical Analysis under Light Microscopy and Scanning Electron Microscopy
2.6. Statistical Analysis
3. Results and Discussion
3.1. Fruit Quality
3.2. Harvest Time and Seed Browning
3.3. Antioxidant Capacity, Total Phenol Content
3.4. Analysis of the Expression Levels of Antioxidant- and Cell Wall-Related Genes During Storage
3.5. Anatomical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- El-Ramady, H.R.; Domokos-Szabolcsy, É.; Abdalla, N.A.; Taha, H.S.; Fári, M. Postharvest management of fruit and vegetables storage. Sustain. Agric. Rev. 2015, 15, 65–152. [Google Scholar]
- Shin, S.Y.; Park, M.H.; Choi, J.W.; Kim, J.G. Gene network underlying the response of harvested pepper to chilling stress. J. Plant Physiol. 2017, 219, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Özden, Ç.; Bayindirli, L. Effects of combinational use of controlled atmosphere, cold storage and edible coating applications on shelf life and quality attributes of green peppers. Eur. Food Res. Technol. 2002, 214, 320–326. [Google Scholar] [CrossRef]
- Lim, C.S.; Woolf, A.B. Varietal differences of chilling-induced physiological responses and quality attributes in pepper (Capsicum annuum L.) cultivars during low temperature storage. Hortic. Environ. Biotechnol. 2010, 51, 531–538. [Google Scholar]
- Boonsiri, K.; Ketsay, S.; Van Doorn, W.G. Seed browning of hot peppers during low temperature storage. Postharvest Biol. Technol. 2007, 45, 358–365. [Google Scholar] [CrossRef]
- Lim, C.S.; Kang, S.M.; Cho, J.L.; Gross, K.C. Antioxidizing enzyme activities in chilling-sensitive and chilling-tolerant pepper fruit as affected by stage of ripeness and storage temperature. J. Am. Soc. Hortic. Sci. 2009, 134, 156–163. [Google Scholar] [CrossRef]
- Wang, Q.; Ding, T.; Gao, L.; Pang, J.; Yang, N. Effect of brassinolide on chilling injury of green bell pepper in storage. Sci. Hortic. 2012, 144, 195–200. [Google Scholar] [CrossRef]
- Wang, F.; Guo, Z.; Li, H.; Wang, M.; Onac, E.; Zhou, J.; Zhou, Y. Phytochrome A and B function antagonistically to regulate cold tolerance via abscisic acid-dependent jasmonate signaling. Plant Physiol. 2016, 170, 459–471. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.G.; Yi, G.; Choi, J.H.; Lee, E.J. Analyses of targeted/untargeted metabolites and reactive oxygen species of pepper fruits provide insights into seed browning induced by chilling. Food Chem. 2020, 332, 127406. [Google Scholar] [CrossRef]
