Sunflower: From Cortuso’s Description (1585) to Current Agronomy, Uses and Perspectives
Abstract
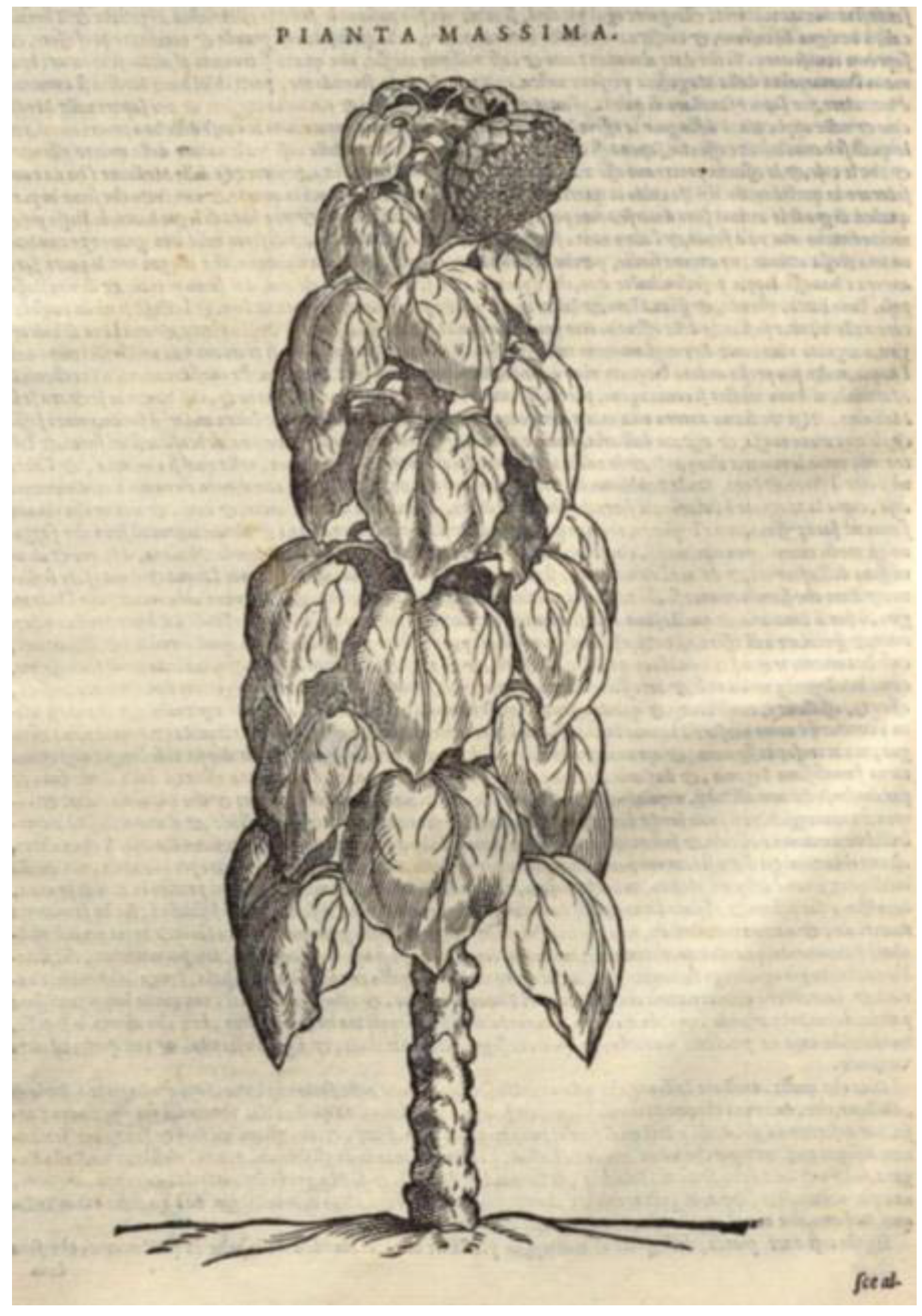
:1. Cortuso and the History of the Sunflower (Helianthus annuus L.)
