Abstract
Glutaredoxins (Grxs) are a class of small, heat-stable, acidic proteins which have been implied in various biological activities in cells, including the defense against oxidative stress induced by various biotic and abiotic factors. In this paper, the effects of RNAi targeting SeGrx1 on the cytotoxicity and insecticide susceptibility of camptothecin (CPT) in Spodoptera exigua were investigated. Results showed that the cytotoxicity of CPT to the cells of S. exigua is heightened significantly by the silencing of SeGrx1. In the larvae of S. exigua, the mortality was significantly increased compared to CPT-alone treatment group at 120 h after knocking down the SeGrx1 gene. Taken together, our results confirmed that SeGrx1 in S. exigua played an important role in protecting the cells from the cytotoxicity induced by CPT, and the sensitivity of S. exigua larvae to CPT was increased by the silencing of SeGrx1. Our findings might provide basic information for understanding the function of Grxs and a strategy in insect pest control of RNAi technology combined with pesticides.
1. Introduction
Glutaredoxins (Grxs) are a class of small and heat-stable oxidoreductases conserved in viruses, eukaryotes, and prokaryotes [1]. They belong to the thioredoxin superfamily and have been proved to play essential functions in cellular redox homeostasis [2]. Until now, eight members of Grxs were found in Saccharomyces cerevisiae and five in Homo sapiens, and they are divided into three major categories based on the structure and catalytic properties [3,4]. The first group, referred to as class I, consists of Grx1 and Grx2, which are the typical Grxs with a characteristic Cys-X-X-Cys (CXXC) active site motif and a thioredoxin/glutaredoxin fold [3]. Grx1 exists widely in the mitochondrial inner membrane space, nucleus, and cytoplasm, and plays a role in oxidative stress and redox homeostasis [1,5]. It has been implicated in the regulation of many cellular processes including apoptosis, oxidation, and inflammation, and is related closely to aging and the pathogenesis of diabetes and cardiovascular diseases caused by oxidative stress in humans [6]. Grx1 can play the regulatory roles through both its enzymatic redox activity and protein–protein interactions with specific proteins, such as Ask1, Ras, Fas, and procaspase-3, which are involved in the apoptosis signal transduction pathway [7]. It has been proved by a growing body of evidence that there is potential clinical and therapeutic application of Grx1 in atherosclerosis, and neurodegenerative disease, and aging-related diseases [8].
In insects, several studies were focused on the identification and classification of Grxs, and few studies investigated the role of Grxs in oxidative stress induced by exposure to biotic and abiotic factors. In order to identify the major components of the antioxidant system in Apis mellifera, three members of Grxs, Grx1, Grx2 and Grx-like-1 were identified by using genome sequence and comparative analysis [9]. In A. cerana cerana, two glutaredoxin genes, AccGrx1 and AccGrx2, were identified and then investigated for their response to abiotic environmental stress such as temperature, H2O2, and pesticides [10]. This study demonstrated that AccGrx1 and AccGrx2 play important roles in antioxidant defense when A. cerana cerana is subjected to oxidative stress [10]. Until now, four genes HaGrx, HaGrx3, HaGrx5, and HaGdccr were identified in Helicoverpa armigera and their role in protecting insects against oxidative stress induced by temperature and H2O2 treatments were confirmed successively [11,12]. In Ostrinia furnacalis, adverse environments (including starvation, ultraviolet light, mechanical injury, Escherichia coli exposure, and high and low temperatures) dramatically induced the transcript expression of OfurGRXGRX2 [13]. These results confirmed that Grxs play important roles in antioxidant defense in insects, as well as highlighted the need and importance for further indepth research in the physiological function of the insect Grxs.
Camptothecin (CPT), an indole alkaloid isolated from Camptotheca acuminate Decaisnean, showed significant biological activities against several insect pests including Brevicoryne brassicae, Empoasca vitis, Nilaparvata lugens, Chilo suppressalis, and Heliothis virescens, which suggested its potential use as a pesticide in the field [14,15]. CPT strongly inhibits the growth, development, and reproduction of S. exigua Hübner larvae [14]. Moreover, it can induce cytotoxic effects against IOZCAS-Spex-II cells derived from S. exigua by promoting the increase of intracellular oxidative stress due to the accumulation of intracellular ROS [16,17]. In our previous study, the full-length cDNA of SeGrx1 was cloned (GenBank accession no.: MK318813) and expressed successfully in vitro, and its enzymatic kinetic parameters were obtained, which provided a foundation for further exploring the biological function of SeGrx1 [18]. In this study, we conducted RNA interference (RNAi) experiments to investigate the role of SeGrx1 on the cytotoxicity and insecticide susceptibility of CPT in S. exigua.
2. Materials and Methods
2.1. Cell Lines and Insects
The IOZCAS-Spex-II cells used in this present study were cultured in Grace’s insect culture medium (Invitrogen Life Technologies, New York, NY, USA) supplemented with 10% fetal bovine serum (Invitrogen Life Technologies, New York, NY, USA) in T25 cm2 tissue culture flasks (Corning, New York, NY, USA) at 27 °C, and were acquired from the Institute of Zoology, Chinese Academy of Sciences (Beijing, China) [13]. The third-instar larvae of S. exigua laboratory strain were obtained from Dr. Cui at the Institute of Plant Protection Chinese Academy of Agricultural Sciences.
2.2. Total RNA Extraction and cDNA Synthesis
Total RNA was extracted from the IOZCAS-Spex-II cells on logarithmic phase using RNasy Mini Kit (QIAGEN, Duesseldorf, Germany) following the manufacturer’s protocol, and the quantity and quality of RNA were assessed by using Infinite M200 Pro NanoQuant (Tecan Trading, Männedorf, Switzerland) and agarose gel electrophoresis. According to the manufacturer’s protocol, 1 μg total RNA was used to synthesize the first-strand cDNA with an EasyScript cDNA Synthesis Supermix Kit (Transgen, Beijing, China).
2.3. Double-Stranded RNA (dsRNA) Synthesis
Primers for dsRNA synthesis corresponding to SeGrx1 and SeGFP were designed using the Primer-BLAST online tool (http://www.ncbi.nlm.nih.gov/tools/primer-blast/ (accessed on 22 November 2018), Table 1) and then synthesized by BGI genomics Co., Ltd. (Beijing, China). The synthesis and purification of dsSeGrx1 and dsSeGFP were performed according to the instructions of the T7 RiboMAXTM Express RNAi System kit (Promega, Madison, WI, USA), after which the quantity and quality of dsRNA were analyzed by Infinite M200 PRO (Tecan, Männedorf, Switzerland) and 1.5% agarose gel electrophoresis, respectively [19,20].

Table 1.
Primers used for dsRNA synthesis and RT-qPCR.
2.4. Effect of RNAi on the Cytotoxicity of CPT
2.4.1. Cell Transfection with dsSeGrx1
For cell transfection [21], the normal cells on logarithmic phase were harvested at a density of 105 cells/mL, which were incubated overnight in 6-well transparent plates (1800 μL/well) without fetal bovine serum and antibiotics. The transfection of 10 μg dsSeGrx1 and dsGFP was conducted using Lipofectamine® 2000 (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions, respectively. Each RNAi treatment was replicated at least three times. The transfection efficiency was detected by RT-qPCR.
2.4.2. CPT Exposure
An amount of 10 μM CPT was added into each group after the cells were treated with dsGFP or dsSeGrx1, respectively. Cells treated with 0.1% DMSO were conducted as the control group [13]. Subsequently, cells of different treatment were collected at 2, 4, 6, 12, 24, and 48 h for the following morphological observation immediately or stored at −80 °C. The morphological changes of IOZCAS-Spex-II cell were recorded by an inverted phase contrast microscope (IX53, Olympus, Japan).