- Seo, J.; Yi, G.; Lee, J.G.; Choi, J.H.; Lee, E.J. Seed browning in pepper (Capsicum annuum L.) fruit during cold storage is inhibited by methyl jasmonate or induced by methyl salicylate. Postharvest Biol. Technol. 2020, 166, 111210. [Google Scholar] [CrossRef]
- Lurie, S.; Watkins, C.B. Superficial scald, its etiology and control. Postharvest Biol. Technol. 2012, 65, 44–60. [Google Scholar] [CrossRef]
- Vega-Garcia, M.O.; Lopez-Espinoza, G.; Ontiveros, J.C.; Caro-Corrales, J.J.; Vargas, F.D.; Lopez-Valenzuela, J.A. Changes in protein expression associated with chilling injury in tomato fruit. J. Am. Soc. Hortic. Sci. 2010, 135, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Saltveit, M.E. Fruit ripening and fruit quality. Crop Prod. Sci. Hortic. 2005, 13, 145. [Google Scholar]
- Yang, J.; Fu, M.R.; Zhao, Y.Y.; Mao, L.C. Reduction of chilling injury and ultrastructural damage in cherry tomato fruits after hot water treatment. Agric. Sci. China 2009, 8, 304–310. [Google Scholar] [CrossRef]
- Park, M.H.; Sangwanangkul, P.; Choi, J.W. Reduced chilling injury and delayed fruit ripening in tomatoes with modified atmosphere and humidity packaging. Sci. Hortic. 2018, 231, 66–72. [Google Scholar] [CrossRef]
- Lin, W.C.; Hall, J.W.; Saltveit, M.E. Ripening stage affects the chilling sensitivity of greenhouse grown peppers. J. Am. Soc. Hortic. Sci. 1993, 118, 791–795. [Google Scholar] [CrossRef] [Green Version]
- Abe, K.; Chachin, K.; Ogata, K. Chilling injury in eggplant fruits. II. The effects of maturation and harvesting season on pitting injury and browning of seeds and pulp during storage. J. Jpn. Soc. Hort. Sci. 1976, 45, 307–312. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Bel, P.; Egea, I.; Sanchez-Ballesta, M.T.; Martinez-Madrid, C.; Fernandez-Garcia, N.; Romojaro, F.; Olmos, E.; Estrella, E.; Bolarín, M.C.; Flores, F.B. Understanding the mechanisms of chilling injury in bell pepper fruits using the proteomic approach. J. Proteom. 2012, 75, 5463–5478. [Google Scholar] [CrossRef] [Green Version]