2. Sunflower Breeding and Implications on the Dynamic of the Cropped Surface
3. Sunflower Cultivation under Climate Change Scenarios
4. Sunflower Uses and Market
4.1. Sunflower Market Development
- ⁃
- The level of seed import in 2010 (220,715 t) was almost identical to that of domestic production (212,900 t) and gradually decreased to a minimum in the last year of the examined period (2021: 153,350 t);
- ⁃
- Conversely, oil imports increased significantly over the same period (+126%).
4.2. Sunflower for Food Consumption
4.3. Biodiesel Production
4.4. Feed Production
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zukosky, P.M. Cultivated Plants and Their Wild Relatives; Commonwealth Agricultural Bureaux: Farnham Royal, UK; Kolos: Leningrad, USSR, 1950. [Google Scholar]
- Mezzalira, F. La scoperta della biodiversità botanica del mondo. In Illustrazioni Dall’epoca Delle Esplorazioni Geografiche; Biblioteca internazionale “La Vigna”: Vicenza, Italy, 2018; pp. 1–25. [Google Scholar]
- Putt, E.D. Early history of sunflower. Sunflower Technol. Prod. 1997, 35, 1–19. [Google Scholar]
- Semelczi-Kovacs, A. Acclimatization and dissemination of the sunflower in Europe. Acta Ethnogr. Acad. Sci. Hung. 1975, 24, 47–88. (In German) [Google Scholar]
- Russo, G.L. (Ed.) Final Report and Scientific Handbook of the Specific Support Action—MAC-Oils—Mapping and Comparing Oils, Sixth Framework Programme—Priority 5—Food Quality and Safety; Institute of Food Sciences—National Research Council: Avellino, Italy, 2009. [Google Scholar]
- Dauguet, S.; Labalette, F.; Fine, F.; Carré, P.; Merrien, A.; Palleau, J.P. Genetic impact on protein content and hullability of sunflower seeds, and on the quality of sunflower meal. OCL Oilseeds Fats Crops Lipids 2016, 23, D205. [Google Scholar] [CrossRef] [Green Version]
- Gulya, T.J.; Sackston, W.E.; Viranyi, F.; Masirevic, S.; Rashid, K.Y. New races of the sunflower downy mildew pathogen (Plasmopara halstedii) in Europe and North and South America. J. Phytopathol. 1991, 132, 303–311. [Google Scholar] [CrossRef]
- Mihaljĉević, M.; Muntañola-Cvetković, M.; Petrov, M. Further studies on the sunflower disease caused by Diaporthe (Phomopsis) helianthi and possibilities of breeding for resistance. In Proceedings of the 10th International Sunflower Conference, Surfers Paradise, Australia, 14–18 March 1982; pp. 157–159. [Google Scholar]
- Viguié, A.; Touvieille de Labrouhe, D.; Vear, F. Inheritance of several sources of resistance to Phomopsis stem canker (Diaporthe helianthi Munt.-Cvet.) in sunflower (Helianthus annuus L.). Euphytica 2000, 116, 167–179. [Google Scholar] [CrossRef]
- Fusari, C.M.; Di Rienzo, J.A.; Troglia, C.; Nishinakamasu, V.; Moreno, M.V.; Maringolo, C.; Quiroz, F.; Álvarez, D.; Escande, A.; Hopp, E.; et al. Association mapping in sunflower for sclerotinia head rot resistance. BMC Plant Biol. 2012, 12, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Vear, F.; Grezes-Besset, B. Progress in breeding sunflowers for resistance to Sclerotinia. In Proceedings of the I.S.A. Symposium “Sunflower Breeding for Resistance to Diseases”, Krasnodar, Russia, 19–21 June 2010; pp. 30–35. [Google Scholar]
- Leclercq, P. Une sterilite male cytoplasmique chez le tournesol. Ann. Amel. Plantes 1969, 19, 99–106. [Google Scholar]
- Kinman, M.L. New Developments in the USDA and State Experiment Station Sunflower Breeding Programs. In Proceedings of the 4th International Sunflower Conference, Memphis, TN, USA, 14–18 March 1970; pp. 181–183. [Google Scholar]
- Škorić, D.; Jocić, S.; Hladni, N.; Vannozzi, G.P. An analysis of heterotic potential for agronomically important traits in Sunflower (Helianthus annuus L.). Helia 2007, 30, 55–74. [Google Scholar]