2.4.3. Cell Viability Assay
The proliferative activity of cells was detected with a Cell Titer 96 Aqueous One Solution Cell Proliferation Assay Kit (Promega, Madison, WI, USA). According to the manufacturer’s instruction, IOZCAS-Spex-II cells were collected at the certain time in 96-well plates (170 μL/well) at a density of 1.00 × 105 cells/ mL. An amount of 30 μL CellTiter 96®AQueous One solution was added to each well and then incubated for 2 h at 27 °C. The formazan product was measured at 490 nm using an Infinite M200 PRO microplate reader (Tecan, Männedorf, Switzerland) [13].
2.5. CPT Sensitivity against S. exigua after RNAi
2.5.1. dsRNA Injection
The method of injection was used to introduce dsRNA into the third-instar larvae of S. exigua with a nanoliter injector (WPI, Beijing, China). A total of 2.5 μL dsSeGrx1, dsGFP or diethylpyrocarbonate (DEPC)-treated water was injected into the third-instar larvae. Each treatment was replicated three times, and for each replication, 20 larvae were injected [22]. RNA was extracted after 24 h to check the efficiency of RNAi by RT-qPCR.
2.5.2. Bioassays
The third-instar larvae of S. exigua were subjected to bioassays after 24 h postinjection by using leaf-dipping method [23]. Briefly, the cabbage leaf discs (7 cm diameter) were cut and dipped in 3.48 mg/L CPT distilled water solutions containing 0.1% DMSO for 30 s and then air dried for 1 h at room temperature. Leaf discs were placed in petri dish (9 cm diameter) and a total of 20 third-instar larvae were introduced into each dish. The control group larvae were fed with leaf discs treated with 0.1% DMSO. Each group was replicated three times. All bioassays were conducted at 25 ± 1 °C, 50–60% RH and under a 16:8 h (light/dark) photoperiod. The mortalities were recorded at 96 and 120 h, and the leaf discs were replaced every day during the bioassays. Additionally, weights of the survivors were measured at 120 h.
2.6. Gene Expression with RT-qPCR
Primers used for the RT-qPCR analysis were designed with Primer Premier 6.0 and synthesized by BGI genomics Co., Ltd. (Beijing, China) (Table 1). Real-time PCR was conducted using a QuanStudio 3 ABI system (Thermo Fisher Scientific, Waltham, MA, USA) with the TransStart Top Green qPCR SuperMix kit (Transgen, Beijing, China). According to the manufacturer’s instructions, a total volume of 20 μL reaction mixture containing 1 μL of cDNA template, 1 μL of each primer, 10 μL of 2 × TransStart® Top Green qPCR SuperMix, and 7.0 μL of distilled ddH2O. The RT-qPCR conditions were 30 s at 94 °C, followed by 40 cycles at 94 °C for 30 s, and then annealing at 72 °C for 30 s. The relative expression was calculated with α-tubulin and GADPH as reference genes according to the methods developed by Vandesompele et al. [24].
2.7. Statistical Analysis
All results were confirmed in at least three independent experiments. Data are presented as mean ± standard error. The SPSS 26.0 Software Package (SPSS Inc., Chicago, IL, USA) was used to perform statistical analyses. Independent samples t-test and one-way ANOVA followed by the Duncan’s multiple range test were performed. Means with the different letters are significantly different at p < 0.05.