- Cao, S.; Zheng, Y.; Wang, K.; Jin, P.; Rui, H. Methyl jasmonate reduces chilling injury and enhances antioxidant enzyme activity in postharvest loquat fruit. Food Chem. 2009, 115, 1458–1463. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Dietz, B.M.; Kang, Y.H.; Liu, G.; Eggler, A.L.; Yao, P.; Chadwick, L.R.; Pauli, G.F.; Farnsworth, N.R.; Mesecar, A.D.; Van Breemen, R.B.; et al. Xanthohumol isolated from Humulus lupulus inhibits menadione-induced DNA damage through induction of quinone reductase. Chem. Res. Toxicol. 2005, 18, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrigrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Livak, K.J.; Schimittgen, T.D. Analysis of relative gene expression data using realtime quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Clément, C.; Burrus, M.; Audran, J.C. Floral organ growth and carbohydrate content during pollen development in Lilium. Am. J. Bot. 1996, 83, 459–469. [Google Scholar] [CrossRef]
- Rao, T.R.; Gol, N.B.; Shah, K.K. Effect of postharvest treatments and storage temperatures on the quality and shelf life of sweet pepper (Capsicum annum L.). Sci. Hortic. 2011, 132, 18–26. [Google Scholar] [CrossRef]
- Panigrahi, J.; Patel, M.; Patel, N.; Gheewala, B.; Gantait, S. Changes in antioxidant and biochemical activities in castor oil-coated Capsicum annuum L. during postharvest storage. 3 Biotech 2018, 8, 280. [Google Scholar] [CrossRef] [Green Version]
- Shewfelt, R.L.; Del Rosario, B.A. The role of lipid peroxidation in storage disorders of fresh fruits and vegetables. HortScience 2000, 35, 575–579. [Google Scholar] [CrossRef]
- Jang, Y.K.; Jung, E.S.; Lee, H.A.; Choi, D.; Lee, C.H. Metabolomic characterization of hot pepper (Capsicum annuum “CM334”) during fruit development. J. Agric. Food Chem. 2015, 63, 9452–9460. [Google Scholar] [CrossRef]
- Vamos Vigyazo, L.; Haard, N.F. Polyphenol oxidases and peroxidases in fruits and vegetables. Crit. Rev. Food Sci. Nutr. 1981, 15, 49–127. [Google Scholar] [CrossRef]
