Growth Responses and Accumulation Characteristics of Three Ornamental Plants to Sn Contamination in Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrates and Plants
2.2. Plant Growth Experiment
2.3. Substrates and Plants
in substrate (in soil))
3. Results
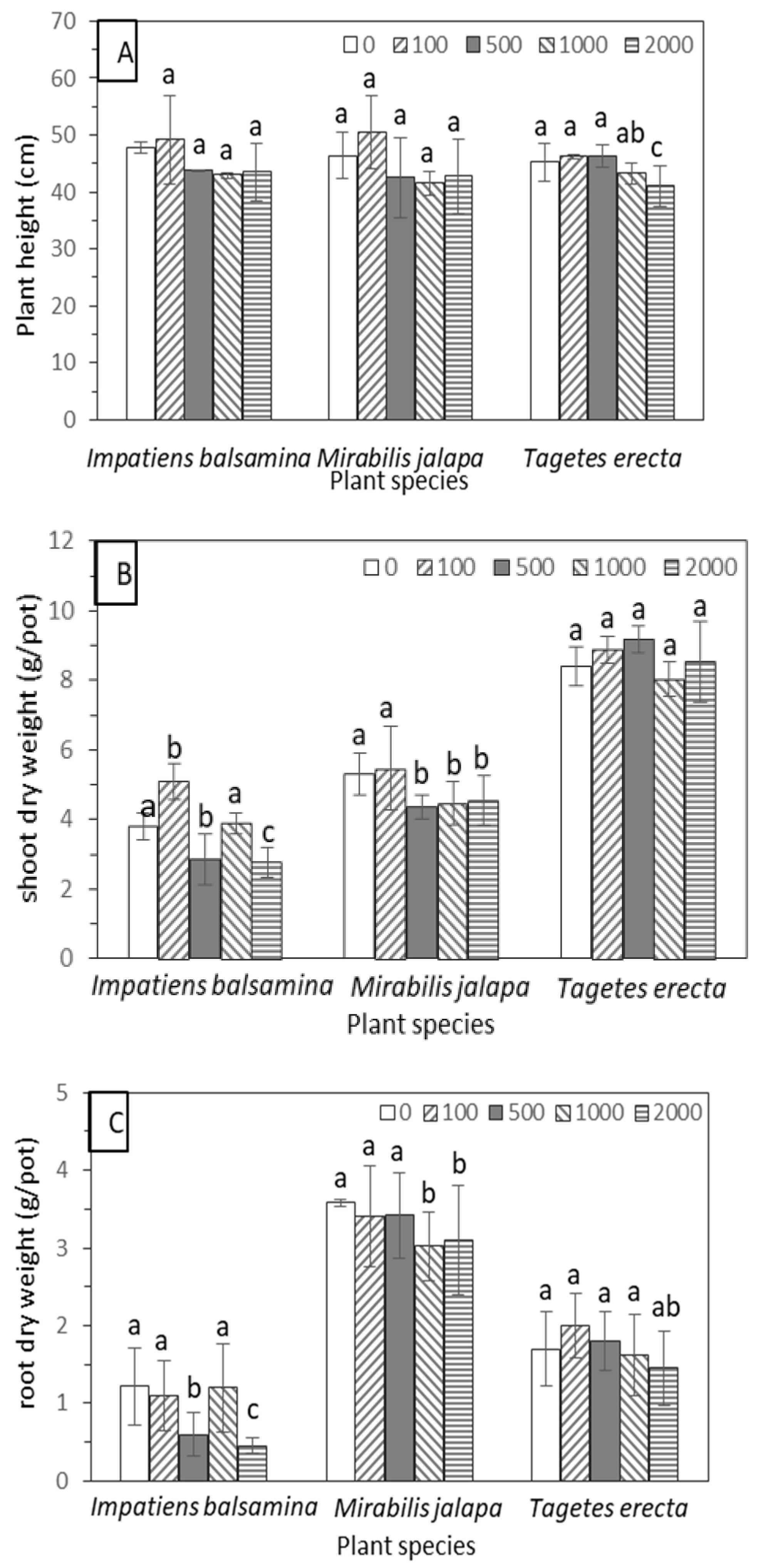
3.1. Sn Tolerance under Soil Culture Conditions
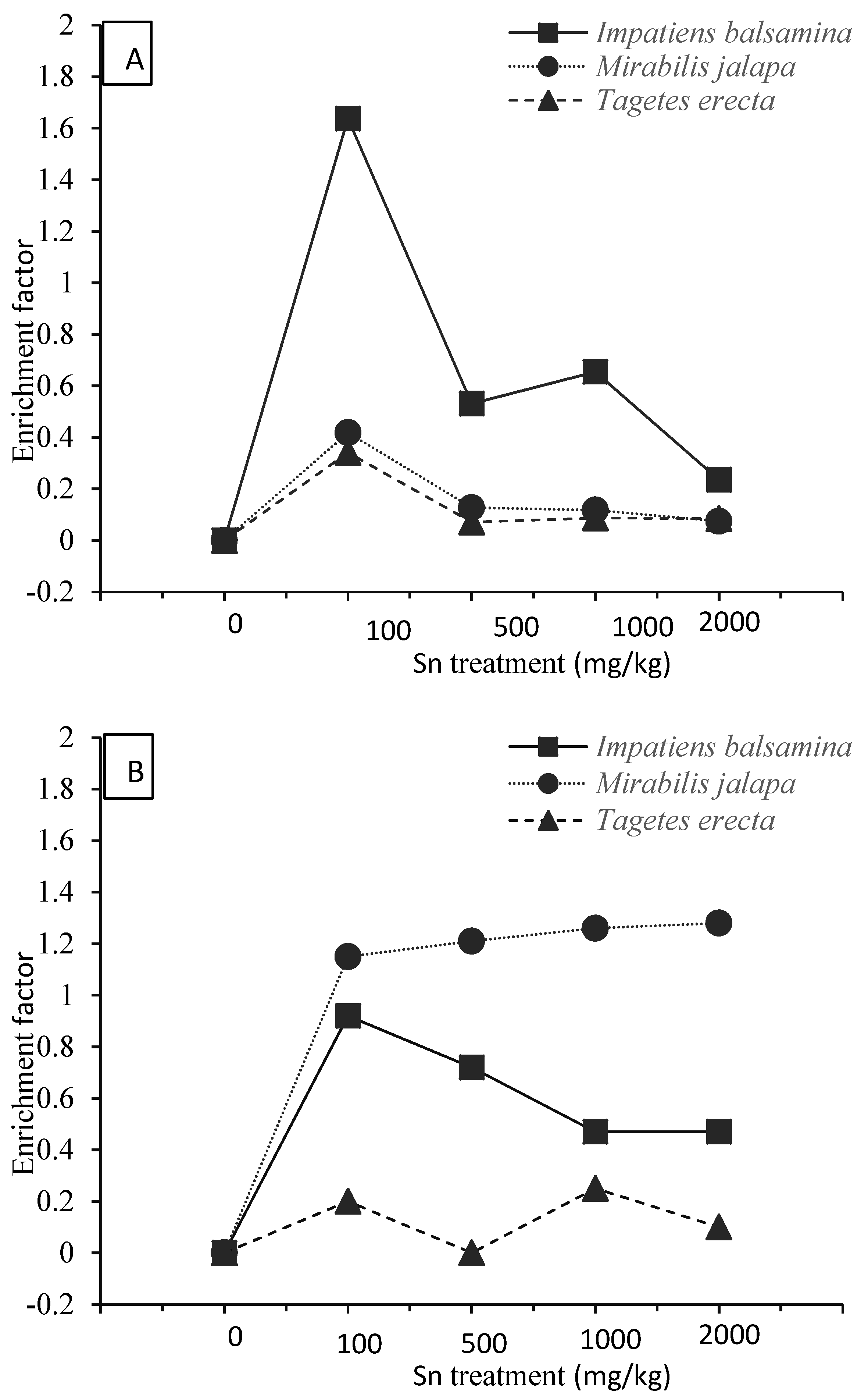
3.2. Sn Accumulation Characteristics under Soil Culture Conditions
3.3. Sn Accumulation Characteristics under Soil Culture Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Howe, P.; Wood, M.; Watts, P. Tin and Inorganic Tin Compounds; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Cooney, J.J.; Wuertz, S. Toxic effects of tin compounds on microorganisms. J. Ind. Microbiol. Biotechnol. 1989, 4, 375–402. [Google Scholar] [CrossRef]
- Harper, C.; Llados, F. Toxicological Profile for Tin and Tin Compounds, in Organotin Compounds; U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2005.