3. Results and Discussion
3.1. RNAi Targeting SeGrx1 Increased the Cytotoxicity of CPT in IOZCAS-Spex-II Cells
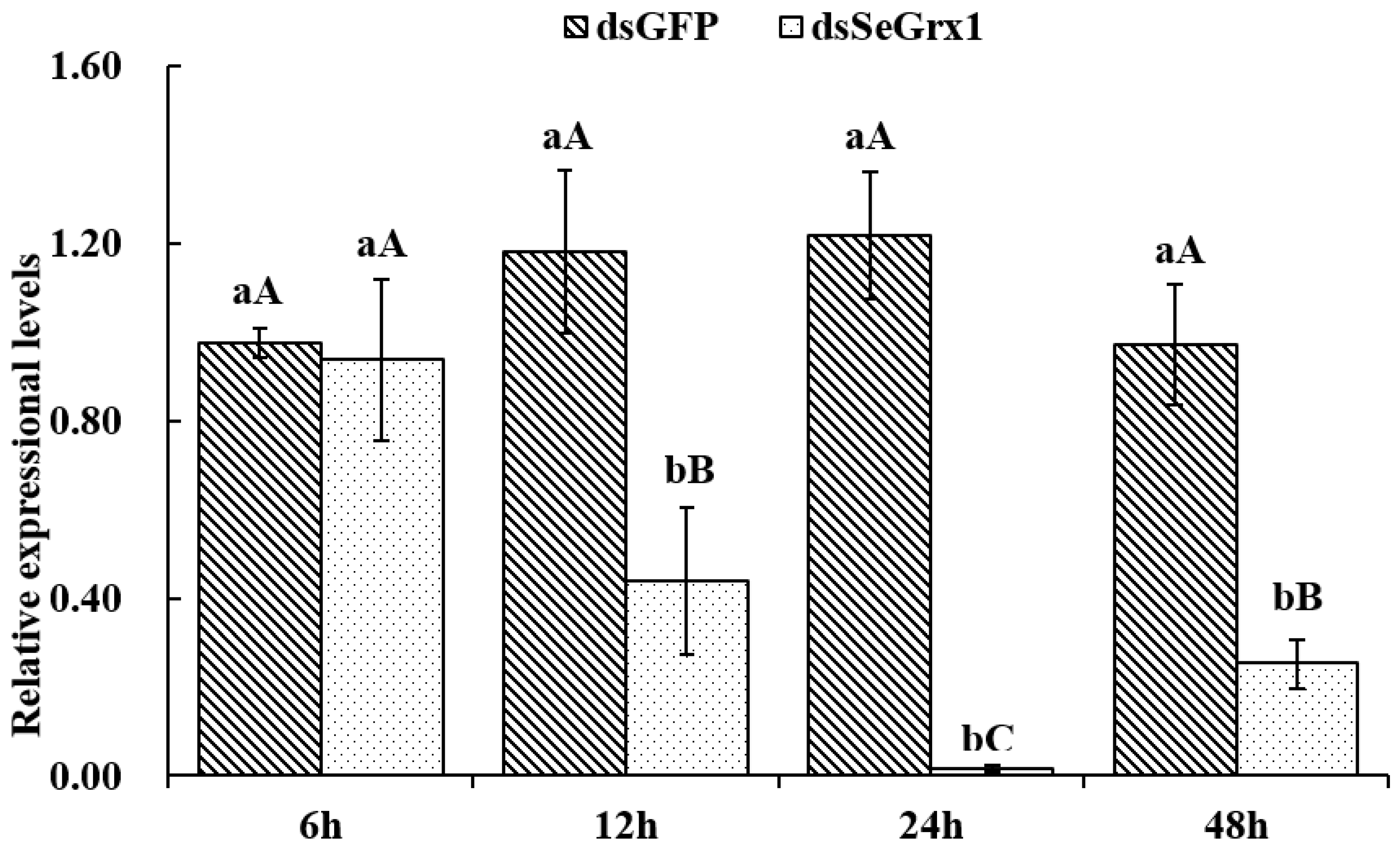
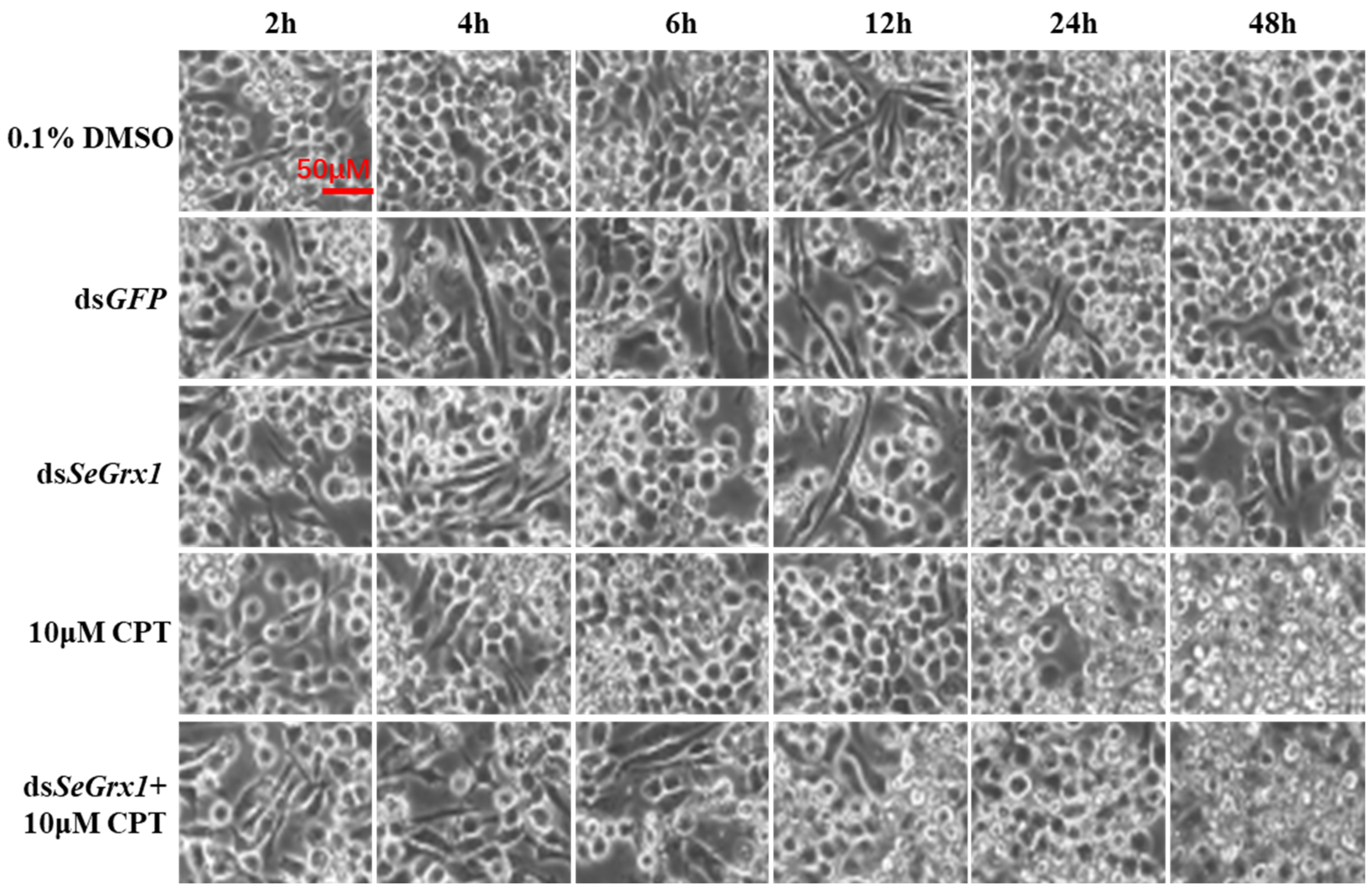
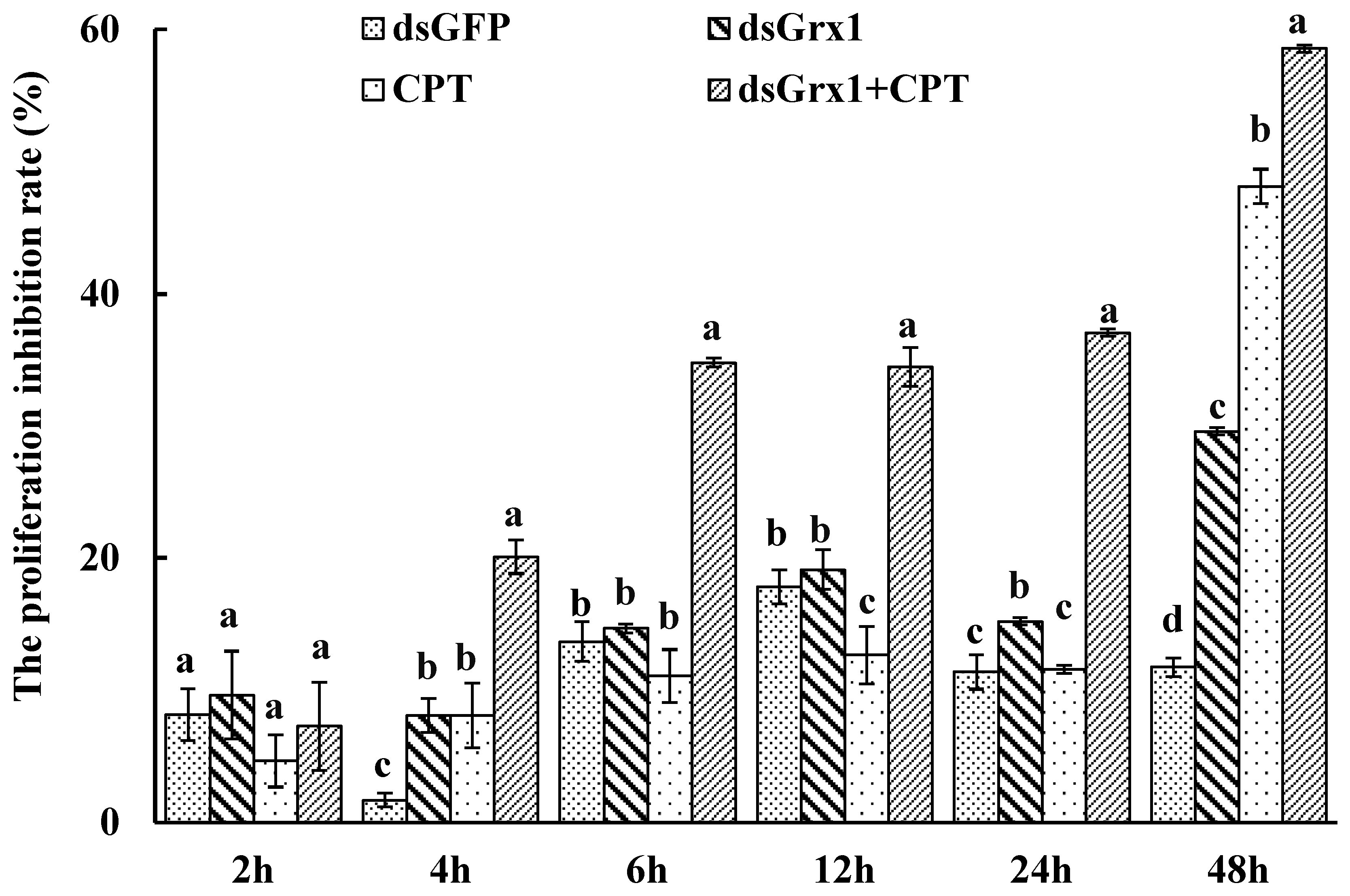
The effect of dsRNA on the mRNA level of SeGrx1 expression change in IOZCAS-Spex-II cells after transfection with dsSeGrx1 was detected using RT-qPCR. The expression level of SeGrx1 in the treatment group decreased 6.33, 56.0, 98.3, and 74.7% compared to the control group transfected with Lipofectamine 2000 Reagent, respectively. Moreover, the relative expression levels of SeGrx1 were changed from 0.99 to 1.20 compared to the control group. This result suggests that transfection of specific dsSeGrx1 is an effective way to silence the expression of SeGrx1 in IOZCAS-Spex-II cells (Figure 1). Therefore, the CPT was added into the cells transfected with dsSeGrx1 or dsGPF for 24 h to ensure the RNAi efficiency. As shown in Figure 2, there was no significant difference in cell morphologies between cells treated with 0.1% DMSO and disrupted with dsGFP. The cell treated with dsSeGrx1 showed some certain morphological changes with apoptotic bodies at 24 and 48 h [13]. In contrast, the cells treated with CPT and dsSeGrx1 + CPT showed typical characteristics of apoptosis in their morphological changes, such as cell shrinkage, gap generation, membrane blebbing, and apoptotic bodies. The inhibition rate of cell viability induced by dsGFP fluctuated between 1.69% and 17.8% (Figure 3). The efficacy of dsSeGrx1 on the cell viability increased gradually with time and reached to 29.6% at 48 h. Consistent with previous reports, CPT showed cytotoxic effects to IOZCAS-Spex-II cells in a time-dependent manner with the inhibition rate increasing from 4.64% at 2 h to 48.1% at 48 h (Figure 3). It was noteworthy that the inhibition of cell viability is heightened significantly by the disruption of SeGrx1 together with the treatment of 10 μM CPT in IOZCAS-Spex-II cells at 6, 12, 24, and 48 h (Figure 3). These results suggest that SeGrx1 may function to protect IOZCAS-Spex-II cells from CPT-induced apoptosis, which confirms previous reports that SeGrx1 plays important roles in antioxidant defense in insects [16,25]. In our previous studies, a significant increase in the level of intracellular ROS was observed, accompanied by markedly increased DNA damage, lipid peroxidation, and protein carbonylation after exposure to CPT in IOZCAS-Spex-II cells [16]. It could be proposed that oxidative stress is more intense in SeGrx1-silenced IOZCAS-Spex-II cells.
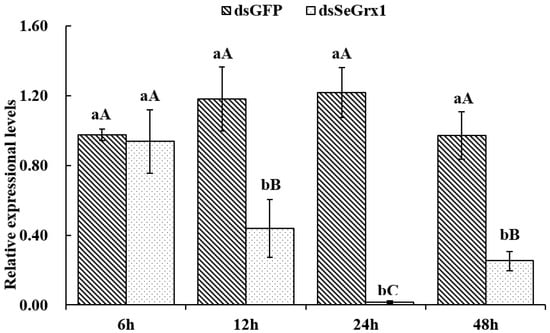
Figure 1.