- Qi, L.L.; Foley, M.E.; Cai, X.W.; Gulya, T.J. Genetics and mapping of a novel downy mildew resistance gene, Pl 18, introgressed from wild Helianthus argophyllus into cultivated sunflower (Helianthus annuus L.). Theor. Appl. Genet. 2016, 129, 741–752. [Google Scholar] [CrossRef]
- Fick, G.N.; Caroline, J.J.; Auwartir, G.E.; Duhig, P.M. Agronomic characteristics and field performance of dwarf sunflower hybrids. In Proceedings of the 11th International Sunflower Conference, Mar del Plata, Argentina, 10–13 March 1985; Volume 2, pp. 739–742. [Google Scholar]
- Cadic, E.; Coque, M.; Vear, F.; Grezes-Besset, B.; Pauquet, J.; Piquemal, J.; Lippi, Y.; Blanchard, P.; Romestant, M.; Pouilly, N.; et al. Combined linkage and association mapping of flowering time in sunflower (Helianthus annuus L.). Theor. Appl. Genet. 2013, 126, 1337–1356. [Google Scholar] [CrossRef]
- Brigham, R.D.; Young, J.K. Inheritance of the “Y”-branched character in sunflower (Helianthus annuus L.) and implication in breeding. In Proceedings of the 9th International Sunflower Conference, Torremolinos, Spain, 8–13 June 1980; Volume 1, pp. 343–346. [Google Scholar]
- Liu, G.S.; Leclercq, P. The expression of the Y-branched character in sunflower (Helianthus annuus L.). In Proceedings of the 12th International Sunflower Conference, Novi-Sad, Serbia, 27 March 1988; Volume 2, pp. 443–447. [Google Scholar]
- Al-Khatib, K.; Baumgartner, J.R.; Peterson, D.E.; Currie, R.S. Imazethapyr resistance in common sunflower (Helianthus annuus). Weed Sci. 1998, 46, 403–407. [Google Scholar] [CrossRef]
- Al-Khatib, K.; Miller, J.F. Registration of four genetic stocks of sunflower resistant to imidazolinone herbicides. Crop Sci. 2000, 40, 869. [Google Scholar]
- Zollinger, R.K. Advances in sunflower weed control in the USA. In Proceedings of the 16th International Sunflower Conference, Fargo, ND, USA, 29 August–2 September 2004; pp. 435–439. [Google Scholar]
- Tuemmler, C.; Schroeder, G. Study on chemical control of common ragweed (Ambrosia artemisiifolia) in sunflower and grain legumes. Proccedings of the International Multidisciplinary Scientific GeoConference: Ambrosia in Germany—Can the Invasion Be Halted? Berlin, Germany, 10–12 September 2013; pp. 105–110. [Google Scholar]
- García-Vila, M.; Fereres, E.; Prieto, M.H.; Ruz, C.; Soriano, M.A. Sunflower. In Crop Yield Response to Water; FAO Irrigation and Drainage: Rome, Italy, 2012; p. 66. [Google Scholar]
- IPCC; Pachauri, R.K.; Meyer, L.A. (Eds.) Climate Change 2014: Synthesis Report. In Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Flagella, Z.; Rotunno, T.; Tarantino, E.; Di Caterina, R.; De Caro, A. Changes in seed yield and oil fatty acid composition of high oleic sunflower (Helianthus annuus L.) hybrids in relation to the sowing date and the water regime. Eur. J. Agron. 2002, 17, 221–230. [Google Scholar] [CrossRef]
- Ebrahimian, E.; Seyyedi, S.M.; Bybordi, A.; Damalas, C.A. Seed yield and oil quality of sunflower, safflower, and sesame under different levels of irrigation water availability. Agric. Water Manag. 2019, 218, 149–157. [Google Scholar] [CrossRef]
- Alberio, C.; Izquierdo, N.G.; Aguirrezábal, L.A.N. Sunflower crop physiology and agronomy. In Sunflower; AOCS Press: Champaign, IL, USA, 2015; pp. 53–91. [Google Scholar]
- Ferreira, A.M.; Abreu, F.G. Description of development, light interception and growth of sunflower at two sowing dates and two densities. Math. Comput. Simul. 2001, 56, 369–384. [Google Scholar] [CrossRef]