- Shao, J.P.; Song, X.B.; Li, G.X.; Zhang, Q.; Fang, X.J.; He, L.P. Evaluation of soil fertility and heavy metal contamination in abandoned regions of tin mine, China. In International Forum on Energy, Environment Science and Materials (IFEESM 2015); Atlantis Press: Shenzhen, China, 2015. [Google Scholar]
- Gan, F.W.; Fang, W.X.; Wang, X.L.; Yang, S.F. The heavy metal contamination in soil-potato and pea of tin tailings. Ecol. Environ. 2008, 5, 1847–1852. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Maah, M.J.; Yusoff, I. Heavy metals accumulation in plants growing in ex tin mining catchment. Int. J. Environ. Sci. Technol. 2011, 8, 401–416. [Google Scholar] [CrossRef] [Green Version]
- Schreck, E.; Foucault, Y.; Sarret, G.; Sobanska, S.; Cécillon, L.; Castrec-Rouelle, M.; Uzu, G.; Dumat, C. Metal and metalloid foliar uptake by various plant species exposed to atmospheric industrial fallout: Mechanisms involved for lead. Sci. Total Environ. 2012, 427, 253–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franzaring, J.; Damsohn, W.; Fangmeier, A.; Schlosser, S.; Kurz, H.; Buttner, P. Phytotoxicity of tin mine waste and accumulation of involved heavy metals in common buckwheat (Fagopyrum esculentum Moench). Int. J. Phytoremediat. 2018, 20, 462–470. [Google Scholar] [CrossRef]
- Hou, A.; Takamatsu, T.; Koshikawa, M.K.; Hosomi, M. Migration of silver, indium, tin, antimony, and bismuth and variations in their chemical fractions on addition to uncontaminated soils. Soil Sci. 2005, 170, 624–639. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; Taylor & Francis Group: New York, NY, USA, 2011. [Google Scholar]
- Elbana, T.A.; Sparks, D.L.; Selim, H.M. Transport of tin and lead in soils: Miscible displacement experiments and second-order modeling. Soil Sci. Soc. Am. J. 2014, 78, 701–712. [Google Scholar] [CrossRef]
- Murata, T.; Koshikawa, M.K.; Watanabe, M.; Hou, H.; Takamatsu, T. Migration of Ag, In, Sn, Sb, and Bi and their chemical forms in a monolith lysimeter filled with a contaminated andosol. Arch. Environ. Contam. Toxicol. 2018, 74, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Nakamaru, Y.; Uchida, S. Distribution coefficients of tin in Japanese agricultural soils and the factors affecting tin sorption behavior. J. Environ. Radioact. 2008, 99, 1003–1010. [Google Scholar] [CrossRef]
- Pulford, I.D.; Watson, C. Phytoremediation of heavy metal-contaminated land by trees—A review. Environ. Int. 2003, 29, 529–540. [Google Scholar] [CrossRef]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, A. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, B.; Wang, L.A.; Urbanovich, O.; Nagorskaya, L.; Li, X.; Tang, L. A review on phytoremediation of mercury contaminated soils. J. Hazard. Mater. 2020, 400, 123138. [Google Scholar] [CrossRef] [PubMed]
- Perrino, E.V.; Brunetti, G.; Farrag, K. Plant communities in multi-metal contaminated soils: A case study in the national park of Alta Murgia (Apulia region—southern Italy). Int. J. Phytorem. 2014, 16, 871–888. [Google Scholar] [CrossRef]
- Liu, J.N.; Xin, X.; Zhou, Q.X. Phytoremediation of contaminated soils using ornamental plants. Environ. Rev. 2017, 26, 43–54. [Google Scholar] [CrossRef]