The relative expression of SeGrx1 after RNAi in IOZCAS-Spex-II cells. Data are presented as mean ± standard error. Independent samples t-test and one-way ANOVA, followed by a Duncan’s multiple range test were performed by using SPSS 26.0 Software Package (SPSS Inc., Chicago, IL, USA). Data followed by the same lowercase letters indicate no significant difference at 0.05 level between dsGFP group and dsSeGrx1 treatment at the same time. Data followed by the same capital letters indicate no significant difference at 0.05 level among the same group at different times.
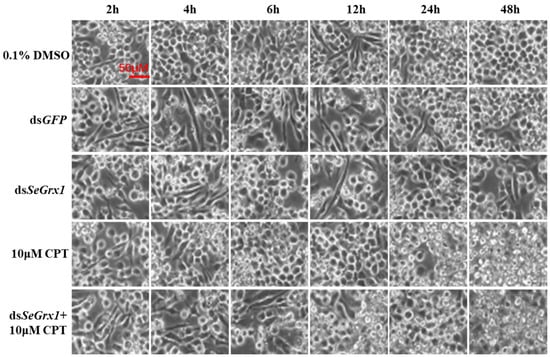
Figure 2.
Morphology observation of IOZCAS-Spex-II cells treated with dsGFP, dsSeGrx1, 10 μM CPT, and deSeGrx1 + 10 μM CPT. 0.1% DMSO was used as a control. The scale bar is 50 μm. The morphological changes of IOZCAS-Spex-II cell were recorded by an inverted phase contrast microscope with 400× magnification (IX53, Olympus, Tokyo, Japan).
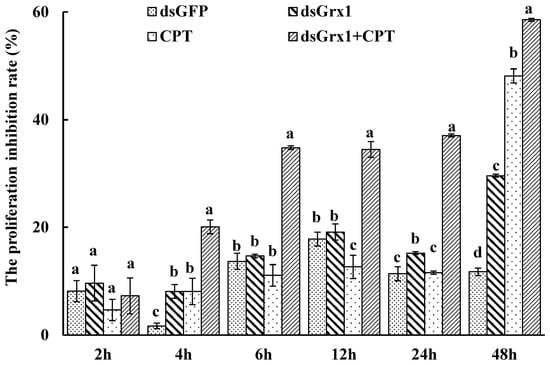
Figure 3.
Inhibitory effects of dsRNA and CPT to S. exigua cell line IOZCAS-Spex-II. Inhibition (%) = (OD490 of the 0.1% DMSO-treated cells − OD490 of CPT or/and dsRNA-treated cells)/OD of 0.1% DMSO-treated cells × 100%. Values are shown as mean ± SEM. Data followed by the same lowercase letters among different treatment at the same time indicate no significant difference at 0.05 level.
Previous studies demonstrated that Grx1 is highly sensitive to oxidants and specifically catalyzes the reduction of specific target proteins, which have important functions of cell protection and antioxidation [6,7,26]. In A. cerana cerana, it has been showed that AccGrx1 may play critical roles in antioxidant defense against lower temperature (4 °C), H2O2, HgCl2, and pesticide cyhalothrin and phoxime treatments [10]. Our previous studies showed that CPT treatment can induce the overproduction of ROS accompanied by markedly increased DNA damage, lipid peroxidation, and protein carbonylation in IOZCAS-Spex-II cells [16]. These results indicated that oxidative stress induced by CPT played an essential role in the toxicity and mode of action of CPT at the cellular level. In this study, the cytotoxicity of CPT was heightened significantly by the disruption of SeGrx1 together with the treatment of 10 μM CPT in IOZCAS-Spex-II cells. These results suggest that SeGrx1 may function to protect IOZCAS-Spex-II cells from CPT-induced oxidative stress, which confirms previous reports that Grx1 plays important roles in antioxidant defense in insects [26]. A similar result was reported that the levels of ROS are controlled by the activities of Grx2 in mitochondria, which can help to modulate the susceptibility of a cell to apoptosis [27]. Prior studies have noted the importance of Grx2 in mitochondrial redox status, when Grx2 knock-down resulted in increasing the sensitivity to cell death induced by doxorubicin/adriamycin and phenylarsin [28]. Taken with the above studies, Grxs as the major antioxidant enzyme families were involved in regulating cellular redox homeostasis and in defense of enhanced oxidative stress induced by adverse factors including temperatures, ultraviolet light, pesticides, and so on. In this study, we confirmed that SeGrx1 is involved in the defense of CPT-induced oxidative stress in IOZCAS-Spex-II cells.