- Balalić, I.; Zorić, M.; Branković, G.; Terzić, S.; Crnobarac, J. Interpretation of hybrid× sowing date interaction for oil content and oil yield in sunflower. Field Crop. Res. 2012, 137, 70–77. [Google Scholar] [CrossRef]
- Giannini, V.; Mula, L.; Carta, M.; Patteri, G.; Roggero, P.P. Interplay of irrigation strategies and sowing dates on sunflower yield in semi-arid Mediterranean areas. Agric. Water Manag. 2022, 260, 107287. [Google Scholar] [CrossRef]
- Debaeke, P.; Casadebaig, P.; Langlade, N. New challenges for sunflower ideotyping in changing environments and more ecological cropping systems. OCL Oilseeds Fats Crops Lipids 2021, 28, 1–23. [Google Scholar] [CrossRef]
- Hussain, M.; Farroq, S.; Hasan, W.; Ul-allah, S.; Tanveer, M.; Farroq, M.; Nawaz, A. Drought stress in sunflower: Physiological effects and its management through breeding and agronomic alternatives. Agric. Water Manag. 2018, 201, 152–166. [Google Scholar] [CrossRef]
- Göksoy, A.T.; Demir, A.O.; Turan, Z.M.; Dağüstü, N. Responses of sunflower (Helianthus annuus L.) to full and limited irrigation at different growth stages. Field Crop. Res. 2004, 87, 167–178. [Google Scholar] [CrossRef]
- Browne, C.L. Effect of date and final irrigation on yield and yield components of sunflowers in a semi-arid environment. Aust. J. Exp. Agr. 1997, 17, 482–488. [Google Scholar] [CrossRef]
- Rawson, H.M.; Turner, N.C. Irrigation timing and relationships between leaf area and yield in sunflower. Irrig. Sci. 1983, 4, 167–175. [Google Scholar] [CrossRef]
- Hussain, M.; Malik, M.A.; Farooq, M.; Ashraf, M.Y.; Cheema, M.A. Improving drought tolerance by exogenous application of glycinebetaine and salicylic acid in sunflower. J. Agron. Crop Sci. 2008, 194, 193–199. [Google Scholar] [CrossRef]
- Iqbal, N.; Ashraf, Y.; Ashraf, M. Modulation of endogenous levels of some key organic metabolites by exogenous application of glycine betaine in drought stressed plants of sunflower (Helianthus annuus L.). Plant Growth Regul. 2011, 63, 7–12. [Google Scholar] [CrossRef]
- Singh, N.B.; Singh, D.; Singh, A. Biological seed priming mitigates the effects of water stress in sunflower seedlings. Physiol. Mol. Biol. Plants 2015, 21, 207–214. [Google Scholar] [CrossRef]
- Langeroodia, A.S.; Tedeschi, P.; Allevato, E.; Stazi, S.R.; Aadil, R.M.; Mancinelli, R.; Radicetti, E. Agronomic response of sunflower subjected to biochar and arbuscular mycorrhizal fungi application under drought conditions. Ital. J. Agron. 2022, 17, 2086. [Google Scholar] [CrossRef]
- Trudgill, D.L.; Squire, G.R.; Thompson, K. A thermal time basis for comparing the germination requirements of some British herbaceous plants. New Phytol. 2000, 145, 107–114. [Google Scholar] [CrossRef]
- Poonia, R.; Chaudhary, O.P. Unraveling the mystery of non extraction of honey from modern sunflower hybrids. J. Apic. Res. 2022, 61, 557–566. [Google Scholar] [CrossRef]
- Vear, F. Changes in sunflower breeding over the last fifty years. OCL Oilseeds Fats Crops Lipids 2016, 23, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Deppermann, A.; Balkovič, J.; Bundle, S.C.; Di Fulvio, F.; Havlik, P.; Leclère, D.; Lesiv, M.; Prishchepov, A.V.; Schepaschenko, D. Increasing crop production in Russia and Ukraine—Regional and global impacts from intensification and recultivation. Environ. Res. Lett. 2018, 13, 025008. [Google Scholar] [CrossRef]
- Velasco, L.; Fernández-Martínez, J.M.; Fernández, J. Sunflower production in the European Union. In Sunflower; AOCS Press: Champaign, IL, USA, 2015; pp. 555–573. [Google Scholar]