- Chandrasekhar, C.; Ray, J.G. Lead accumulation, growth responses and biochemical changes of three plant species exposed to soil amended with different concentrations of lead nitrate. Ecotox. Environ. Saf. 2019, 171, 26–36. [Google Scholar] [CrossRef]
- Ancona, V.; Caracciolo, A.B.; Campanale, C.; Rascio, I.; Grenni, P.; Di Lenola, M.; Bagnuolo, G.; Uricchio, V.F. Heavy metal phytoremediation of a poplar clone in a contaminated soil in southern Italy. J. Chem. Technol. Biotechnol. 2020, 95, 940–949. [Google Scholar] [CrossRef]
- Mun, H.W.; Hoe, A.L.; Koo, L.D. Assessment of Pb uptake, translocation and immobilization in kenaf (Hibiscus cannabinus L.) for phytoremediation of sand tailings. J. Environ. Sci. 2008, 20, 1341–1347. [Google Scholar] [CrossRef]
- Awa, S.H.; Hadibarata, T. Removal of heavy metals in contaminated soil by phytoremediation mechanism: A review. Water Air Soil Pollut. 2020, 231, 47. [Google Scholar] [CrossRef]
- Penalosa, J.M.; Carpena, R.O.; Vazquez, S.; Agha, R.; Granado, A.; Sarro, M.J.; Esteban, E. Chelate-assisted phytoextraction of heavy metals in a soil contaminated with a pyritic sludge. Sci. Total Environ. 2007, 378, 199–204. [Google Scholar] [CrossRef]
- Beiyuan, J.; Tsang, D.C.W.; Bolan, N.S.; Baek, K.; OK, Y.S.; Li, X.D. Interactions of food waste compost with metals and metal-chelant complexes during soil remediation. J. Clean. Prod. 2018, 192, 199–206. [Google Scholar] [CrossRef]
- Wei, S.H.; Xu, L.; Dai, H.P.; Hu, Y.H. Ornamental hyperaccumulator Mirabilis jalapa L. phytoremediating combine contaminated soil enhanced by some chelators and surfactants. Environ. Sci. Pollut. Res. 2018, 25, 29699–29704. [Google Scholar] [CrossRef] [PubMed]
- Gul, I.; Manzoor, M.; Kallerhoff, J.; Arshad, M. Enhanced phytoremediation of lead by soil applied organic and inorganic amendments: Pb phytoavailability, accumulation and metal recovery. Chemosphere 2020, 258, 127405. [Google Scholar] [CrossRef]
- Simiele, M.; Lebrun, M.; Miard, F.; Trupiano, D.; Poupart, P.; Forestier, O.; Scippa, G.S.; Bourgerie, S.; Morabito, D. Assisted phytoremediation of a former mine soil using biochar and iron sulphate: Effects on As soil immobilization and accumulation in three Salicaceae species. Sci. Total Environ. 2020, 710, 136203. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, F.; Liu, Q.; Chen, M.; Liu, X.L.; Wang, Y.Y.; Sun, Y.; Zhang, L.L. Nanomaterials and plants: Positive effects, toxicity and the remediation of metal and metalloid pollution in soil. Sci. Total Environ. 2019, 662, 414–421. [Google Scholar] [CrossRef]
- Jusselme, M.D.; Miambi, E.; Mora, P.; Diouf, M.; Rouland-Lefevre, C. Increased lead availability and enzyme activities in root-adhering soil of Lantana camara during phytoextraction in the presence of earthworms. Sci. Total Environ. 2013, 445, 101–109. [Google Scholar] [CrossRef]
- Petriccione, M.; Di Patre, D.; Ferrante, P.; Papa, S.; Bartoli, G.; Fioretto, A.; Scortichini, M. Effects of Pseudomonas fluorescens seed bioinoculation on heavy metal accumulation for Mirabilis jalapa phytoextraction in smelter-contaminated soil. Water Air Soil Pollut. 2013, 224, 1645. [Google Scholar] [CrossRef] [Green Version]
- Yasin, N.A.; Khan, W.U.; Ahmad, S.R.; Ali, A.; Ahmad, A.; Akram, W. Effect of Enterobacter sp. Cs2 and edta on the phytoremediation of Ni-contaminated soil by Impatiens balsamina. Pol. J. Environ. Stud. 2019, 28, 425–433. [Google Scholar] [CrossRef]