3.2. RNAi Targeting SeGrx1 Increased the Sensitivity of CPT against S. exigua
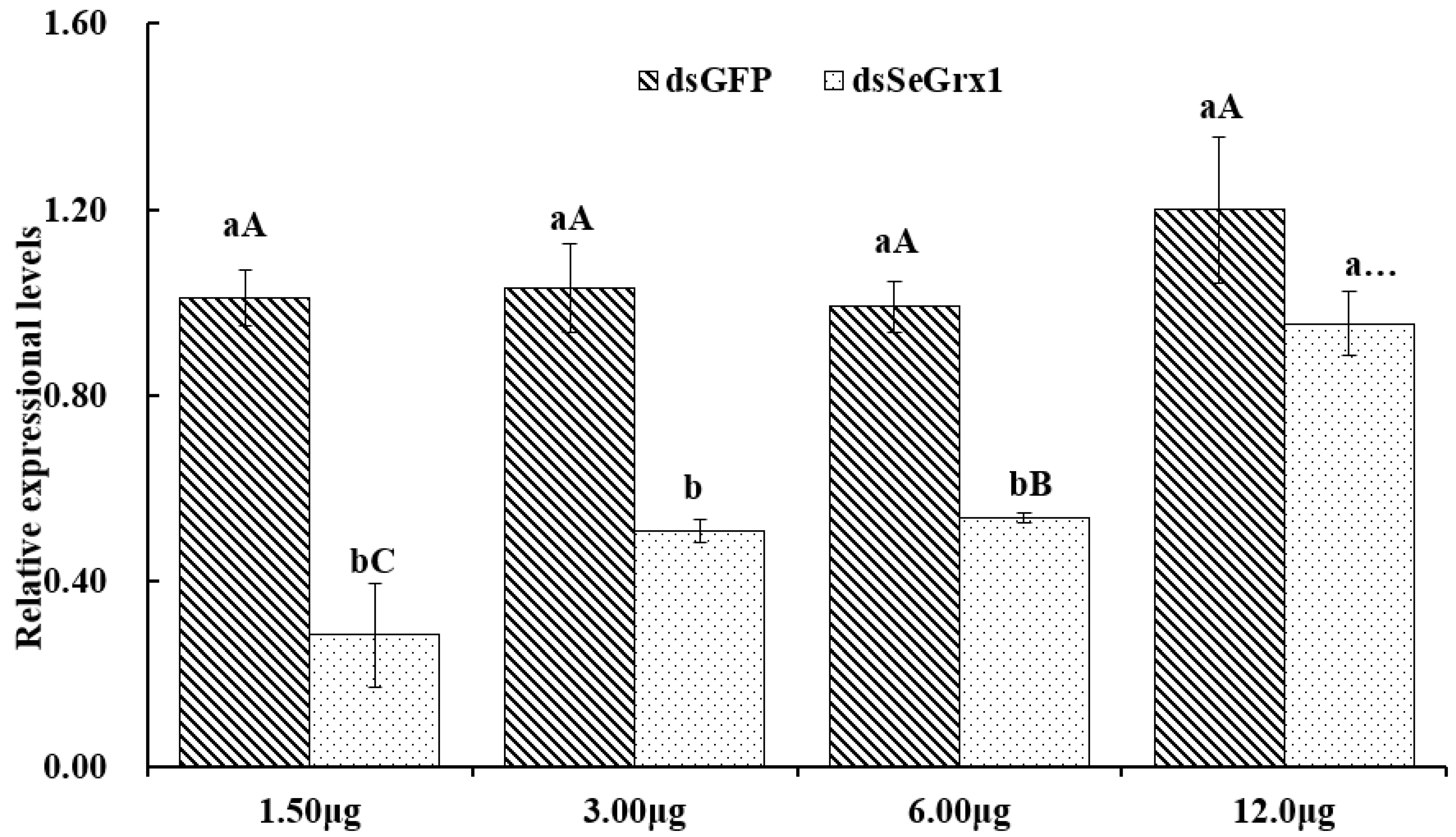
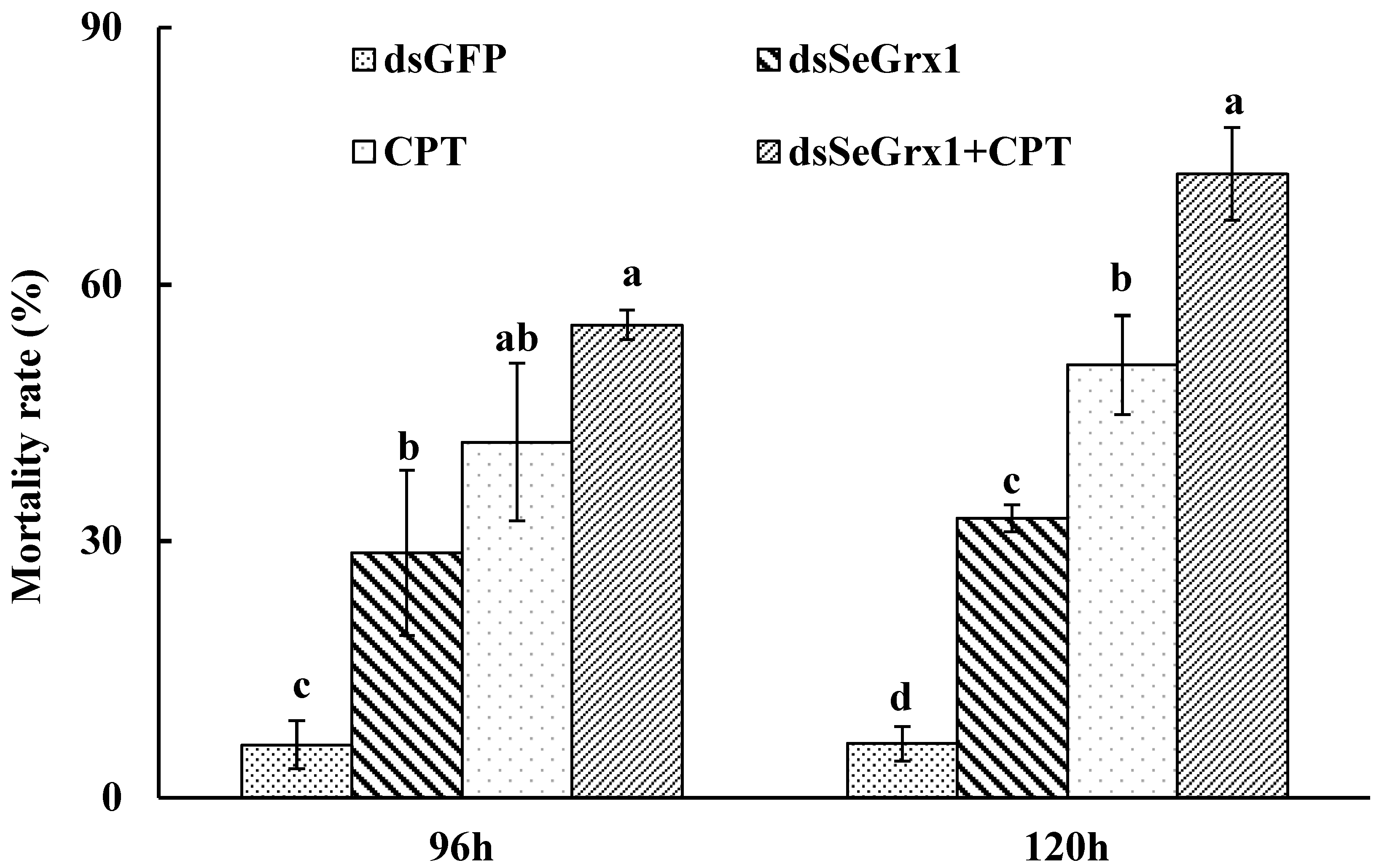
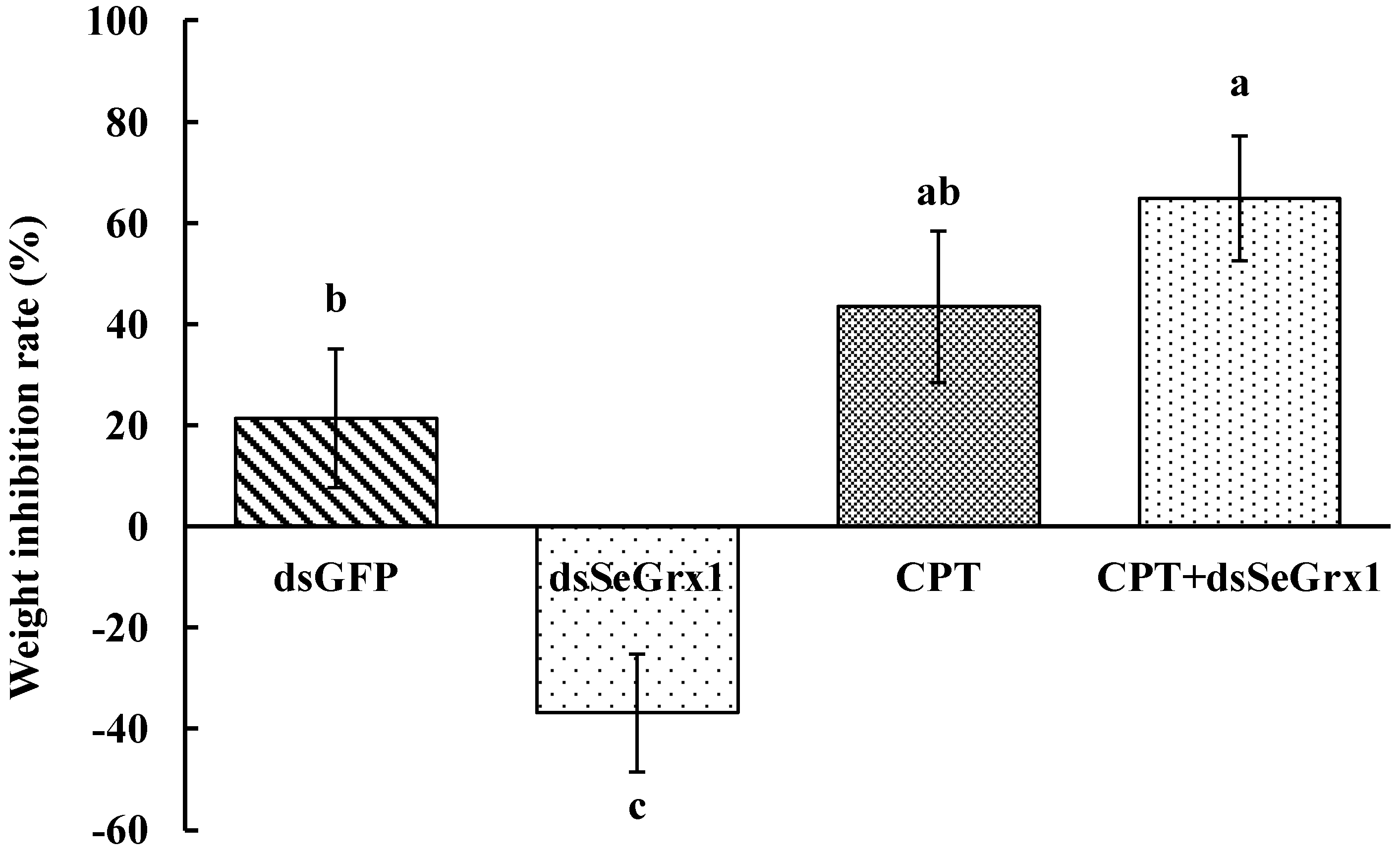
The RT-qPCR results showed that the expression levels of SeGrx1 were significantly downregulated at 24 h after silencing with 1.50, 3.00, and 6.00 μg/larvae dsSeGrx1, respectively (Figure 4). The highest RNAi efficiency was 72.1% at the concentration of 1.5 μg/larvae dsSeGrx1. This result suggested that RNAi of specific dsSeGrx1 is also an effective way to silence the expression of SeGrx1 in the larvae of S. exigua. Furthermore, the bioassays test was conducted to examine the effect of RNAi on the survival and weight of CPT-treated S. exigua larvae. The cumulative mortality of S. exigua larvae was 6.32, 32.6, 50.6, and 72.9% in the dsGFP, dsSeGrx1, CPT, and dsSeGrx1 + CPT group, respectively (Figure 5) The results showed that after knocking down the SeGrx1 for 24 h, the mortality was significantly increased compared to the CPT-alone treatment group at 96 h, which suggested that RNAi targeting SeGrx1 increased the sensitivity of CPT against S. exigua. In addition, the weight of the survivors was measured (Figure 5). Interestingly, the weight of the surviving larvae treated with dsSeGrx1 alone was increased significantly. However, after feeding with leaves treated with CPT, the weight of the S. exigua larvae decreased compared to that treated with CPT alone, although there was no significant difference (p > 0.05) (Figure 6). These observations are consistent with previous findings in other insects, including Spodoptera frugiperda [29,30]. CPT diets induced weight loss of the larvae of S. frugiperda and the molecular basis for the impact of CPT on S. frugiperda was explored by comparative transcriptomic analyses among midgut samples. Our results suggested that the inhibition effect on the growth of CPT can be increased by the knocking down of SeGrx1, which showed that CPT as an insecticide may be used with other insecticides for enhanced efficiency in controlling important insect pests in the field. In Homo sapiens, Grxs have been implicated in various physiological and pathological conditions, from immune defense to neurodegeneration and cancer development, which makes Grxs a possible drug target [8,31]. The RNAi-mediated gene knockdown has shown promising results in different insect groups, pointing it to be the upcoming technique for insect control [32,33,34,35,36]. According to the reports of Yoon [37], the inhibitor of apoptosis (IAP) protein, a negative regulator of apoptosis in insects, provides opportunities for developing targets for RNAi-based insect pest control. In this study, RNAi targeting SeGrx1 increased the sensitivity of CPT against S. exigua. According to the reports of Liu et al. (2021), after GmGrx is silenced by RNAi, the percentage of larval survival to emamectin benzoate was significantly decreased, demonstrating that GmGrx contributes to the defense of oxidative damage induced by emamectin benzoate in Grapholita molesta (Busck) [38]. These results provided insights for an innovative strategy in insect control of RNAi technology with the silencing of Grxs combined with pesticides.
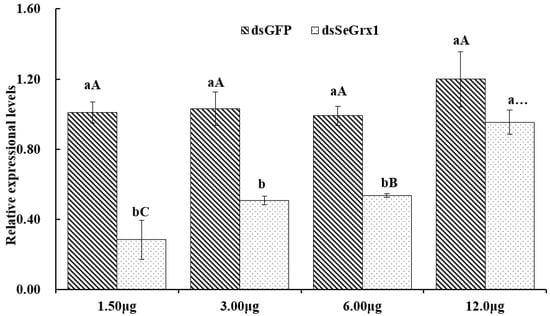
Figure 4.