- Absalome, M.A.; Massara, C.-C.; Alexandre, A.A.; Gervais, K.; Chantal, G.G.-A.; Ferdinand, D.; Rhedoor, A.J.; Coulibaly, I.; George, T.G.; Brigitte, T.; et al. Biochemical properties, nutritional values, health benefits and sustainability of palm oil. Biochimie 2020, 178, 81–95. [Google Scholar] [CrossRef]
- Cuypers, D.; Geerken, T.; Gorissen, L.; Lust, A.; Peters, G.; Karstensen, J.; Prieler, S.; Fischer, G.; Van Velthuizen, H. The Impact of EU Consumption on Deforestation: Comprehensive Analysis of the Impact of EU Consumption on Deforestation; Technical Report (63); European Union: Brussels, Belgium, 2013. [Google Scholar]
- Pendrill, F.; Persson, U.M.; Godar, J.; Kastner, T.; Moran, D.; Schmidt, S.; Wood, R. Agricultural and forestry trade drives large share of tropical deforestation emissions. Glob. Environ. Change 2019, 56, 1–10. [Google Scholar] [CrossRef]
- Pilorgé, E. Sunflower in the global vegetable oil system: Situation, specificities and perspectives. OCL Oilseeds Fats Crops Lipids 2020, 27, 34. [Google Scholar] [CrossRef]
- FDA. 2018. Available online: https://www.foodnavigatorusa.com/Article/2018/11/19/FDAapprovesqualifiedhealthclaimforhigholeicoilsandreducedriskofcoronaryheartdisease (accessed on 27 September 2022).
- Warner, K.; Vick, B.A.; Kleingartner, L.; Isaac, I.; Doroff, K. Composition of sunflower NuSun (mid-oleic sunflower), and high-oleic sunflower oils. In Proceedings of the Sunflower Res. Workshop, Fargo, ND, USA, 16–17 January 2003; National Sunflower Association: Mandan, ND, USA, 2003. Available online: http://www.sunflowernsa.com/research/research-workshop/documents/107.PDF (accessed on 27 September 2022).
- Anushree, S.; André, M.; Guillaume, D.; Frédéric, F. Stearic sunflower oil as a sustainable and healthy alternative to palm oil. A review. Agron. Sustain. Dev. 2017, 37, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Mba, O.I.; Dumont, M.J.; Ngadi, M. Palm oil: Processing, characterization and utilization in the food industry–A review. Food Biosci. 2015, 10, 26–41. [Google Scholar] [CrossRef]
- Parsons, S.; Raikova, S.; Chuck, C.J. The viability and desirability of replacing palm oil. Nat. Sustain. 2020, 3, 412–418. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Babalola, O.O. Oilseed crop sunflower (Helinathus annuus) as a source of food: Nutritional and healths benefits. Food Sci. Nutr. 2020, 8, 4666–4684. [Google Scholar] [CrossRef]
- Binici, H.; Eken, M.; Kara, M.; Dolaz, M. An environment-friendly thermal insulation material from sunflower stalk, textile waste and stubble fibers. In Proceedings of the 2013 International Conference on Renewable Energy Research and Applications (ICRERA), Madrid, Spain, 20–23 October 2013; pp. 833–846. [Google Scholar]
- Mathias, J.D.; Alzina, A.; Grédiac, M.; Michaud, P.; Roux, P.; De Baynast, H.; Delattre, C.; Dumoulin, N.; Faure, T.; Larrey-Lassalle, P.; et al. Upcycling sunflower stems as natural fibers for biocomposite applications. BioResources 2015, 10, 8076–8088. [Google Scholar] [CrossRef]
- Bona, S.; Mosca, G.; Vamerali, T. Oil crops for biodiesel production in Italy. Renew Energy 1999, 16, 1053–1056. [Google Scholar] [CrossRef]
- Mahmood, A.; Awan, M.I.; Sadaf, S.; Mukhtar, A.; Wang, X.; Fiaz, S.; Khan, S.A.; Ali, H.; Muhammad, F.; Hayat, Z.; et al. Bio-diesel production of sunflower through sulphur management in a semi-arid subtropical environment. Environ. Sci. Pollut. Res. 2022, 29, 13268–13278. [Google Scholar] [CrossRef]
- Kallivroussis, L.; Natsis, A.; Papadakis, G. RD—Rural development: The energy balance of sunflower production for biodiesel in Greece. Biosyst. Eng. 2002, 81, 347–354. [Google Scholar] [CrossRef]