- Trainotti, L.; Ferrarese, L.; Poznanski, E.; Dalla Vecchia, F. Endo-β-1, 4-glucanase activity is involved in the abscission of pepper flowers. J. Plant Physiol. 1998, 152, 70–77. [Google Scholar] [CrossRef]
- Lashbrook, C.C.; Gonzalez-Bosch, C.; Bennett, A.B. Two divergent endo-beta-1, 4-glucanase genes exhibit overlapping expression in ripening fruit and abscising flowers. Plant Cell 1994, 6, 1485–1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, J.A.; Gonzalez-Carranza, Z. Plant Cell Separation and Adhesion; Blackwell: Oxford, UK, 2007. [Google Scholar]
- Ogasawara, S.; Abe, K.; Nakajima, T. Pepper beta-galactosidase 1 (PBG1) plays a significant role in fruit ripening in bell pepper (Capsicum annuum). Biosci. Biotechnol. Biochem. 2007, 71, 309–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, Z.M.; Chin, L.H.; Marimuthu, M.; Lazan, H. Low temperature storage and modified atmosphere packaging of carambola fruit and their effects on ripening related texture changes, wall modification and CI symptoms. Postharvest Biol. Technol. 2004, 22, 181–192. [Google Scholar] [CrossRef]
- Pagamas, P.; Nawata, E. Sensitive stages of fruit and seed development of chili pepper (Capsicum annuum L. var. Shishito) exposed to high-temperature stress. Sci. Hortic. 2008, 117, 21–25. [Google Scholar] [CrossRef]
- Tully, R.E.; Musgrave, M.E.; Leopold, A.C. The seed coat as a control of imbibitional chilling injury. Crop Sci. 1981, 21, 312–317. [Google Scholar] [CrossRef]
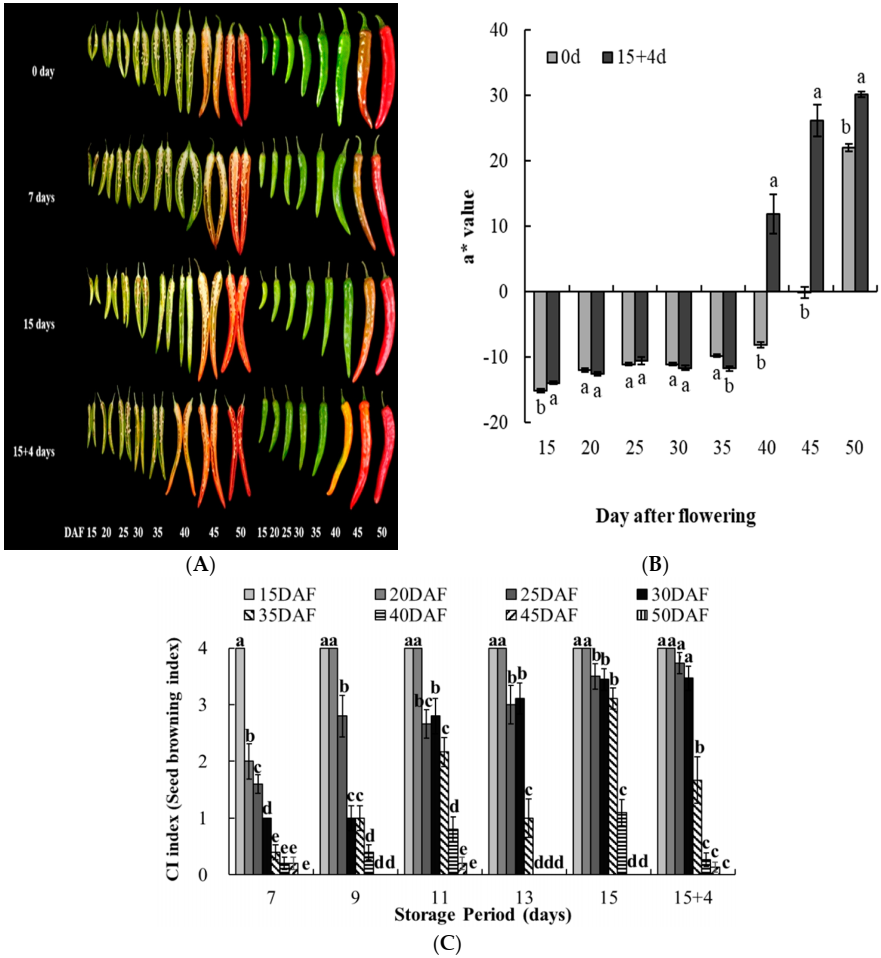
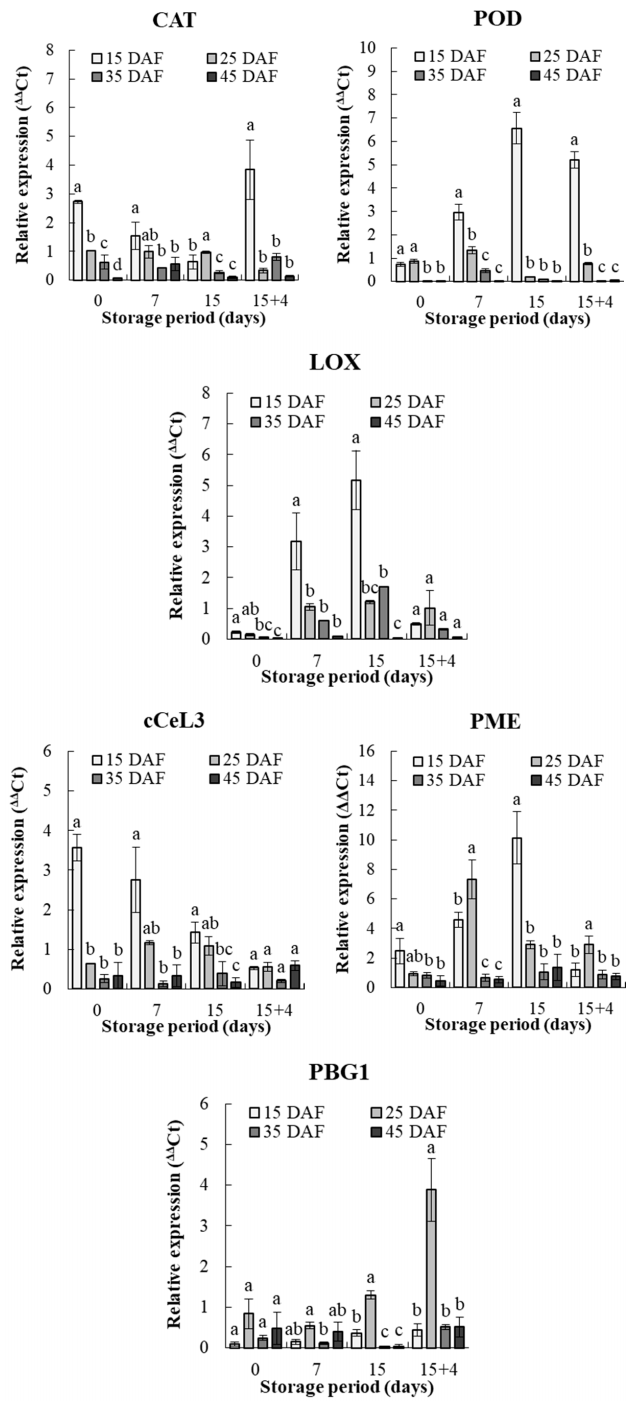


| 0 day | 7 days | 15 days | 15 + 4 days | |
|---|---|---|---|---|
| Firmness (N) | ||||
| 15 DAF | 9.63 ± 0.34 Fa | 10.46 ± 0.32 Da | 6.51 ± 0.43 CDb | 5.12 ± 0.24 CDc |
| 20 DAF | 11.05 ± 0.37 Ea | 10.63 ± 0.25 Da | 6.08 ± 0.26 Db | 4.85 ± 0.20 Dc |
| 25 DAF | 12.94 ± 0.33 Da | 12.62 ± 0.39 Ca | 7.28 ± 0.24 Cb | 5.35 ± 0.26 CDc |
| 30 DAF | 13.80 ± 0.31 CDa | 11.82 ± 0.33 Cb | 8.67 ± 0.37 Bc | 5.15 ± 0.28 CDd |
| 35 DAF | 15.76 ± 0.40 Ba | 11.94 ± 0.43 Cb | 9.23 ± 0.28 Bc | 6.55 ± 0.25 Bd |
| 40 DAF | 18.91 ± 0.49 Aa | 14.42 ± 0.49 Bb | 10.71 ± 0.21 Ac | 7.55 ± 0.34 Ad |
| 45 DAF | 18.16 ± 0.43 Aa | 15.97 ± 0.44 Ab | 11.20 ± 0.25 Ac | 7.94 ± 0.38 Ad |