The relative expression of SeGrx1 after RNAi in the larvae of S. exigua. Data are presented as mean ± standard error. Independent samples t-test and one-way ANOVA, followed by the Duncan’s multiple range test were performed by using SPSS 26.0 Software Package (SPSS Inc., Chicago, IL, USA). Data followed by the same lowercase letters indicate no significant difference at 0.05 level between dsGFP group and dsSeGrx1 treatment at the same time. Data followed by the same capital letters indicate no significant difference at 0.05 level among the same group treated with different concentration of dsRNA.
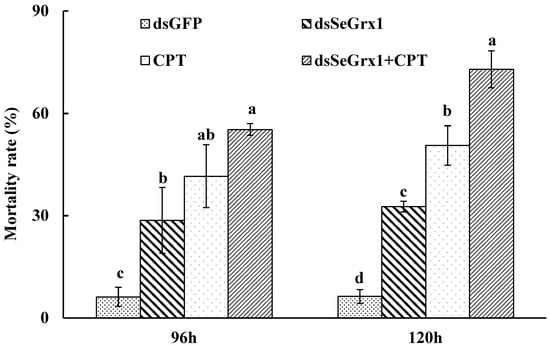
Figure 5.
Mortality of S. exigua larvae treated by dsRNA and/or CPT at 96 h and 120 h. Values are shown as mean ± SEM. Data followed by the same lowercase letters among different treatments at the same time indicate no significant difference at 0.05 level.
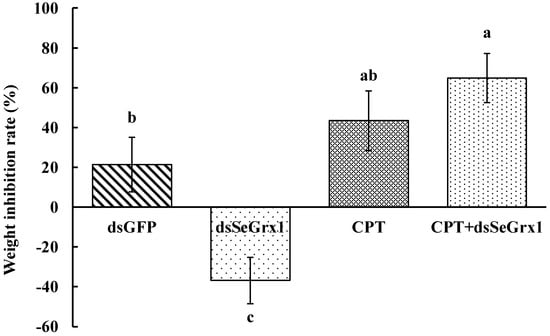
Figure 6.
Weight inhibition rate of S. exigua larvae treated by dsRNA and/or CPT at 120 h. Values are shown as mean ±SEM. Data followed by the same lowercase letters indicate no significant difference at 0.05 level.
4. Conclusions
In conclusion, our results confirmed that SeGrx1 played an important role in defending against the oxidative stress induced by CPT in S. exigua, and the sensitivity of larvae to CPT was increased by the silencing of SeGrx1. It could be proposed that oxidative stress is more intense in insects. These results provide a strategy in insect pest control of RNAi technology combined with pesticides.
Author Contributions
F.Y. and L.Z. (Lan Zhang) conceived and designed the research. F.Y. and Y.Z. performed the experiments. F.Y. analyzed the data and wrote the manuscript. L.Z. (Lizhen Zhu) and H.J. reviewed the manuscript. X.L. and L.M. helped analysis with constructive discussions. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Chinese National Natural Science Foundation (31672059).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank Li Cui for kindly providing S. exigua larvae.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fernandes, A.P.; Holmgren, A. Glutaredoxins: Glutathione-Dependent Redox Enzymes with Functions Far Beyond a Simple Thioredoxin Backup System. Antioxid. Redox Signal. 2004, 6, 63–74. [Google Scholar] [CrossRef]
- Ogata, F.T.; Branco, V.; Vale, F.F.; Coppo, L. Glutaredoxin: Discovery, Redox Defense and Much More. Redox Biol. 2021, 43, 101975. [Google Scholar] [CrossRef]
- Abdalla, M.; Eltayb, W.A.; Yousif, A. Comparison of Structures among Saccharomyces cerevisiae Grxs Proteins. Genes Environ. 2018, 40, 17. [Google Scholar] [CrossRef]
- Lillig, C.H.; Berndt, C.; Holmgren, A. Glutaredoxin Systems. Biochim. Biophys. Acta. 2008, 1780, 1304–1317. [Google Scholar] [CrossRef]
- Maghool, S.; La Fontaine, S.; Maher, M.J. High-Resolution Crystal Structure of the Reduced Grx1 from Saccharomyces cerevisiae. Acta Crystallogr. F Struct. Biol. Commun. 2019, 75 Pt 5, 392–396. [Google Scholar] [CrossRef]
- Matsui, R.; Ferran, B.; Oh, A.; Croteau, D.; Shao, D.; Han, J.; Pimentel, D.R.; Bachschmid, M.M. Redox Regulation via Glutaredoxin-1 and Protein S-Glutathionylation. Antioxid. Redox Signal. 2020, 32, 677–700. [Google Scholar] [CrossRef]
- Sun, J.; Wei, X.; Lu, Y.; Cui, M.; Li, F.; Lu, J.; Liu, Y.; Zhang, X. Glutaredoxin 1 (GRX1) Inhibits Oxidative Stress and Apoptosis of Chondrocytes by Regulating CREB/HO-1 in Osteoarthritis. Mol. Immunol. 2017, 90, 211–218. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, Y.; Zhao, S.; Xu, W.; Li, Y.; Zhao, P.; Wang, D.; Cheng, H.; Ke, Y.; Zhang, X. Oxidative Stress-Induced FABP5 S-Glutathionylation Protects Against Acute Lung Injury by Suppressing Inflammation in Macrophages. Nat. Commun. 2021, 12, 7094. [Google Scholar] [CrossRef]
- Lozano, R.M.; Yee, B.C.; Buchanan, B.B. Thioredoxin-Linked Reductive Inactivation of Venom Neurotoxins. Arch. Biochem. Biophys. 1994, 309, 356–362. [Google Scholar] [CrossRef]
- Yao, P.; Chen, X.; Yan, Y.; Liu, F.; Zhang, Y.; Guo, X.; Xu, B. Glutaredoxin 1, Glutaredoxin 2, Thioredoxin 1, and Thioredoxin Peroxidase 3 Play Important Roles in Antioxidant Defense in Apis cerana cerana. Free Radic. Biol. Med. 2014, 68, 335–346. [Google Scholar] [CrossRef]
- Zhang, S.D.; Shen, Z.J.; Liu, X.M.; Li, Z.; Zhang, Q.W.; Liu, X.X. Molecular Identification of Three Novel Glutaredoxin Genes that Play Important Roles in Antioxidant Defense in Helicoverpa armigera. Insect Biochem. Mol. Biol. 2016, 75, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.J.; Zhang, S.D.; Liu, Y.J.; Liu, X.M.; Li, Z.; Zhang, Q.W.; Liu, X.X. Functional Analysis by RNAi of an Glutaredoxin Gene in Helicoverpa armigera. J. Insect Physiol. 2018, 106, 98–105. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Zhang, Y.; Wang, T.; Luo, M.; Li, C. Molecular Characterization of Glutaredoxin 2 from Ostrinia furnacalis. Integr. Zool. 2013, 8 (Suppl. 1), 30–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Y.; He, W.; Ma, D.; Jiang, H. Effects of Camptothecin and Hydroxycamptothecin on Insect Cell Lines Sf21 and IOZCAS-Spex-II. Pest Manag. Sci. 2011, 68, 652–657. [Google Scholar] [CrossRef]