- Porte, A.F.; de Souza Schneider, R.D.C.; Kaercher, J.A.; Klamt, R.A.; Schmatz, W.L.; Da Silva, W.L.T.; Severo Filho, W.A. Sunflower biodiesel production and application in family farms in Brazil. Fuel 2010, 89, 3718–3724. [Google Scholar] [CrossRef]
- López Granados, M.; Poves, M.Z.; Alonso, D.M.; Mariscal, R.; Galisteo, F.C.; Moreno-Tost, R.; Santamaría, J.; Fierro, J.L.G. Biodiesel from sunflower oil by using activated calcium oxide. Appl. Catal. B 2007, 73, 317–326. [Google Scholar] [CrossRef]
- Frohlich, A.; Rice, B. Evaluation of Camelina sativa oil as a feedstock for biodiesel production. Ind. Crops Prod. 2005, 21, 25–31. [Google Scholar] [CrossRef]
- Cardone, M.; Mazzoncini, M.; Menini, S.; Rocco, V.; Senatore, A.; Seggiani, M.; Vitolo, S. Brassica carinata as an alternative oil crop for the production of biodiesel in Italy: Agronomic evaluation, fuel production by transesterification and characterization. Biomass Bioenerg. 2003, 25, 623–636. [Google Scholar] [CrossRef]
- Geller, D.P.; Goodrum, J.W.; Knapp, S.J. Fuel properties of oil from genetically altered Cuphea viscosissima. Ind. Crops Prod. 1999, 9, 85–91. [Google Scholar] [CrossRef]
- Auld, D.L.; Peterson, C.L.; Korus, R.A. Production, processing, and utilization of rapeseed oil as a diesel fuel substitute. In Energy from Biomass and Wastes XII; Institute of Gas Technology: Chicago, IL, USA, 1989; pp. 1187–1207. [Google Scholar]
- Tashtoush, G.M.; Al Widyan, M.I.; Al Jarrah, M.M. Experimental study on evaluation and optimization of conversion of waste animal fat into biodiesel. Energy Convers. Manag. 2004, 45, 2697–2711. [Google Scholar] [CrossRef]
- Carraretto, C.; Macor, A.; Mirandola, A.; Stoppato, A.; Tonon, S. Biodiesel as alternative fuel: Experimental analysis and energetic evaluations. Energy Oxf. 2004, 29, 2195–2211. [Google Scholar] [CrossRef]
- Zou, L.; Atkinson, S. Characterising vehicle emissions from the burning of biodiesel made from vegetable oil. Environ. Technol. 2003, 24, 1253–1260. [Google Scholar] [CrossRef]
- Krahl, J.; Munack, A.; Stein, H.; Schroder, O.; Hassaneen, A. Fuel economy and environment characteristics of biodiesel and low sulfur fuels in diesel engines. Landbauforsch. Volkenrode 2005, 55, 99–106. [Google Scholar]
- Goodrum, J.W.; Geller, D.P. Influence of fatty acid methyl esters from hydroxylated vegetable oils on diesel fuel lubricity. Bioresour. Technol. 2005, 96, 851–855. [Google Scholar] [CrossRef]
- Scrosta, V. Buone prospettive per il biodiesel ma solo a certe condizioni. Inf. Agrar. 2003, 59, 29–31. [Google Scholar]
- Chiu, C.W.; Schumacher, L.G.; Suppes, G.J. Impact of cold flow improvers on soybean biodiesel blend. Biomass Bioenerg. 2004, 27, 485–491. [Google Scholar] [CrossRef]
- Szeto, W.; Leung, D.Y. Is hydrotreated vegetable oil a superior substitute for fossil diesel? A comprehensive review on physicochemical properties, engine performance and emissions. Fuel 2022, 327, 125065. [Google Scholar] [CrossRef]
- Riello, L.; Bona, S. Life Cycle Assessment for Evaluating On-farm Energy Production: The Case of Sunflower Oil. Ital. J. Agron. 2006, 1, 705–709. [Google Scholar] [CrossRef]
- OECD. Consensus document on compositional considerations for new varieties of sunflower: Key food and feed nutrients, anti-nutrients and toxicants. In Environment Directorate, Joint Meeting of the Chemicals Committee and the Working Party on Chemicals, Pesticides and Biotechnology; Series on the Safety of Novel Foods and Feeds, No. 16; OECD: Paris, France, 2007. [Google Scholar]
- Heuzé, V.; Tran, G.; Vittet, M.A. Sunflower (general). In Feedipedia, a Programme by INRAE, CIRAD, AFZ and FAO. 2015. Available online: https://www.feedipedia.org/node/6225 (accessed on 10 August 2015).