| 50 DAF | 14.75 ± 0.33 BCa | 13.97 ± 0.30 Bb | 8.65 ± 0.25 Bc | 5.73 ± 0.20 Cd |
| Soluble solids content (%) | ||||
| 15 DAF | 5.53 ± 0.12 Dd | 6.40 ± 0.12 DEc | 6.90 ± 0.00 Cb | 7.70 ± 0.00 Da |
| 20 DAF | 5.30 ± 0.06 Ec | 6.67 ± 0.03 Dab | 6.60 ± 0.00 Cb | 6.83 ± 0.09 Ea |
| 25 DAF | 5.30 ± 0.00 Ed | 6.07 ± 0.13 EFc | 6.60 ± 0.06 Cb | 7.17 ± 0.15 DEa |
| 30 DAF | 5.33 ± 0.03 Ed | 5.93 ± 0.03 Fc | 6.60 ± 0.06 Cb | 7.07 ± 0.15 DEa |
| 35 DAF | 5.37 ± 0.03 DEc | 6.20 ± 0.17 EFb | 6.50 ± 0.06 Cb | 6.97 ± 0.18 Ea |
| 40 DAF | 5.90 ± 0.00 Cc | 7.20 ± 0.12 Cb | 6.87 ± 0.15 Cb | 8.87 ± 0.50 Ca |
| 45 DAF | 7.33 ± 0.07 Bc | 8.53 ± 0.15 Bb | 9.60 ± 0.12 Ba | 9.80 ± 0.25 Ba |
| 50 DAF | 9.83 ± 0.03 Ad | 10.60 ± 0.17 Ac | 11.97 ± 0.32 Ab | 13.50 ± 0.00 Aa |
| Titratable acidity (%) | ||||
| 15 DAF | 0.12 ± 0.00 Ga | 0.07 ± 0.00 Fb | 0.07 ± 0.00 Gb | 0.07 ± 0.00 Db |
| 20 DAF | 0.13 ± 0.00 Gb | 0.17 ± 0.01 Ea | 0.08 ± 0.00 Fc | 0.07 ± 0.00 Dc |
| 25 DAF | 0.21 ± 0.00 Fa | 0.21 ± 0.01 Da | 0.10 ± 0.00 DEb | 0.10 ± 0.02 Cb |
| 30 DAF | 0.25 ± 0.02 Ea | 0.23 ± 0.01 Da | 0.09 ± 0.00 Eb | 0.12 ± 0.02 Cb |
| 35 DAF | 0.29 ± 0.01 Da | 0.25 ± 0.00 Db | 0.10 ± 0.00 Dc | 0.11 ± 0.01 Cc |
| 40 DAF | 0.34 ± 0.00 Ca | 0.34 ± 0.01 Ca | 0.11 ± 0.00 Cc | 0.20 ± 0.01 Bb |
| 45 DAF | 0.45 ± 0.01 Ba | 0.45 ± 0.01 Ba | 0.19 ± 0.00 Bc | 0.23 ± 0.00 ABb |
| 50 DAF | 0.52 ± 0.03 Aa | 0.54 ± 0.01 Aa | 0.22 ± 0.00 Ab | 0.24 ± 0.00 Ab |
| 0 day | 7 days | 15 days | 15 + 4 days | |
|---|---|---|---|---|
| DPPH (Trolox equivalent, μmol/g DW) | ||||
| 15 DAF | 5.19 ± 0.07 Aa | 4.79 ± 0.10 Db | 4.76 ± 0.02 Cb | 3.64 ± 0.05 Cc |
| 20 DAF | 5.13 ± 0.08 Ab | 5.42 ± 0.03 Aa | 5.20 ± 0.02 Ab | 3.18 ± 0.04 Dc |
| 25 DAF | 4.59 ± 0.09 Cb | 5.01 ± 0.02 Ca | 4.94 ± 0.04 Ba | 3.73 ± 0.02 Cc |
| 30 DAF | 4.60 ± 0.06 Cc | 5.23 ± 0.02 Ba | 4.94 ± 0.02 Bb | 3.90 ± 0.03 Bd |
| 35 DAF | 4.93 ± 0.04 Bb | 5.08 ± 0.03 Ca | 4.85 ± 0.03 BCb | 4.18 ± 0.07 Ac |
| 40 DAF | 3.88 ± 0.02 Eb | 4.44 ± 0.07 Ea | 4.44 ± 0.06 Da | 3.98 ± 0.03 Bb |
| 45 DAF | 4.36 ± 0.04 Da | 4.03 ± 0.03 Fc | 4.20 ± 0.03 Eb | 3.90 ± 0.06 Bc |
| 50 DAF | 3.89 ± 0.05 Eb | 4.04 ± 0.04 Fa | 3.93 ± 0.04 Fab | 3.99 ± 0.04 Bab |
| ABTS (Trolox equivalent, μmol/g DW) | ||||