- Salvia, R.; Grossi, G.; Amoresano, A.; Scieuzo, C.; Nardiello, M.; Giangrande, C.; Laurenzana, I.; Ruggieri, V.; Bufo, S.A.; Vinson, S.B.; et al. The Multifunctional Polydnavirus TnBVANK1 Protein: Impact on Host Apoptotic Pathway. Sci. Rep. 2017, 7, 11775. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, L.; Zhang, Y.; Mao, L.; Jiang, H. Oxidative Stress Induced by Camptothecin and Hydroxyl-Camptothecin in IOZCAS-Spex-II Cells of Spodoptera exigua Hübner. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 216, 52–59. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, L.; Zhang, Y.; Mao, L.; Jiang, H. Mitochondria Response to Camptothecin and Hydroxycamptothecine-Induced Apoptosis in Spodoptera exigua Cells. Pestic. Biochem. Physiol. 2017, 140, 97–104. [Google Scholar] [CrossRef]
- Yang, F.L.; Zhao, Z.Z.; Wang, Y.H.; Zhang, L.; Zhang, Y.N.; Mao, L.G.; Jiang, H.Y. Prokaryotic Expression, Purification and Enzymatic Characterization of SeGrx1 of the Beet Armyworm, Spodoptera exigua (Lepidoptera: Noctuidae). Acta Entomol. Sin. 2020, 63, 952–960. [Google Scholar]
- Tang, B.; Wang, S.; Zhang, F. Two Storage Hexamerins from the Beet Armyworm Spodoptera exigua: Cloning, Characterization and the Effect of Gene Silencing on Survival. BMC Mol. Biol. 2010, 11, 65. [Google Scholar] [CrossRef]
- Ren, X.L.; Ma, Y.; Cui, J.J.; Li, G.Q. RNA Interference-Mediated Knockdown of Three Putative Aminopeptidases N Affects Susceptibility of Spodoptera exigua Larvae to Bacillus thuringiensis Cry1Ca. J. Insect Physiol. 2014, 67, 28–36. [Google Scholar] [CrossRef]
- Gurusamy, D.; Mogilicherla, K.; Shukla, J.N.; Palli, S.R. Lipids Help Double-Stranded RNA in Endosomal Escape and Improve RNA Interference in the Fall Armyworm, Spodoptera frugiperda. Arch. Insect Biochem. Physiol. 2020, 104, e21678. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, M.; Liu, S.; Jan, S.; Shi, L.; Fernández-Grandon, G.M.; Gulzar, A.; Ali, B.; Rehman, M.; Wang, M. Knock-Down of Gossypol-Inducing Cytochrome P450 Genes Reduced Deltamethrin Sensitivity in Spodoptera exigua (Hübner). Int. J. Mol. Sci. 2019, 20, 2248. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.Y.; Huang, J.L.; Wang, J.; Feng, Y.; Han, T.T.; Wu, Y.D.; Yang, Y.H. Knockout of a P-Glycoprotein Gene Increases Susceptibility to Abamectin and Emamectin Benzoate in Spodoptera exigua. Insect Mol. Biol. 2018, 27, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate Normalization of Real-time Quantitative RT-PCR Data by Geometric Averaging of Multiple Internal Control Genes. Genome Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef]
- Huvenne, H.; Smagghe, G. Mechanisms of dsRNA Uptake in Insects and Potential of RNAi for Pest Control: A Review. J. Insect Physiol. 2010, 56, 227–235. [Google Scholar] [CrossRef]
- Manta, B.; Möller, M.N.; Bonilla, M.; Deambrosi, M.; Grunberg, K.; Bellanda, M.; Comini, M.A.; Ferrer-Sueta, G. Kinetic Studies Reveal a Key Role of a Redox-Active Glutaredoxin in the Evolution of the Thiol-Redox Metabolism of Trypanosomatid Parasites. J. Biol. Chem. 2019, 294, 3235–3248. [Google Scholar] [CrossRef]
- Butt, J.N. Explorations of time and electrochemical potential: Opportunities for fresh perspectives on signalling proteins. Biochem. Soc. Trans. 2014, 42, 47–51. [Google Scholar] [CrossRef]
- Lillig, C.H.; Lönn, M.E.; Enoksson, M.; Fernandes, A.P.; Holmgren, A. Short Interfering RNA-Mediated Silencing of Glutaredoxin 2 Increases the Sensitivity of HeLa Cells Toward Doxorubicin and Phenylarsine Oxide. Proc. Natl. Acad. Sci. USA 2004, 101, 13227–13232. [Google Scholar] [CrossRef]
- Linares, G.R.; Xing, W.; Govoni, K.E.; Chen, S.T.; Mohan, S. Glutaredoxin 5 Regulates Osteoblast Apoptosis by Protecting Against Oxidative Stress. Bone 2009, 44, 795–804. [Google Scholar] [CrossRef][Green Version]
- Shu, B.; Yang, X.; Dai, J.; Yu, H.; Yu, J.; Li, X.; Cao, L.; Lin, J. Effects of Camptothecin on Histological Structures and Gene Expression Profiles of Fat Bodies in Spodoptera frugiperda. Ecotoxicol. Environ. Saf. 2021, 228, 112968. [Google Scholar] [CrossRef]
- Shu, B.; Zou, Y.; Yu, H.; Zhang, W.; Li, X.; Cao, L.; Lin, J. Growth Inhibition of Spodoptera frugiperda Larvae by Camptothecin Correlates with Alteration of the Structures and Gene Expression Profiles of the Midgut. BMC Genomics 2021, 22, 391. [Google Scholar] [CrossRef] [PubMed]
- Christiaens, O.; Niu, J.; Nji Tizi Taning, C. RNAi in Insects: A Revolution in Fundamental Research and Pest Control Applications. Insects 2020, 11, 415. [Google Scholar] [CrossRef] [PubMed]
- Katoch, R.; Sethi, A.; Thakur, N.; Murdock, L.L. RNAi for Insect Control: Current Perspective and Future Challenges. Appl. Biochem. Biotechnol. 2013, 171, 847–873. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, B.; Grossi, G.; Falabella, P.; Liu, Y.; Yan, S.; Lu, J.; Xi, J.; Wang, G. Molecular Basis of Alarm Pheromone Detection in Aphids. Curr. Biol. 2017, 27, 55–61. [Google Scholar] [CrossRef]
- Liu, D.; De Schutter, K.; Chen, P.; Smagghe, G. The N-Glycosylation-Related Genes as Potential Targets for RNAi-Mediated Pest Control of the Colorado Potato Beetle (Leptinotarsa Decemlineata). Pest Manag. Sci. 2021. [Google Scholar] [CrossRef]
- Yu, R.; Xu, X.; Liang, Y.; Tian, H.; Pan, Z.; Jin, S.; Wang, N.; Zhang, W. The Insect Ecdysone Receptor is a Good Potential Target for RNAi-Based Pest Control. Int. J. Biol. Sci. 2014, 10, 1171–1180. [Google Scholar] [CrossRef]
- Yoon, J.S.; Koo, J.; George, S.; Palli, S.R. Evaluation of Inhibitor of Apoptosis Genes as Targets for RNAi-Mediated Control of Insect Pests. Arch. Insect Biochem. Physiol. 2020, 104, e21689. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, F.; Shen, Z.; Moural, T.W.; Liu, L.; Li, Z.; Liu, X.; Xu, H. Glutaredoxins and Thioredoxin Peroxidase Involved in Defense of Emamectin Benzoate Induced Oxidative Stress in Grapholita molesta. Pestic. Biochem. Physiol. 2021, 176, 104881. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).