- Putnam, D.H.; Oplinger, E.S.; Hicks, D.R.; Durgan, B.R.; Noetzel, D.M.; Meronuck, R.A.; Doll, J.D.; Schulte, E.E. Sunflower. In Alternative Field Crop Manual; University of Wisconsin-Exension, Cooperative Extension: Madison, WI, USA, 1990. [Google Scholar]
- Dohme-Meier, F.; Bee, G. Feeding unprotected CLA methyl esters compared to sunflower seeds increased milk CLA content level but inhibited milk fat synthesis in cows. Asian-Aust. J. Anim. Sci. 2012, 25, 75–85. [Google Scholar] [CrossRef] [Green Version]
- Shingfield, K.J.; Reynolds, C.K.; Hervás, G.; Griinari, J.M.; Grandison, A.S.; Beever, D.E. Examination of the persistency of milk fatty acid composition responses to fish oil and sunflower oil in the diet of dairy cows. J. Dairy Sci. 2006, 89, 714–732. [Google Scholar] [CrossRef]
- INRA. INRA Feeding System for Ruminants; Wageningen Academic Publishers: Wageningen, The Netherlands, 2018; 640p. [Google Scholar]
- Finoli, C.; Vecchio, A.; Sirtori, C.; Vercesi, A. Fungal contamination and mycotoxin occurrence in raw materials and finished feeds. Tecn. Molit. 2004, 55, 223–234. [Google Scholar]
- National Research Council. Nutrient Requirements of Dairy Cattle, 8th revised ed.; The National Academies Press: Washington, DC, USA, 2001. [Google Scholar]



| Year | Harvested Area (ha) | Production (t) | Yield (t ha−1) |
|---|---|---|---|
| 2010 | 100,500 | 212,900 | 2.12 |
| 2011 | 118,067 | 274,414 | 2.32 |
| 2012 | 111,678 | 185,494 | 1.66 |
| 2013 | 127,628 | 285,233 | 2.23 |
| 2014 | 111,350 | 250,377 | 2.25 |
| 2015 | 114,449 | 248,007 | 2.17 |
| 2016 | 110,716 | 268,331 | 2.42 |
| 2017 | 114,446 | 243,671 | 2.13 |
| 2018 | 103,870 | 250,460 | 2.41 |
| 2019 | 118,520 | 294,730 | 2.49 |
| 2020 | 122,770 | 299,880 | 2.44 |
| 2021 | 116,990 | 282,400 | 2.41 |
| 2022 | 109,460 | 267,931 * | 2.45 |
| Year | Seed Production (t) | 2010 = 100 | Seed Import (t) | 2010 = 100 | Oil Import (t) | 2010 = 100 |
|---|---|---|---|---|---|---|
| 2010 | 212,900 | 100 | 220,715 | 100 | 251,286 | 100 |
| 2011 | 274,414 | 129 | 228,061 | 103 | 204,207 | 81 |
| 2012 | 185,494 | 87 | 214,622 | 97 | 282,522 | 112 |
| 2013 | 285,233 | 134 | 249,223 | 113 | 286,680 | 114 |
| 2014 | 250,377 | 118 | 176,223 | 80 | 410,638 | 163 |
| 2015 | 248,007 | 116 | 159,743 | 72 | 369,281 | 147 |
| 2016 | 268,331 | 126 | 225,316 | 102 | 454,525 | 181 |
| 2017 | 243,671 | 114 | 222,568 | 101 | 550,365 | 219 |
| 2018 | 250,460 | 118 | 224,012 | 101 | 609,185 | 242 |
| 2019 | 294,730 | 138 | 237,390 | 108 | 658,813 | 262 |
| 2020 | 299,880 | 141 | 159,737 | 72 | 589,559 | 235 |
| 2021 | 282,400 | 133 | 153,350 | 69 | 567,656 | 226 |
| Year | Production (t) | Seed Import (t) | Oil Import (t) 1 | Domestic Consumption (t) | Land Equivalent (ha) 2 | Land Footprint Abroad (ha) 3 | % |
|---|---|---|---|---|---|---|---|
| 2010 | 212,900 | 220,715 | 195,377 | 628,992 | 296,917 | 196,417 | 195.4 |