| 15 DAF | 15.78 ± 1.09 ABa | 15.65 ± 1.05 Ca | 13.33 ± 0.72 CDa | 14.02 ± 0.91 ABa |
| 20 DAF | 17.40 ± 1.15 Ab | 21.16 ± 0.61 Aa | 17.50 ± 0.73 Ab | 12.13 ± 0.67 Cc |
| 25 DAF | 14.69 ± 0.95 Bb | 17.60 ± 0.36 Ba | 17.12 ± 0.50 ABa | 14.71 ± 0.34 Ab |
| 30 DAF | 16.96 ± 0.58 ABab | 18.39 ± 0.73 Ba | 16.35 ± 0.57 ABb | 14.08 ± 0.49 ABc |
| 35 DAF | 14.97 ± 0.78 ABa | 15.58 ± 0.72 Ca | 15.18 ± 0.74 BCa | 12.77 ± 0.53 BCb |
| 40 DAF | 7.33 ± 0.43 Db | 11.11 ± 0.46 Da | 11.62 ± 1.00 Da | 8.50 ± 0.60 Db |
| 45 DAF | 10.94 ± 0.58 Ca | 9.53 ± 0.47 Dab | 8.06 ± 0.57 Eb | 7.89 ± 0.63 Db |
| 50 DAF | 8.55 ± 0.62 Dab | 9.29 ± 0.47 Da | 7.29 ± 0.62 Eb | 8.69 ± 0.64 Dab |
| Total phenolics (gallic acid equivalent, mg/g DW) | ||||
| 15 DAF | 4.66 ± 0.01 Aa | 4.57 ± 0.00 Ab | 4.53 ± 0.00 Ac | 3.33 ± 0.00 Ad |
| 20 DAF | 3.67 ± 0.02 Bb | 4.01 ± 0.01 Ba | 4.04 ± 0.04 Ba | 2.66 ± 0.01 Bc |
| 25 DAF | 2.65 ± 0.02 Db | 2.56 ± 0.01 Cc | 3.41 ± 0.01 Ca | 2.54 ± 0.01 Cc |
| 30 DAF | 2.73 ± 0.02 Ca | 2.44 ± 0.00 Db | 2.46 ± 0.01 Db | 2.20 ± 0.00 Dc |
| 35 DAF | 1.88 ± 0.02 Ec | 2.22 ± 0.01 Eb | 2.29 ± 0.01 Ea | 1.86 ± 0.01 Ec |
| 40 DAF | 1.11 ± 0.01 Gd | 1.52 ± 0.01 Fb | 1.66 ± 0.01 Fa | 1.31 ± 0.01 Fc |
| 45 DAF | 1.54 ± 0.01 Fa | 1.20 ± 0.01 Gb | 1.15 ± 0.00 Gc | 1.21 ± 0.01 Gb |
| 50 DAF | 1.12 ± 0.01 Ga | 1.10 ± 0.02 Hab | 1.12 ± 0.01 Ga | 1.08 ± 0.01 Hb |
| 0 day | 7 days | 15 days | 15 + 4 days | |
|---|---|---|---|---|
| DPPH (Trolox equivalent, μmol/g DW) | ||||
| 15 DAF | 28.74 ± 1.21 Da | 26.05 ± 0.68 Cb | 20.08 ± 1.00 Ec | 29.17 ± 0.52 Da |
| 20 DAF | 35.96 ± 1.16 BCa | 27.01 ± 0.39 Cb | 29.70 ± 1.79 Cb | 28.03 ± 0.42 Db |
| 25 DAF | 34.43 ± 0.29 Ca | 28.23 ± 0.87 BCb | 28.23 ± 0.95 Cb | 36.06 ± 0.54 Ba |
| 30 DAF | 24.89 ± 0.33 Ec | 27.58 ± 0.46 BCb | 24.68 ± 0.35 Dc | 32.58 ± 0.95 Ca |
| 35 DAF | 23.67 ± 0.56 Ec | 26.97 ± 0.43 Cb | 22.24 ± 0.31 DEc | 31.65 ± 0.77 Ca |
| 40 DAF | 27.72 ± 0.98 Da | 22.13 ± 0.94 Db | 27.93 ± 0.65 Ca | 29.40 ± 0.34 Da |
| 45 DAF | 37.09 ± 0.49 Bab | 30.27 ± 1.60 Bc | 38.02 ± 0.42 Ba | 35.30 ± 0.50 Bb |
| 50 DAF | 42.64 ± 0.81 Ab | 39.99 ± 1.29 Ac | 47.83 ± 0.51 Aa | 40.61 ± 0.68 Abc |
| ABTS (Trolox equivalent, μmol/g DW) | ||||