- Elhindi, K.M.; Al-Mana, F.A.; El-Hendawy, S.; Al-Selwey, W.A.; Elgorban, A.M. Arbuscular mycorrhizal fungi mitigates heavy metal toxicity adverse effects in sewage water contaminated soil on Tagetes erecta L. Soil Sci. Plant Nutr. 2018, 64, 662–668. [Google Scholar] [CrossRef] [Green Version]
- Jin, Z.M.; Deng, S.Q.; Wen, Y.C.; Jin, Y.F.; Pan, L.; Zhang, Y.F.; Black, T.; Jones, K.C.; Zhang, H.; Zhang, D.Y. Application of Simplicillium chinense for Cd and Pb biosorption and enhancing heavy metal phytoremediation of soils. Sci. Total Environ. 2019, 697, 134148. [Google Scholar] [CrossRef]
- White, J.S.; Tobin, J.M. Inorganic tin and organotin interactions with Candida maltosa. Appl. Microbiol. Biotechnol. 2004, 63, 445–451. [Google Scholar] [CrossRef]
| Plant Species | Treatment (mg/kg) | The Concentration of Sn (mg/kg) | |||||
|---|---|---|---|---|---|---|---|
| Root | Stem | Leaf | Inflorescence | Shoot 1 | Soil | ||
| Impatiens balsamina | 0 | nd | nd | nd | nd | nd | nd |
| 100 | 86.2 ± 18.6 | 72.6 ± 4.9 | 79.1 ± 10.8 | 80.7 ± 1.7 | 77.4 ± 2.5 | 52.7 ± 2.5 | |
| 500 | 140.7 ± 1.8 | 95.3 ± 13.0 | 98.0 ± 9.3 | 111.6 ± 24.8 | 100.7 ± 14.4 | 265 ± 14.4 | |
| 1000 | 340.7 ± 23.8 | 229.9 ± 22.2 | 125.0 ± 29.9 | 122.5 ± 1.1 | 161.4 ± 19.9 | 520.7 ± 19.9 | |
| 2000 | 244.8 ± 12.9 | 95.5 ± 2.9 | 114.3 ± 23.8 | 133.5 ± 52.4 | 114.4 ± 26.4 | 1040 ± 26.4 | |
| Mirabilis jalapa | 0 | nd | nd | nd | nd | nd | nd |
| 100 | 22.2 ± 38.4 | 23.9 ± 22.3 | 23.2 ± 27.3 | 100.0 ± 58.5 | 25.5 ± 24.2 | 53.1 ± 24.2 | |
| 500 | 34.5 ± 23.8 | 12.4 ± 11.4 | 41.2 ± 16.8 | 110.8 ± 27.1 | 35.3 ± 10.4 | 271.6 ± 10.4 | |
| 1000 | 64.2 ± 20.7 | 55.5 ± 47.7 | 58.8 ± 14.0 | 131.0 ± 32.9 | 65.7 ± 40.8 | 547.5 ± 40.8 | |
| 2000 | 74.3 ± 28.0 | 76.6 ± 27.2 | 93.7 ± 23.3 | 91.0 ± 72.4 | 90.0 ± 16.6 | 1000 ± 16.6 | |
| Tagetes erecta | 0 | nd | nd | nd | nd | nd | nd |
| 100 | 18.0 ± 20.5 | 9.9 ± 17.1 | 5.4 ± 9.4 | 7.0 ± 10.4 | 7.4 ± 12.3 | 52.9 ± 12.3 | |
| 500 | 17.9 ± 28.2 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 252 ± 22.4 | |
| 1000 | 31.2 ± 25.2 | 0.0 ± 0.0 | 5.3 ± 6.1 | 0.0 ± 0.0 | 1.8 ± 2.0 | 360 ± 2.0 | |
| 2000 | 95.8 ± 42.4 | 5.1 ± 7.7 | 14.7 ± 11.5 | 14.1 ± 6.9 | 11.3 ± 9.9 | 1130 ± 9.9 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Xu, W.; Wang, Y.; Hao, W.; Zhou, Q.; Liu, J. Growth Responses and Accumulation Characteristics of Three Ornamental Plants to Sn Contamination in Soil. Agriculture 2021, 11, 205. https://doi.org/10.3390/agriculture11030205
Liu Y, Xu W, Wang Y, Hao W, Zhou Q, Liu J. Growth Responses and Accumulation Characteristics of Three Ornamental Plants to Sn Contamination in Soil. Agriculture. 2021; 11(3):205. https://doi.org/10.3390/agriculture11030205
Chicago/Turabian StyleLiu, Yuxia, Weili Xu, Yi Wang, Weiduo Hao, Qixing Zhou, and Jianv Liu. 2021. "Growth Responses and Accumulation Characteristics of Three Ornamental Plants to Sn Contamination in Soil" Agriculture 11, no. 3: 205. https://doi.org/10.3390/agriculture11030205
APA StyleLiu, Y., Xu, W., Wang, Y., Hao, W., Zhou, Q., & Liu, J. (2021). Growth Responses and Accumulation Characteristics of Three Ornamental Plants to Sn Contamination in Soil. Agriculture, 11(3), 205. https://doi.org/10.3390/agriculture11030205