| 2011 | 274,414 | 228,061 | 158,773 | 661,248 | 284,503 | 166,436 | 141.0 |
| 2012 | 185,494 | 214,622 | 219,663 | 619,779 | 373,143 | 261,465 | 234.1 |
| 2013 | 285,233 | 249,223 | 222,896 | 757,352 | 338,878 | 211,250 | 165.5 |
| 2014 | 250,377 | 176,223 | 319,274 | 745,874 | 331,712 | 220,362 | 197.9 |
| 2015 | 248,007 | 159,743 | 287,119 | 694,869 | 320,665 | 206,216 | 180.2 |
| 2016 | 268,331 | 225,316 | 353,397 | 847,044 | 349,499 | 238,783 | 215.7 |
| 2017 | 243,671 | 222,568 | 427,913 | 894,152 | 419,960 | 305,514 | 267.0 |
| 2018 | 250,460 | 224,012 | 473,646 | 948,118 | 393,201 | 289,331 | 278.6 |
| 2019 | 294,730 | 237,390 | 512,233 | 1,044,353 | 419,966 | 301,446 | 254.3 |
| 2020 | 299,880 | 159,737 | 458,387 | 918,004 | 375,828 | 253,058 | 206.1 |
| 2021 | 282,400 | 153,350 | 441,357 | 877,107 | 363,360 | 246,370 | 210.6 |
| Total Annual Embodied Deforestation per Sector (ha) | ||||
|---|---|---|---|---|
| Sector/Country | Italy | France | Germany | Spain |
| Food | 42,932 | 9367 | 53,124 | 23,809 |
| Energy | 52,593 | 19,853 | 12,971 | 49,970 |
| Oleochemicals | 10,193 | 1215 | 16,010 | 6618 |
| Mean Annual Embodied Deforestation per Sector (ha Year−1) | ||||
| Sector/Country | Italy | France | Germany | Spain |
| Food | 2147 | 468 | 2656 | 1190 |
| Energy | 2630 | 993 | 649 | 2498 |
| Oleochemicals | 510 | 61 | 801 | 331 |
| Properties | Sunflower Oil | Diesel Oil |
|---|---|---|
| Density at 20 °C (kg dm−3) | 0.92 | 0.82 |
| Viscosity at 38 °C (cSt) | 37.1 | 2.7 |
| Lower calorific value (MJ dm−3) | 32.9 | 35.8 |
| (CN) Cetane number | 37 | 47 |
| (FP) Flash point (°C) | 274 | 68 |
| Solidification point (°C) | −18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannini, V.; Maucieri, C.; Vamerali, T.; Zanin, G.; Schiavon, S.; Pettenella, D.M.; Bona, S.; Borin, M. Sunflower: From Cortuso’s Description (1585) to Current Agronomy, Uses and Perspectives. Agriculture 2022, 12, 1978. https://doi.org/10.3390/agriculture12121978
Giannini V, Maucieri C, Vamerali T, Zanin G, Schiavon S, Pettenella DM, Bona S, Borin M. Sunflower: From Cortuso’s Description (1585) to Current Agronomy, Uses and Perspectives. Agriculture. 2022; 12(12):1978. https://doi.org/10.3390/agriculture12121978
Chicago/Turabian StyleGiannini, Vittoria, Carmelo Maucieri, Teofilo Vamerali, Giuseppe Zanin, Stefano Schiavon, Davide Matteo Pettenella, Stefano Bona, and Maurizio Borin. 2022. "Sunflower: From Cortuso’s Description (1585) to Current Agronomy, Uses and Perspectives" Agriculture 12, no. 12: 1978. https://doi.org/10.3390/agriculture12121978
APA StyleGiannini, V., Maucieri, C., Vamerali, T., Zanin, G., Schiavon, S., Pettenella, D. M., Bona, S., & Borin, M. (2022). Sunflower: From Cortuso’s Description (1585) to Current Agronomy, Uses and Perspectives. Agriculture, 12(12), 1978. https://doi.org/10.3390/agriculture12121978