| 15 DAF | 42.49 ± 1.44 Bb | 30.51 ± 0.49 Gc | 27.62 ± 0.19 Fd | 45.44 ± 0.53 Ca |
| 20 DAF | 45.40 ± 1.47 Ba | 38.69 ± 0.11 Eb | 45.98 ± 0.54 Ca | 44.29 ± 0.30 Ca |
| 25 DAF | 35.67 ± 1.53 Cc | 45.04 ± 0.52 Cb | 46.49 ± 0.26 Cb | 57.53 ± 0.12 Aa |
| 30 DAF | 33.58 ± 0.61 Cd | 46.13 ± 0.24 Bb | 37.20 ± 0.57 Ec | 52.33 ± 0.35 Ba |
| 35 DAF | 34.02 ± 1.96 Cc | 38.38 ± 0.13 Eb | 36.31 ± 0.33 Ebc | 44.07 ± 0.56 Ca |
| 40 DAF | 34.98 ± 1.76 Cb | 34.24 ± 0.18 Fb | 41.89 ± 0.50 Da | 40.78 ± 0.52 Da |
| 45 DAF | 42.87 ± 0.83 Bb | 41.69 ± 0.39 Db | 51.22 ± 0.32 Ba | 50.87 ± 0.89 Ba |
| 50 DAF | 50.53 ± 0.81 Ab | 50.58 ± 0.34 Ab | 55.76 ± 0.11 Aa | 51.80 ± 0.65 Bb |
| Total phenol (gallic acid equivalent, mg/g DW) | ||||
| 15 DAF | 3.71 ± 0.13 Cab | 3.46 ± 0.01 Eb | 3.70 ± 0.12 Dab | 3.82 ± 0.03 Da |
| 20 DAF | 3.91 ± 0.05 Ba | 3.53 ± 0.01 Dec | 3.76 ± 0.01 Db | 3.42 ± 0.02 Fd |
| 25 DAF | 3.71 ± 0.01 Cc | 3.79 ± 0.02 Cb | 3.82 ± 0.01 Db | 4.42 ± 0.02 Aa |
| 30 DAF | 3.23 ± 0.02 Dd | 4.00 ± 0.05 Ab | 3.46 ± 0.00 Ec | 4.24 ± 0.01 Ba |
| 35 DAF | 3.31 ± 0.01 Dc | 3.59 ± 0.02 Db | 3.13 ± 0.02 Fd | 3.88 ± 0.02 Ca |
| 40 DAF | 3.37 ± 0.01 Dc | 3.39 ± 0.02 Fc | 4.18 ± 0.02 Ca | 3.65 ± 0.01 Eb |
| 45 DAF | 3.67 ± 0.06 Cd | 3.93 ± 0.01 Bc | 4.39 ± 0.01 Ba | 4.27 ± 0.02 Bb |
| 50 DAF | 4.19 ± 0.01 Ac | 4.06 ± 0.02 Ad | 4.75 ± 0.02 Aa | 4.41 ± 0.00 Ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, M.-H.; Lee, J.-S.; Yang, E.-Y.; Do, G.-R.; Hong, Y.-P. Optimal Harvest Time for Preventing Hot Pepper Seed Browning during Cold Storage Is Associated with Seed Maturity. Agriculture 2020, 10, 585. https://doi.org/10.3390/agriculture10120585
Park M-H, Lee J-S, Yang E-Y, Do G-R, Hong Y-P. Optimal Harvest Time for Preventing Hot Pepper Seed Browning during Cold Storage Is Associated with Seed Maturity. Agriculture. 2020; 10(12):585. https://doi.org/10.3390/agriculture10120585
Chicago/Turabian StylePark, Me-Hea, Jung-Soo Lee, Eun-Young Yang, Gyung-Ran Do, and Yoon-Pyo Hong. 2020. "Optimal Harvest Time for Preventing Hot Pepper Seed Browning during Cold Storage Is Associated with Seed Maturity" Agriculture 10, no. 12: 585. https://doi.org/10.3390/agriculture10120585




